Published online by Cambridge University Press: 13 December 2004
Estimates of the intensity and abundance of species provide essential data for ecological, evolutionary and epidemiological studies of gastrointestinal nematode communities. These estimates are typically derived from the species composition of adult males when only males have readily scorable species-specific morphological traits. Such estimation assumes that all species in the community have the same adult sex ratio. We evaluated this assumption for the trichostrongyle nematodes Ostertagia gruehneri and Marshallagia marshalli in infracommunities in Svalbard reindeer by identifying to species adult females using a polymerase chain reaction assay. The proportion of males was found to be slightly higher in O. gruehneri than in M. marshalli. Evidence for seasonal variation and density dependence in the adult sex ratio was only found for O. gruehneri. Possible demographic mechanisms for such sex ratio variation are discussed, and stochastic models that generate density-dependent sex ratios proposed. Sex ratio variation caused substantial bias in some male-based estimates of intensity of infection, while substantial and consistent bias in estimates of abundances was only evident in late winter samples. Our results suggest that estimating sex ratios can be particularly important in individual host level studies of nematode species of low abundance.
Infrapopulations of parasitic nematodes usually consist of more female than male adult individuals (Poulin, 1997a,b), but both inter- and intra-specific variation in sex ratios has been observed (Crofton & Whitlock, 1969; Haukisalmi, Henttonen & Vikman, 1996; Stien, Halvorsen & Leinaas, 1996; Poulin, 1997a,b). Intraspecific variation in sex ratios can be seasonal and density dependent (Singhvi & Johnson, 1976; Haukisalmi et al. 1996) while interspecific variation has been associated with abundances of infection and sexual size dimorphism (Poulin, 1997a,b). Population dynamic consequences of sex ratio variation arise from effects on mating probabilities and egg production (May & Woolhouse, 1993). For gastrointestinal (GI) nematodes of ruminants, knowledge of sex ratio variation is mainly available from single species infection experiments (Crofton & Whitlock, 1969; Borgsteede & Hendriks, 1979) due to difficulties in identifying to species females. In trichostrongyloid GI nematodes, males may be identified to species from the morphology of the spicules and the genital cone and females from the pattern of cuticular ridges (synlope) and structure of the oesophagus (Gibbons & Khalil, 1983; Lichtenfels, 1983). The morphology of the male bursa, the spicules and the genital cone has traditionally been included in species descriptions and is available for most species, whereas good descriptions of the synlope and structure of the oesophagus are mostly lacking. The male characters are also technically more accessible. In natural mixed species infections, estimates of species abundances are therefore usually derived from the species composition of adult males by assuming that all species in the community have the same sex ratio. However, violations of the assumption of a common sex ratio cause estimates of species abundances to be biased. For many GI nematodes, both sexes can now be identified to species using molecular assays (McKeand, 1998; Zarlenga & Higgins, 2001).
The population dynamics of Svalbard reindeer (Rangifer tarandus platyrhynchus) and its GI nematode community has recently been the focus of intensive studies (Halvorsen et al. 1999; Irvine et al. 2000, 2001; Albon et al. 2002; Stien et al. 2002a,b). In this GI nematode community, just two species, Ostertagia gruehneri and Marshallagia marshalli, contribute more than 99% of the individuals (Bye & Halvorsen, 1983; Halvorsen & Bye, 1999; Dallas, Irvine & Halvorsen, 2000a, 2001; Irvine et al. 2000). Adult males of these species are identifiable using genital morphology (Drózdz, 1965). The synlope structure of both species has also been studied, but only compared between dimorphs within species, making female species identification difficult (Lichtenfels & Pilitt, 1989; Lichtenfels, Pilitt & Fruetel, 1990). Both sexes of the species may, however, be identified using a polymerase chain reaction (PCR) assay (Dallas et al. 2000b). In O. gruehneri but not M. marshalli, fecundity is under density-dependent regulation (Irvine et al. 2001), and intensity of infection is inversely related to host body condition and fecundity (Stien et al. 2002b). The latter two effects give O. gruehneri the potential to regulate the size of its host population (Albon et al. 2002). However, all these findings are based on male-derived estimates of intensities of infection for O. gruehneri and M. marshalli. Any interspecific differences in sex ratio would make the estimates of intensities of infection biased.
