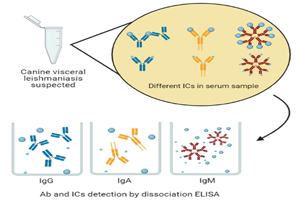
Introduction
Visceral leishmaniasis (VL) is an endemic and highly prevalent parasitic disease in tropical areas, including countries with economic vulnerability. Despite underreporting, the annual occurrence of cases is approximately 50 000–90 000 per year (WHO, 2019). Brazil emerges as one of the countries with the highest prevalence of cases, a condition associated with several characteristics, among which stands out its territorial extension and the socioeconomic disparities of the population. Latin America's VL is caused by the species Leishmania (Leishmania) infantum (Marcondes and Day, Reference Marcondes and Day2019), transmitted by the bite of contaminated female sandflies of the genus Lutzomyia.
Dogs are the main zoonotic reservoir of L. L. infantum parasites, and their presence in endemic areas represents a high risk for the development of human VL (Brodskyn and Kamhawi, Reference Brodskyn and Kamhawi2018); intense cutaneous parasitism favours the infection of insect vectors and the spread of the disease (Diniz et al., Reference Diniz, Silva, Carvalho Neta, Bueno, Guerra, Abreu-Silva and Santos2008). Canine visceral leishmaniasis (CVL) is spectral; some dogs present a subclinical form of the disease (initial or permanent during the infection), and some dogs develop clinical manifestations (Maia and Campino, Reference Maia and Campino2018). The disease causes weight loss, lymphadenomegaly, hepatosplenomegaly, keratoconjunctivitis, onychogryphosis, alopecia, dermatitis, skin ulcers, secondary bacterial infections, coagulopathy, anaemia (Baneth et al., Reference Baneth, Koutinas, Solano-Gallego, Bourdeau and Ferrer2008) and polyclonal hyperglobulinaemia (Freitas et al., Reference Freitas, Nunes-Pinheiro, Lopes Neto, Santos, Abreu, Braga, Campos and Oliveira2012).
CVL clinical evolution is associated with the balance of inflammatory and regulatory immune responses; these processes involve the mechanisms of macrophage activation, cytokine profile modulation and proliferation of T CD4 and B cells (Toepp and Petersen, Reference Toepp and Petersen2020). The few resistant dogs develop a Th1-type immune response with the production of inflammatory cytokines such as interferon gamma (IFN-γ) and tumour necrosis factor alpha (TNF-α) that act directly to control parasitic proliferation (Hosein et al., Reference Hosein, Blake and Solano-Gallego2017). Most dogs present a Th2-type response with the production of interleukin 4 (IL-4), interleukin 5 (IL-5) and interleukin 6 (IL-6) cytokines that activate the proliferation of B cells, inducing the production of high levels of antibodies and uncontrolled parasite proliferation (Pinelli et al., Reference Pinelli, van der Kaaij, Slappendel, Fragio, Ruitenberg, Bernadina and Rutten1999). High levels of specific antibodies with no defined role in infection control are characteristics found in CVL, and this scenario is often associated with high-parasitic loads and exacerbation of the disease (Di Pietro et al., Reference Di Pietro, Crinò, Falcone, Crupi, Francaviglia, Vitale and Giudice2020).
Anti-leishmania antibody detection is widely used to study the humoral immune response of CVL and in laboratory diagnosis through the quantification and identification of immunoglobulin classes (Batista et al., Reference Batista, Torrecilha, Silva, Utsunomiya, Silva, Tomokane, Pacheco, Bosco, Paulan, Rossi, Costa, Marcondes, Ciarlini, Nunes, Matta and Laurenti2020). IgG antibodies are most often used for the diagnosis of CVL due to their quantitative availability and circulation period (Olías-Molero et al., Reference Olías-Molero, Moreno, Corral, Jiménez-Antón, Day, Domínguez and Alunda2020), while other classes, such as IgA and IgM, are less explored for that purpose (de Freitas et al., Reference de Freitas, Lopes-Neto, de Abreu, Coura-Vital, Braga, Reis and Nunes-Pinheiro2012). High availability of anti-Leishmania antibodies produced during infection, associated with intense host parasitaemia, facilitates the formation of immune complexes (ICs). Th1 or Th2 polarization of the immune response in VL triggers a series of mechanisms that affect the regulation of the expression of immunoglobulin receptors in macrophages, which directly affects the elimination of circulating ICs (Guilliams et al., Reference Guilliams, Bruhns, Saeys, Hammad and Lambrecht2014). IC biological properties depend on the factors such as isotype and affinity of antibodies and molecular composition of the antigen. IgG antibodies often form ICs, but other antibodies, such as IgM, IgA and IgE, also have this ability (Aibara and Ohyama, Reference Aibara and Ohyama2020). The binding of specific antibodies to protein or glycan antigens forms different ICs in VL and represents a limiting factor for conventional serological methods (de Carvalho et al., Reference de Carvalho, Partata, Hiramoto, Borborema, Meireles, Nascimento and de Andrade2013, Reference de Carvalho, Ferrão, de Freitas and de Andrade2019). Acid dissociation in serum samples is an alternative for indirect detection of protein or glycan ICs in VL and is performed by ELISA, as previously reported (de Carvalho et al., Reference de Carvalho, Partata, Hiramoto, Borborema, Meireles, Nascimento and de Andrade2013; Aparecida de Carvalho et al., Reference Aparecida de Carvalho, Fidelis Ferrão, Mitsuyoshi Hiramoto, Kelsei Partata and de Andrade Júnior2019). Glycan haptens from L. (L.) infantum promastigotes conjugated to a carrier protein allow their application to solid support in ELISA and the detection of specific anti-Leishmania antibodies and ICs in experimental hamster infection (de Carvalho et al., Reference de Carvalho, Ferrão, de Freitas and de Andrade2019).
