INTRODUCTION
A common protozoan parasite Toxoplasma gondii (worldwide prevalence varies between 20 and 70% in different countries, depending on climate, hygiene standards and eating habits) (Tenter et al. Reference Tenter, Heckeroth and Weiss2000) is known to induce behavioural and neurophysiological changes in infected human or animal hosts (Webster, Reference Webster2001). Increased dopamine and testosterone levels are suspected to play an important role in the observed changes (Flegr, Reference Flegr2007). However, the association between toxoplasmosis and increased testosterone concentration was only postulated on the basis of indirect evidence. Infected men are taller, have a lower left hand 2D:4D ratio (Flegr et al. Reference Flegr, Hruskova, Hodny, Novotna and Hanusova2005) and higher perceived dominance and masculinity scores (Hodkova et al. Reference Hodkova, Kolbekova, Skallova, Lindová and Flegr2007). Infected women have a (non-significantly) lower left hand 2D:4D ratio and are more likely to give birth to a boy than a girl (Kaňková et al. Reference Kaňková, Šulc, Nouzová, Fajfrlík, Frynta and Flegr2007). All these traits are known to be positively associated with testosterone concentration in humans (James, Reference James1986, Reference James1996; Drop et al. Reference Drop, De Waal and Keizer-Schrama1998; Brown et al. Reference Brown, Hines, Fane and Breedlove2002; Okten et al. Reference Okten, Kalyoncu and Yaris2002; Neave et al. Reference Neave, Laing, Fink and Manning2003; Lutchmaya et al. Reference Lutchmaya, Baron-Cohen, Raggatt, Knickmeyer and Manning2004). However, no studies directly comparing testosterone levels in Toxoplasma-infected and Toxoplasma-free subjects have been available to date and analogous studies in animal models are missing as well.
Here, we searched for direct evidence of the changed testosterone levels in Toxoplasma-infected men and women by testing the concentration of testosterone in saliva in a population of 174 female and 91 male students screened for Toxoplasma infection.
MATERIALS AND METHODS
Subjects
Undergraduate biology students of the Faculty of Sciences, Charles University, Prague, were addressed during regular biology lectures and were invited to participate in the study on a voluntary basis. In total, 91 male students and 174 female students were enrolled in the study and signed an informed consent form. All participants provided 2 ml of blood for serological testing and 3 saliva samples of approximately 200 μl (collected approximately at 09.00, 11.30 and 13.00). Meanwhile, all participants performed the same panel of psychological and ethological tests. All blood and saliva samples were stored in a freezer at −20°C until assayed. The recruitment of the study subjects and data handling practices complied with the Czech regulations currently in force.
Immunological tests for toxoplasmosis
All serological tests were carried out in the National Reference Laboratory for Toxoplasmosis, National Institute of Public Health, Prague. Specific anti-Toxoplasma IgG ELISA (in-house test NRL TOXO – Pokorný et al. Reference Pokorný, Fruhbauer, Polednakova, Sykora, Zastera and Fialova1989); and the complement fixation test (CFT – SEVAPHARMA, Prague) which is more reliable in old T. gondii infections as decrease in CFT titres is more regular (Warren and Sabin, Reference Warren and Sabin1942; Kodym et al. Reference Kodym, Machala, Rohacova, Sirocka and Maly2007) were performed in all subjects. Anti-Toxoplasma IgM antibodies were determined by ELISA (TestLine, Brno) optimized for early detection of acute toxoplasmosis in subjects with CFT titres of ⩾1:64. Toxoplasma antibody titres in the sera were measured at dilutions between 1:8 and 1:1024. The subjects with positive IgG ELISA values, CFT titres higher or equal than 1:8 and testing IgM negative by ELISA (positivity index<0·9) were considered latent toxoplasmosis positive.