The goals of this study were to characterize interspecific differences and intraspecific variation in the sex ratio in O. gruehneri and M. marshalli from Svalbard reindeer, and to assess the degree of any resulting bias in species-specific estimates of intensity of infection (i.e. number of parasites in one host) and abundance of infection (i.e. average number of parasites in a population of hosts including uninfected animals). Estimates of sex ratios in individual hosts were obtained using a combination of morphological identification of male nematodes and PCR identification of females. The sampling design used to obtain the species-specific estimates of sex ratios involved several levels of subsampling. We suggest a statistical method that takes into account this sampling design and gives valid estimates of the sampling variance associated with the sex ratio estimates. The association between estimated sex ratios, intensity of infection, study site, season of sampling, and anthelmintic treatment of the host were assessed separately for each nematode species. Male-derived estimates of intensities and abundances of infection were assessed for bias by comparing them with estimates derived from both sexes. Finally, we suggest some simple demographic processes that may cause observed density-dependent patterns in the sex ratio of GI nematodes.
Female Svalbard reindeer aged 1 year or older were culled in 2 valleys, Colesdalen and Sassendalen in Nordenskjiöldland, Spitsbergen (77°50′–78°20′N and 15°00′–17°30′E). Colesdalen is approximately 20 km southwest, and Sassendalen, approximately 40 km east, of Longyearbyen, the main settlement on Svalbard. Reindeer were culled in April 1999 (n=9), July–early August 1999 (n=14), October 1999 (n=20), April 2000 (n=8) and October 2000 (n=11). Twelve reindeer were culled in Sassendalen and the remaining 50 were culled in Colesdalen. The anthelmintic moxidectin was administered to 12 reindeer in Colesdalen in late April 1999. These reindeer form a subsample of 6 animals in each of the samples of reindeer culled during the following July and October (Irvine et al. 2000). Moxidectin treatment causes expulsion of nematodes and prevents reinfection for at least 5 weeks subsequently (Irvine, 2000). Thus, the treated animals sampled in late July 1999 were exposed to reinfection for 7 weeks and those sampled in late October 1999 were exposed to reinfection for 19 weeks.
The abomasum of each culled animal was ligated, extracted, and frozen within 3 h of death. Each abomasum was thawed in the laboratory, opened along the greater curvature and its contents washed out into 4 litres of water. The suspension was mixed thoroughly and one or more 5% subsamples were taken using a vacuum pump and passed through a 150 μm sieve. Nematodes were sampled from the retained material and those with clearly defined genital structures were categorized as adults.
Population sizes of adult males and females of O. gruehneri and M. marshalli in each reindeer were estimated as follows. Numbers of adult male (nm) and female (nf) nematodes in 1–4 abomasal subsamples (nsub) were counted until at least 100 male and 100 females were obtained or until 20% of the abomasal contents had been examined. A subsample of sm males (range 74–148 individuals) of the nm males counted were identified to species according to genital morphology (Drózdz, 1965), yielding sm,i number of males of species i. Similarly, a subsample of sf females (range 43–49 individuals) of the nf females counted were identified to species according to a PCR assay of the second internal transcribed spacer of ribosomal DNA (Dallas et al. 2000b), yielding sf,i number of females of species i.
The sex ratio of species i in each host (Pi) was estimated as the proportion of males in the total number of adults of that species:

where pm is the proportion of male nematodes in adults, pm,i is the proportion of species i in males, and pf,i is the proportion of species i in females. The sample estimates of the unknown proportions in this equation were estimated as

,

and

.
When estimating the sample variance of

, we assumed that subsampling was random with respect to sex and species. The variables nm, sm,i and sf,i could then be regarded as binomial random variables:



This model (eq. 1–4) was implemented in the program WinBUGS (Lunn et al. 2000). WinBUGS is available from http://www.mrc-bsu.cam.ac.uk/bugs/, and the code for fitting the model is available from the senior author. The parameters pm, pm,i and pf,i were assumed to have independent, uniform prior distributions bounded by 0 and 1 when estimating

and

using Markov chain Monte Carlo (MCMC) methodology (Gilks, Richardson & Spiegelhalter, 1996). The model was run with a burn-in period of 5000 iterations followed by sampling from the posterior distribution of

for 25000 iterations. Convergence was assessed in WinBUGS using the Gelman-Rubin convergence statistic, as modified by Brooks & Gelman (1998).
Associations of

with year, season, study site, anthelmintic treatment and intensity of infection were analysed using weighted least-squares regression, with weights given by

. Two reindeer with outlying estimates of the proportion of males of O. gruehneri and M. marshalli (Fig. 1) were assumed to result from mislabelling of tubes of separated male and female nematodes, and were not analysed further. In O. gruehneri, the proportion of males increased asymptotically with intensity of infection (I), and this relationship fitted well to the model:

where πl is the expected proportion of males in reindeer individual l, β0 and β1 are parameters assumed to be constant across hosts, and αuvwx is the asymptotic male proportion with increasing intensity of infection for month u, valley v, year w, and anthelmintic treatment x.

Fig. 1. Estimated proportions of males in adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli (with 95% confidence limit bars) plotted against the estimated intensity of infection of the nematode species in individual reindeer. Closed squares indicate nematodes from reindeer treated with an anthelmintic in April or May of the year of sampling, and open squares indicate untreated reindeer. Crosses indicate 2 outlying observations.
Previously, we (Irvine et al. 2000, 2001; Albon et al. 2002; Stien et al. 2002a,b) used the following estimator for the intensity of infection of species i (Ii):

This estimator is based on the species composition from male nematodes only and assumes that the sex ratios of the species in the infrapopulations are the same. When the species composition of both the adult male and female nematodes are available, intensities of infection can be estimated as:
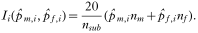
To evaluate the estimator based on males,

, we compared it with the estimator that use information on both sexes,

, by calculating the percentage bias:

With two species in an infracommunity, the true intensity of infection of species 2, I2, can be described as a factor cI,1 of I1 (I2=cI,1I1, subscript 1 and 2 denoting species number), and the true male proportion in species 2, P2 a factor cP,1 of P1 (P2=cP,1P1). Using this parameterization, the expression for percentage bias (eq. 8) in the estimated intensity of species 1 depend only on cI,1 and cP,1:

In other words, in two species communities the bias depends only on the relative difference between the species in overall male proportions and intensities of infection. The bias will be positive when the male proportion in species 1 is higher than in species 2 (cP,1<1) and negative if opposite (cP,1>1). Also, for cP,1≠1 the bias increase with increasing cI,1.
Demographic processes in the population dynamics of parasitic nematodes can cause density-dependent sex ratios according to the model used by Stien et al. (1996) for the number of larvae (Li) and adults (Ai) of sex i. Assuming that an uninfected host was introduced into an environment with infective stages, through birth or release after anthelmintic treatment, they (Stien et al. 1996) described the population dynamics of a parasitic nematode species in this host using the simple deterministic model:


where Λ is the infection rate, pi is the proportion of infectious larvae of sex i, mi is the sex-specific per capita rate at which parasitic larvae develop into adults, and

, and

are the sex-specific per capita mortality rates of parasitic larvae and adults, respectively. With Li(0)=0 and Ai(0)=0, the intensity of adults increases with time towards an asymptote given by

. Stien et al. (1996) pointed out that in the early phase of infection the proportion of adult males,

(subscript M and F denoting males and females respectively), depends only on pi and the ratio mF/mM,

, and approaches the asymptote with time,

. Thus, M(t) increases with time (but not necessarily monotonically) when

and decreases when the inequality goes the opposite way. Note that neither

nor

depends on the infection rate (Λ). If time in this model is interpreted as time period during which hosts have been exposed to infection, both the overall intensity of infection and male proportion increase with time if