Radioimmunoassay (RIA) test for testosterone
All testosterone assays were performed at the Institute of Endocrinology, Prague. Saliva samples and controls or blanks (double-distilled water), 1·0 ml each, were spiked with [3H] testosterone (Radiochemical Centre, Amersham, UK, 1200 dpm/sample), and extracted in duplicate with diethyl ether (4 ml) in stoppered glass tubes. The aqueous phase was frozen in solid carbon dioxide, the organic phase was decanted and the ether was evaporated to dryness. The extracts were dissolved in ethanol (500 μl), and then 100 μl of the solution were removed for determination of the losses during the extraction, while the rest was evaporated again and taken for radioimmunoassay. A standard curve consisting of 0·025, 0·05, 0·1, 0·2, 0·4, 0·8, 1·6 and 3·2 nmol/l testosterone in duplicate was prepared. Antiserum (rabbit anti-testosterone-3-CMO: BSA, working dilution 1:100 000) and the tracer ([125I] iodohistaminyl testosterone derivative, 15 000 cpm), 100 μl each, were added, the volume was adjusted to 300 μl with the working buffer (20 mmol sodium phosphate saline containing sodium azide and BSA, 0·1% each) and the tubes were equilibrated at room temperature for 1 h or overnight at 4°C. After incubation, 1 ml of dextran-coated charcoal suspension (0·025 and 0·25 g/100 ml, respectively) was added to each tube to separate the free fraction and the radioactivity of 125I was measured in the supernatant using a 12-channel gamma counter (Berthold, FRG). Results were calculated from the standard curve using a log-logit transformation, corrected for recovery and expressed as nmol of testosterone per litre of sample. The samples were assayed in triplicate. Because of the low volume of some saliva samples (the minimum limit for a reliable assay was 100 μl) the complete testosterone concentration data (all 3 samples) were not obtained for all subjects.
Statistics
Differences in age and testosterone concentration between men and women were measured with t-tests. The relation between the average testosterone concentration in all (usually 3) samples of a particular subject and independent factors sex, age and Toxoplasma infection was tested with General Linear Models (GLM). The difference was also analysed with repeated measures GLM, with concentration of testosterone in samples 1–3 as a dependent variable and sex, age and toxoplasmosis as independent variables, in 236 subjects with available data from all 3 saliva samples (126 Toxoplasma-infected and 26 Toxoplasma-free women, 62 Toxoplasma-infected and 22 Toxoplasma-free men). If the Mauchly's test indicated violation of the sphericity assumption, the degrees of freedom were corrected using the Greenhouse-Geisser method. The separate analyses for men and women were performed with GLM (with the covariate age) and (nonparametric) Mann-Whitney U test (without any covariate). All parametric statistical tests were performed with log-transformed testosterone concentrations; however, there was practically no qualitative difference between results of statistical tests performed either with or without log transformation of the testosterone concentration data. All analyses including the tests of analyses assumptions (Levene tests of homogeneity, univariate and multivariate Lack of fit tests, Mauchly's tests of sphericity) were performed with SPSS 12.0.
RESULTS
The average age of 174 women was 21.03 years, range 18–28, median 21, lower quartile 20, upper quartile 22, average concentration of testosterone was 0·230 ng/ml, range 0·035–0·860, median 0·201, lower quartile 0·148, upper quartile 0·275, The average age of 91 men was 20.91 years, range 19–27, median 20, lower quartile 20, upper quartile 22, average concentration of testosterone was 0·387 ng/ml, range 0·083–1·088, median 0·358, lower quartile 0·266, upper quartile 0·485. The difference in age between women and men was non-significant t263=0·494, P=0·62. However, we found significantly higher levels of testosterone (t263=−8·133, P<0·0001) in men than in women.
Twenty-nine (16·7%) women and 23 (25·3%) men were Toxoplasma-infected. The difference between Toxoplasma-infected and Toxoplasma-free subjects in average testosterone concentration estimated by GLM with independent factors sex, age and toxoplasmosis was nonsignificant (F1,260=0·906, P=0·342, η2=0·003). At the same time, the effect of the toxoplasmosis-sex interaction on the testosterone concentration was highly significant (F1,260=13·434, P<0·001, η2=0·049). Infected men had higher and infected women had lower testosterone levels than Toxoplasma-free men and women, respectively (Fig. 1). The difference in testosterone concentration was significant for women (GLM: F1,171=12·643, P<0·001, η2=0·069, M-W: U=1298, P=0·001) and non-significant for men (F1,88=2·678, P=0·105, η2=0·030, M-W: U=645, P=0·211).

Fig. 1. Average testosterone levels in saliva of Toxoplasma-free and Toxoplasma-infected men and women. Boxes represent standard errors; spreads represent standard deviations.