. Thus, a simple difference between male and female nematodes in rates of development and/or mortality may cause density-dependent patterns in sex ratios when hosts differ in their period of exposure to infection.
To evaluate scenarios in which variation in infection intensity is due to heterogeneity in infection rates, we simulated the nematode population dynamics in individual reindeer (subscript r) using stochastic discrete-time versions of eq. 10–11 with a fixed time step of 1 day. We used a variable infection rate that had a negative binomial distribution with mean λr and variance parameter k, Λr(t)~Negative binomial(λr, k) (Venables & Ripley, 1999). We explored the situation where the average infection rate was constant across reindeer individuals, λr=λ, and where it varied between reindeer individuals following a gamma distribution, λr~gamma(a, b) with E(λr)=a/b. The latter scenario included a density-dependent adult female mortality rate, which was a linear function of adult intensity of infection at time t−1,

with I(t−1)=AM(t−1)+AF(t−1) as suggested for adult O. ostertagi by Anderson & Michel (1977). The adult male mortality rate was assumed to be constant to keep the model simple. However, models with density dependence in both male and female mortality rates can be formulated that give similar results to those presented. Simulations consisted of 500 hosts and were run for 135 time-steps corresponding to period of exposure to infection of the treated reindeer sampled in October 1999. The relationship between intensities of infection and male proportion in the simulated infrapopulations was investigated at the end of each simulation.
Sex ratios in O. gruehneri and M. marshalli from individual reindeer were in general female biased, with average proportions of males of 0·42 (S.E.=0·01) in O. gruehneri and 0·35 (S.E.=0·01) in M. marshalli. Proportions of males in O. gruehneri were positively and significantly associated with O. gruehneri intensity (Fig. 1A, F2,51=10·63, P=0·0001). This relationship reached asymptotic levels at intensities of approximately 5000. Nonetheless, the proportion of males in O. gruehneri was not significantly associated with O. gruehneri intensity in the subset of reindeer that had not been treated with anthelmintic (F2,49=0·40, P=0·67). Thus, the intensity-dependent relationship was due to the low-intensity infections in the treated reindeer (Fig. 1A). It was evident in the treated groups from both July 1999 (F2,3=24·66, P=0·01), and October 1999 (F2,3=23·21, P=0·01), with no evidence that the relationship differed between these groups (F2,6=2·26, P=0·19). Proportions of males in M. marshalli were not significantly associated with M. marshalli intensity (Fig. 1B, F2,51=1·17, P=0·19).
After accounting for the association between sex ratio and intensity in O. gruehneri using eq. 5, the asymptotic proportion of males (α) was found to differ significantly among sampling sites and seasons (Fig. 2A, F8,51=5·31, P=0·0001). The differences were due mainly to the samples collected in April having higher proportions of males (α=0·45, S.E.=0·02) than samples collected in July (α=0·38, S.E.=0·01) and October (α=0·41, S.E.=0·01). Untreated reindeer sampled in Colesdalen in October 1999 (α=0·49, S.E.=0·02) were the exception. The average proportion of males in M. marshalli also differed significantly among sampling sites and seasons (Fig. 2B, F8,53=4·57, P=0·0003). The differences were mainly due to samples from untreated reindeer collected in Colesdalen in October 1999 having a lower average proportion of males (mean=0·20, S.E.=0·03) than the other samples (mean=0·39, S.E.=0·01).

Fig. 2. Average proportions of males (with S.E. bars) in adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli in the 9 month-year-site-treatment groups of reindeer. In (A), proportions of males were estimated as the asymptotic male proportion (αuvw) using eq. 5, and the sample sizes are given above the bars.
The male-derived estimates of intensity of infection,

, for each nematode species in individual reindeer were highly correlated with the estimates derived from both sexes,

(Fig. 3A,B, r=0·99 for O. gruehneri, r=0·96 for M. marshalli). However, since O. gruehneri in general had a higher male proportion than M. marshalli (Fig. 2), male-derived estimates of O. gruehneri intensities tended in general to have a positive bias (Fig. 3A,C), while estimates for M. marshalli tended to have negative bias (Fig. 3B,D). Estimates for some anthelmintic-treated reindeer differed from this pattern by having a substantial negative bias in estimates of O. gruehneri (Fig. 3C) and positive bias in estimates of M. marshalli (Fig. 3D). This pattern was due to a relatively low male proportion in O. gruehneri (Fig. 1A, cp>1 in Fig. 3C) and a higher intensity of M. marshalli than O. gruehneri (cI>1 in Fig. 3C). For most samples the higher intensity of O. gruehneri than M. marshalli infection resulted in little bias in male-derived estimates of O. gruehneri infection (cI[Lt ]1 in Fig. 3C), while the bias in many estimates of M. marshalli infection was substantial (Fig. 3D). However, high intensities of M. marshalli infection in March caused a substantial positive bias also in some estimates of O. gruehneri infection.
Male-derived estimates of abundance of infection in the samples from each year-month-site-treatment combination were also highly correlated with estimates derived from both sexes (Fig. 3, r=0·99 for O. gruehneri, r=0·99 for M. marshalli). For O. gruehneri the bias in estimates of abundance of infection were greatest in March (range=10–17%), estimates for July were unbiased due to the low intensities of M. marshalli infection, while the bias in estimates for October were positive and low (range=0–8%) with the exception of the negative bias (−9%) found for the anthelmintic-treated reindeer. In comparison, the negative bias in estimates of the abundance of M. marshalli were more pronounced, particularly in March (range=12–21%) and in the untreated reindeer from October 1999 (−43%).
In the scenario with constant adult mortality rates and the same infection rate across hosts, λr=λ, a combination of a high variance in the infection rate over time (k[Lt ]1), high adult female and higher adult male mortality rates

, and a high development rate of larvae (mF=mm=0·5 d−1) was found to generate a pattern (Fig. 4A) similar to the observed one (Fig. 1A). This model generated an infection dynamics characterized by large, rare infection pulses followed by periods of adult mortality with falling intensities of infection. Low intensities of infection were associated with a long time-period since the previous large infection event, and the low male proportion in these infrapopulations was due to the higher adult mortality rate in males than in females.
The scenario with a density-dependent adult female mortality rate

, a constant adult male mortality rate, and differences in infection rates among hosts, λr=Gamma(a,b), could also generate a density-dependent pattern in sex ratios (Fig. 4B). Density-dependent patterns were generated even at low larval development rates (mF=mm=1/21) and when differences in infection rates among hosts approached a Poisson distribution (k→∞). In this scenario, most of the variability in intensities of infection was generated by variability in the average infection rate, λr, and the associated variability in the ratio

caused correlated variation in the average sex ratio. The shape of the density-dependent pattern in the sex ratio will also depend on this ratio, with nonlinear models for

able to generate patterns closer to the observed one (Fig. 1A).

Fig. 3. Estimates of intensity of infection of adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli based on males (, eq. 6), plotted against the estimate based on both sexes (, eq. 7) for single hosts (small symbols) and the abundance of infection in the 9 year-month-treatment groups of reindeer (large symbols). The solid line indicates the optimal 1[ratio ]1 relationship. Estimates of cp plotted against cI for (C) O. gruehneri and (D) M. marshalli with contour lines for 10–50% positive and negative bias in calculated as (eq. 9). Samples from March are shown as squares, July as circles, October as triangles and samples from anthelmintic-treated animals are shown as filled symbols in all plots (A–D).