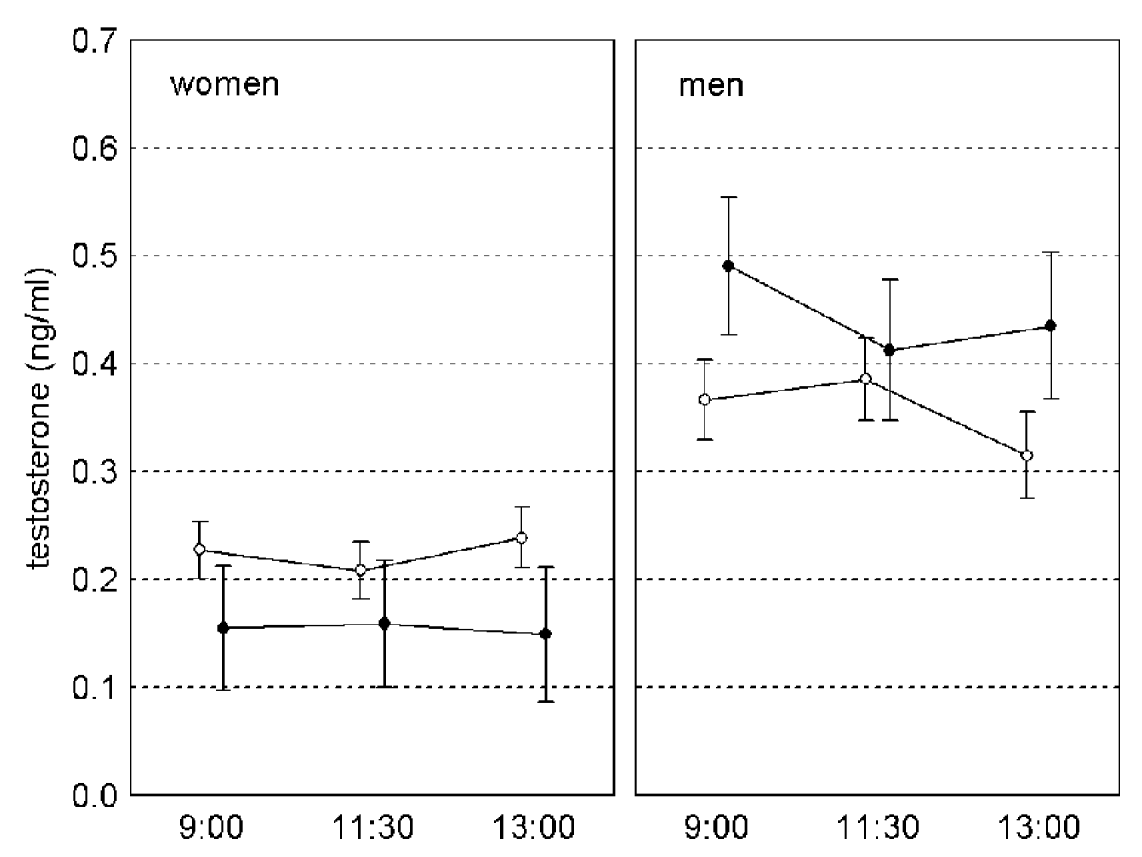
The results of repeated measures GLM, with concentration of testosterone in samples 1–3 as a dependent variable and sex, age and toxoplasmosis as independent variables, were similar to those of GLM analyses with the average testosterone concentration for samples 1–3 as a dependent variable. The effect of toxoplasmosis was non-significant (F1,231=1·155, P=0·284, η2=0·005) while the effects of toxoplasmosis-sex interaction (F1,231=16·347, P<0·001, η2=0·066) was significant. The effect of time-toxoplasmosis-sex interaction was non-significant (F1·86,429·55=2·464, P=0·090, η2=0·011, linear contrast: F1,231=0·207, P=0·649, η2 <0·001, quadratic contrast: F1,231=6·047, P=0·015, η2=0·026). Separate repeated measures GLM analyses for women and men have shown significant effects of toxoplasmosis in women (F1,149=15·579, P<0·001, η2=0·095) and of time-toxoplasmosis interaction in men (F2,162=3·283, P<0·040, η2=0·039, linear contrast: F1,81=0·095, P=0·759, η2=0·001, quadratic contrast: F1,81=7·959, P=0·006, η2=0·089), (Fig. 2).
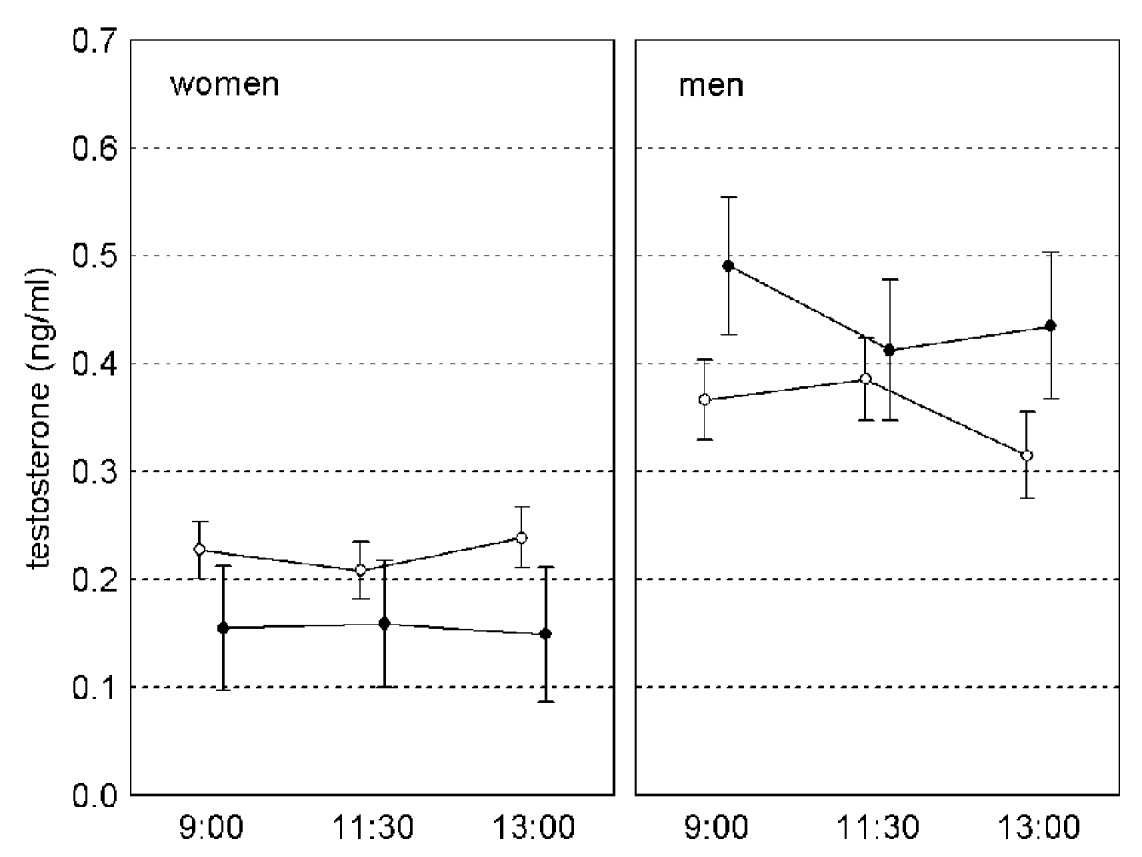
Fig. 2. Changes of testosterone level in saliva of Toxoplasma-free (empty circles) and Toxoplasma-infected (black circles) subjects between 09.00 and 13.00. Vertical bars denote 0·95 confidence intervals.
DISCUSSION
We have found Toxoplasma-infected men to have a higher concentration of testosterone and Toxoplasma-infected women to have a lower concentration of testosterone than Toxoplasma-free subjects. The difference was significant for all 3 saliva samples in women but only for saliva samples 1 (collected at 09.00) and 3 (collected at 13.00) in men.
The proximal mechanism of association between testosterone and toxoplasmosis is not clear. As stressed in previous articles (Flegr et al. Reference Flegr, Hruskova, Hodny, Novotna and Hanusova2005; Hodkova et al. Reference Hodkova, Kolbekova, Skallova, Lindová and Flegr2007), a case control study cannot decide whether Toxoplasma infection induces changes in testosterone concentration or whether low-testosterone and high-testosterone subjects differ in the probability of acquiring Toxoplasma infection or both. High concentrations of testosterone are known to have immunosuppressive effects (Roberts et al. Reference Roberts, Walker and Alexander2001; Schuster and Schaub, Reference Schuster and Schaub2001). The results of the present study, in particular the lower concentration of testosterone in Toxoplasma infected women, however, make the immunosuppression-based explanation of the association between Toxoplasma infection and testosterone concentration rather unlikely.