Fig. 4. The adult male proportion in 500 infrapopulations plotted against their intensity of infection after 135 days of exposure to infection. In (A) the infrapopulations were generated using the stochastic discrete time version of eq. 8–9, assuming Λr(t)~Negative binomial(400,0·01), , mF=mM=1/2 d−1, pF=0·5, and . In (B) the infrapopulations were generated using the stochastic discrete time version of eq. 8–9, assuming Λr(t)~Negative binomial(λr,1), λr~Gamma(6,0·01), , mF=mM=1/21 d−1, pF=0·5, with β0=1/55 and β1=0·000004, and .
The sex ratios in GI nematodes from Svalbard reindeer were found to be female-biased and to differ slightly between O. gruehneri and M. marshalli. Despite the latter finding, estimates of intensities and abundances of infection derived from males and estimates derived from both sexes were highly correlated. This supports the use of the more easily obtainable male-derived estimates used in published studies. However, substantial bias was found in the estimated intensity of some infrapopulations, particularly when the species was the subdominant one in the host. Such errors will affect studies conducted at the level of the individual host e.g. studies of correlations between infection intensities and host fitness traits (e.g. Stien et al. 2002b). Due to its effect on host fitness traits, much focus has been on the population dynamics of O. gruehneri in Svalbard reindeer (Albon et al. 2002; Stien et al. 2002b). The numerical dominance of O. gruehneri during most of the year causes estimates of its intensities and abundances of infection to be affected little by bias. The bias in estimates of O. gruehneri abundance was most pronounced in samples from March, when the male-derived estimates over-estimate the abundance by 10–17%. This suggests that the fecundity of O. gruehneri in late winter is slightly higher than that estimated by Stien et al. (2002a). The bias in estimates of O. gruehneri abundance for July and October was, however, small, suggesting that analyses based on samples from these months are largely unbiased (e.g. Albon et al. 2002).
As in most parasitic nematodes (Poulin, 1997a,b), the average sex ratio was female-biased in both O. gruehneri and M. marshalli. Female-biased sex ratios are usually interpreted as resulting from adult males having lower survival than the females (Poulin, 1997b). In M. marshalli the male proportion was on average slightly lower than in O. gruehneri, suggesting a more pronounced difference between male and female survival rates in M. marshalli than in O. gruehneri. In O. gruehneri the average male proportion was slightly higher in the samples collected in April than in samples collected later in the year. This finding is consistent with an early pulse of infection, or development of arrested larvae, that minimize the effect a sex difference in adult mortality rates will have on sex ratios in April.
The average proportion of males in M. marshalli also differed between sample dates and locations, but no clear trends with these factors or with prior anthelmintic treatment were apparent. This variation in the average male proportions is therefore not readily explained, and is potentially attributable to stochastic between-year and between-site variation in the transmission and within-host demographic processes that generate the observed sex ratios.
In O. gruehneri, the proportion of males increased asymptotically with intensity of infection in hosts treated with an anthelmintic the same year. The detection of this trend only in treated hosts might have been due the high frequency of hosts with low intensities of infection in this group. The trend was found in samples collected the same time-period after anthelmintic treatment, so differences between hosts in period of exposure (Stien et al. 1996) cannot explain the observed pattern. Variation in rate of infection (Λ) caused by differences between hosts in parasite uptake rates or susceptibility to infection, is likely to be the main cause of observed variation in intensities of infection.
The two model scenarios we investigated provided potential mechanisms for density-dependent sex ratios when variation in intensities of infection was due to heterogeneity in the infection rate. Adult mortality rates at the scale used in the scenario with constant adult mortality rates and no systematic individual variation in infection rates, has been reported for trichostrongyle GI nematodes of sheep (Kao et al. 2000), while the rate of larval development was significantly higher than that generally accepted for trichostrongyle nematodes (Kao et al. 2000). However, the outcome of this scenario depended on a short synchronous pulse of development to adults. Introducing a constant developmental delay at the larval stage before the developmental pulse, to allow for a more reasonable residence time at the larval stage, preserves the outcome of a density-dependent sex ratio. No information is available to evaluate whether large, rare infection pulses (k[Lt ]1) characterize O. gruehneri transmission dynamics in Svalbard reindeer. We may, however, speculate that large infection pulses can occur when infective larvae are distributed in clumps associated with faeces (Pugliese, Rosà & Damaggio, 1998), and faecal avoidance causes foraging hosts to graze these clumps rarely (van der Wal et al. 2000).
The model scenario with constant adult male and density-dependent adult female mortality rates, and host differences in infection rates, is also biologically reasonable. Intensity of infection might affect mortality rates since it also affects size and fecundity in adult female O. gruehneri (Irvine et al. 2001). Furthermore, differences among hosts in infection rates due to individual variation in behaviour, susceptibility or both factors are considered an important cause of individual differences in intensities of infection for macroparasites (Hudson & Dobson, 1995). At present we have no data in favour of a particular scenario explaining the density-dependent sex ratio in O. gruehneri. The main implication of the simulations is that several models of demographic processes involved in the development of infrapopulations of GI nematodes can create density-dependent sex ratios. Identification of which process predominates in a given system will require detailed experimental studies.
We analysed subsamples of nematodes because species identification of the entire infracommunities would have been too laborious. The subsampling design caused sample sizes of males and females identified to species (sm,i and sf,i) to differ significantly between infrapopulations. In the subsequent analysis of the average sex ratio across reindeer, sample sites, and seasons, the use of appropriate estimates of the sampling variance as weights was crucial for reaching our quantitative and qualitative conclusions. Since infection intensities in GI nematode communities often reach several thousands, species identification of all available samples, whether by morphology or by PCR, will often be laborious. Our approach to the estimation of the sampling variance associated with the estimated sex ratios may therefore also be useful in other studies. The non-standard specification and fitting of the model are potential obstacles. However, the use of computer intensive Bayesian methods has become common in epidemiology and ecology (e.g. Gilks et al. 1996; Harwood & Stokes, 2003). This trend is also likely to be seen in parasitological studies (Basanez et al. 2004).
We are grateful to Cathrine Vollelv at the Zoological Museum in Oslo who identified to species the male nematodes. Fred Skanche Hansen and Jørn Dybdahl at UNIS and members of Longyearbyen Hunting and Fishing Association provided help with the culling. The work was funded by the Research Council of Norway (Arktisk Lys programme) and the Natural Environment Research Council, UK (GR3 10811).