The increased level of testosterone in men could be explained by a positive association between testosterone and dopamine (Szczypka et al. Reference Szczypka, Zhou and Palmiter1998; Dominguez and Hull, Reference Dominguez and Hull2005; Hull et al. Reference Hull, Muschamp and Sato2004) that can increase in response to local inflammatory processes in the infected brain (Flegr, Reference Flegr2007). However, we have no explanation for the decreased testosterone in infected women.
It has been shown in several studies that latent toxoplasmosis has gender-different effects on the personality factors A (warmth), G (rule consciousness), L (vigilance, mistrust) and Q3 (self-control, self-image) from Cattell's 16PF Questionnaire (Flegr et al. Reference Flegr, Zitkova, Kodym and Frynta1996, Reference Flegr, Kodym and Tolarova2000). Behavioural experiments also showed gender-different effects in behavioural variables Self-Control and Clothes Tidiness (analogue to the 16PF factors G – conscientiousness and Q3 – self-control), with infected men scoring significantly lower than uninfected men and infected women scoring non-significantly higher than uninfected women (Lindová et al. Reference Lindová, Novotná, Havliček, Jozífková, Skallová, Kolbeková, Hodný, Kodym and Flegr2006).
Biological and psychological hypotheses were suggested to explain the opposite directions of personality factor scores and behavioural shifts in men and women. According to the biological hypothesis, infected intermediate hosts are manipulated by the parasite to behave in a way which makes them easier prey for the final host (Lindová et al. Reference Lindová, Novotná, Havliček, Jozífková, Skallová, Kolbeková, Hodný, Kodym and Flegr2006). In primitive human societies, being reserved, detached and critical of other people, being uncontrolled, lax, disregarding rules (low Self Control) and untidily dressed would isolate males from a group, making them a more probable prey. The opposite traits in infected woman could make her more attractive for potential sexual partners, and in such a way increase the rate of its transmission into the offspring.
The psychological hypothesis assumes that men and women use a different strategy to cope with non-specific stressors (including toxoplasmosis) (Lindová et al. Reference Lindová, Novotná, Havliček, Jozífková, Skallová, Kolbeková, Hodný, Kodym and Flegr2006). There is some evidence that men are socialized to cope with stress differently from women. Men are expected to use more problem-focused forms of coping, while women are brought up to cope with an emotion-focused style (Brems and Johnson, Reference Brems and Johnson1989; Carver et al. Reference Carver, Scheier and Weintraub1989). Thus, coping with toxoplasmosis, men would be expected to withdraw from society in order to concentrate on the ‘problem’, whereas women would be expected to turn to society, where they can express their emotions.
Our present results suggest that the gender-different effects of latent toxoplasmosis on behavioural traits and personality factor scores can be the product of the opposite effects of toxoplasmosis on testosterone levels. The changes in testosterone concentration in men and women can be either side-effects of the Toxoplasma activities or a result of the specific manipulation activity of the parasite aimed to increase the probability of its transmission from intermediate to definitive host (Dawkins, Reference Dawkins1982; Barnard and Behnke, Reference Barnard and Behnke1990). Therefore, our testosterone hypothesis is compatible with the biological explanation mentioned above.
Although several pieces of indirect evidence for increased testosterone in Toxoplasma-infected men have been recently reported, we present here the first piece of direct (biochemical) evidence for the changed testosterone concentration in saliva of infected subjects. Surprisingly, our study has not only confirmed a positive association between latent toxoplasmosis and testosterone level in infected men but also has shown a negative association between latent toxoplasmosis and testosterone level in infected women. Obviously, it is not possible to perform an infection experiment in the human-Toxoplasma system. The relatively low incidence of toxoplasmosis would also make a longitudinal study rather inefficient. Therefore, the direction of causality between toxoplasmosis and testosterone level will probably need to be studied in experimentally infected laboratory animals.
We thank Aleš Kuběna for his help with statistical analysis. This research was supported by grant no. 406/07/0581 of the Grant Agency of the Czech Republic, 160/2006/B-B10/PrF GAUK and grant no. 0021620828 of the Czech Ministry of Education.