Fig. 1. Estimated proportions of males in adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli (with 95% confidence limit bars) plotted against the estimated intensity of infection of the nematode species in individual reindeer. Closed squares indicate nematodes from reindeer treated with an anthelmintic in April or May of the year of sampling, and open squares indicate untreated reindeer. Crosses indicate 2 outlying observations.

Fig. 2. Average proportions of males (with S.E. bars) in adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli in the 9 month-year-site-treatment groups of reindeer. In (A), proportions of males were estimated as the asymptotic male proportion (αuvw) using eq. 5, and the sample sizes are given above the bars.

Fig. 3. Estimates of intensity of infection of adult (A) Ostertagia gruehneri and (B) Marshallagia marshalli based on males (, eq. 6), plotted against the estimate based on both sexes (, eq. 7) for single hosts (small symbols) and the abundance of infection in the 9 year-month-treatment groups of reindeer (large symbols). The solid line indicates the optimal 1[ratio ]1 relationship. Estimates of cp plotted against cI for (C) O. gruehneri and (D) M. marshalli with contour lines for 10–50% positive and negative bias in calculated as (eq. 9). Samples from March are shown as squares, July as circles, October as triangles and samples from anthelmintic-treated animals are shown as filled symbols in all plots (A–D).

Fig. 4. The adult male proportion in 500 infrapopulations plotted against their intensity of infection after 135 days of exposure to infection. In (A) the infrapopulations were generated using the stochastic discrete time version of eq. 8–9, assuming Λr(t)~Negative binomial(400,0·01), , mF=mM=1/2 d−1, pF=0·5, and . In (B) the infrapopulations were generated using the stochastic discrete time version of eq. 8–9, assuming Λr(t)~Negative binomial(λr,1), λr~Gamma(6,0·01), , mF=mM=1/21 d−1, pF=0·5, with β0=1/55 and β1=0·000004, and .