ICs are little explored in CVL, and knowledge on aspects related to the presence and composition of these elements directly contributes to the understanding of interaction mechanisms between antibodies and antigens and their role in the pathogenesis and serology of the disease. This study evaluated the presence of antibodies blocked by nonspecific antigens or glycan haptens in dog serum samples from endemic areas for VL. These monovalent ICs were composed of IgG, IgA and IgM anti-Leishmania antibodies detected indirectly by conventional ELISA (cELISA) and dissociative ELISA (dELISA) using soluble promastigote extract (PSE) and glycan-BSA complex (GBC).
Materials and methods
Samples
Our sample composed of 184 serum samples from dogs from endemic areas (174) or from a nonendemic area as controls (10) for VL in the state of São Paulo, Brazil. Control samples were used to calculate the reactivity threshold for serology in each assay.
Antigens
Soluble PSE was produced by L. (L.) infantum promastigote cultures, as described in a previous study (de Carvalho et al., Reference de Carvalho, Partata, Hiramoto, Borborema, Meireles, Nascimento and de Andrade2013). In parallel with the use of PSE, we used a glycan antigen conjugated to bovine serum albumin (BSA) or GBC. Briefly, GBC was produced by a low molecular weight fraction of PSE, which we characterized as a glycan antigen that was chemically linked to a support protein, as described in a previously published work (de Carvalho et al., Reference de Carvalho, Ferrão, de Freitas and de Andrade2019).
Antibody detection and immune complex determination by ELISA
Ninety-six-well plates were coated with PSE (0.6 μg mL−1) and GBC (0.03 μg mL−1) for both cELISA and dELISA. Each sample dilution (1/200) was tested in duplicate for each ELISA (conventional or dissociative). For dELISAs, we added 50 μL of half diluted serum samples per well, followed by 25 μL well−1 of 0.1 m glycine (pH 2.0) and stirred for 5 min for acid dissociation. We then added 25 μL of 0.5 M TRIS/HCl (pH 8.0) to the wells and reacted for 1 h at 37°C. For control wells, instead of acid and buffer, we added 50 μL of phosphate buffered saline with 0.3% skimmed milk and 0.05% Tween 20 (de Carvalho et al., Reference de Carvalho, Ferrão, de Freitas and de Andrade2019). Anti-dog peroxidase conjugates were used at specific dilutions: IgG (1/20 000), IgA (1/10 000) and IgM (1/80 000). The reaction was revealed with 100 μL well−1 3,3′,5,5′-tetramethylbenzidine commercial substrate and interrupted with 50 μL 4 N HCl, with A 450 nm determined on a microplate reader. Specific cut-off values for each test were determined using the 95% confidence interval (95% CI) based on the mean and standard deviation of negative control samples and subsequently expressed as artificial units (AUs), which is the ratio of observed sample optical density and each assays’ determined cut-off. All individual quantitative measurements were estimated using AU values, mostly defined as the percentage of dELISA AU compared to cELISA AU, using simple calculation (dELISA AU × 100/cELISA AU = % ICs). We determined the frequency of samples in which the result of the reference test (cELISA or dELISA) was negative and the experimental test (dELISA or cELISA) was positive using the values of AUs with individual cut-off values. Relative seroconversion was determined in two assays for the same class of immunoglobulins. In this analysis, we excluded concordant negative samples in both assays. The greatest relative seroconversion of an experimental assay represents its efficiency in specific serology. Relative seroconversion was a discordant result between both methods (cELISA and dELISA) for each class of immunoglobulin tested.
Statistical analysis
We initially used the sample distribution and compared samples by analysis of variance (ANOVA) or t-tests after checking variance uniformity. The correlation between data was performed using Pearson correlation and expressed as r 2. The extent of significance is expressed as asterisks (*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001) or not significant (NS) in the absence of a difference. All of the assays must be similar, as they use the same leishmaniasis dog samples, and their comparison demands the detection of subtle differences. Proportions of positive and negative results for each method and class of immunoglobulins were analysed in contingency tables employing bicaudal chi-squared test and 90% power, and estimation of serological parameters as positive-predictive value (PPV) and likelihood ratio (LR). Statistical analyses were calculated using GRAPHPAD PRISM 6.0 software (GraphPad Prism, San Diego, CA).
Results
As proposed, we evaluated the presence of IgG, IgA and IgM antibodies and their ICs in 174 serum samples from dogs suspected of VL using PSE and GBC in cELISA and dELISA. The qualitative intra- and intertest reproducibility was high, with less than 10% variation in the quantitative comparison of the results. IgG detection (Fig. 1) by cELISA and dELISA with PSE and GBC showed similar reactivity. IgG anti-PSE antibodies in cELISA were similar to those in dELISA (Fig. 1A), qualitatively distinguishing positive and negative samples. The IgG anti-PSE increment (Fig. 1B) revealed by dELISA was identified in 64.9% of the samples. cELISA and dELISA results for the detection of IgG anti-PSE (Fig. 1C) showed a high correlation (r 2 = 0.81). IgG anti-GBC (Fig. 1D) again showed similar results and was able to identify qualitative positive and negative samples. dELISA showed an increase in anti-GBC IgG in 81.6% of the samples (Fig. 1E), as in PSE without large increments. cELISA and dELISA in the detection of IgG anti-GBC antibodies (Fig. 1F) were highly correlated, with an r 2 of 0.90. The comparative analysis between the cELISA and dELISA methods with PSE or GBC did not show any statistically significant difference.
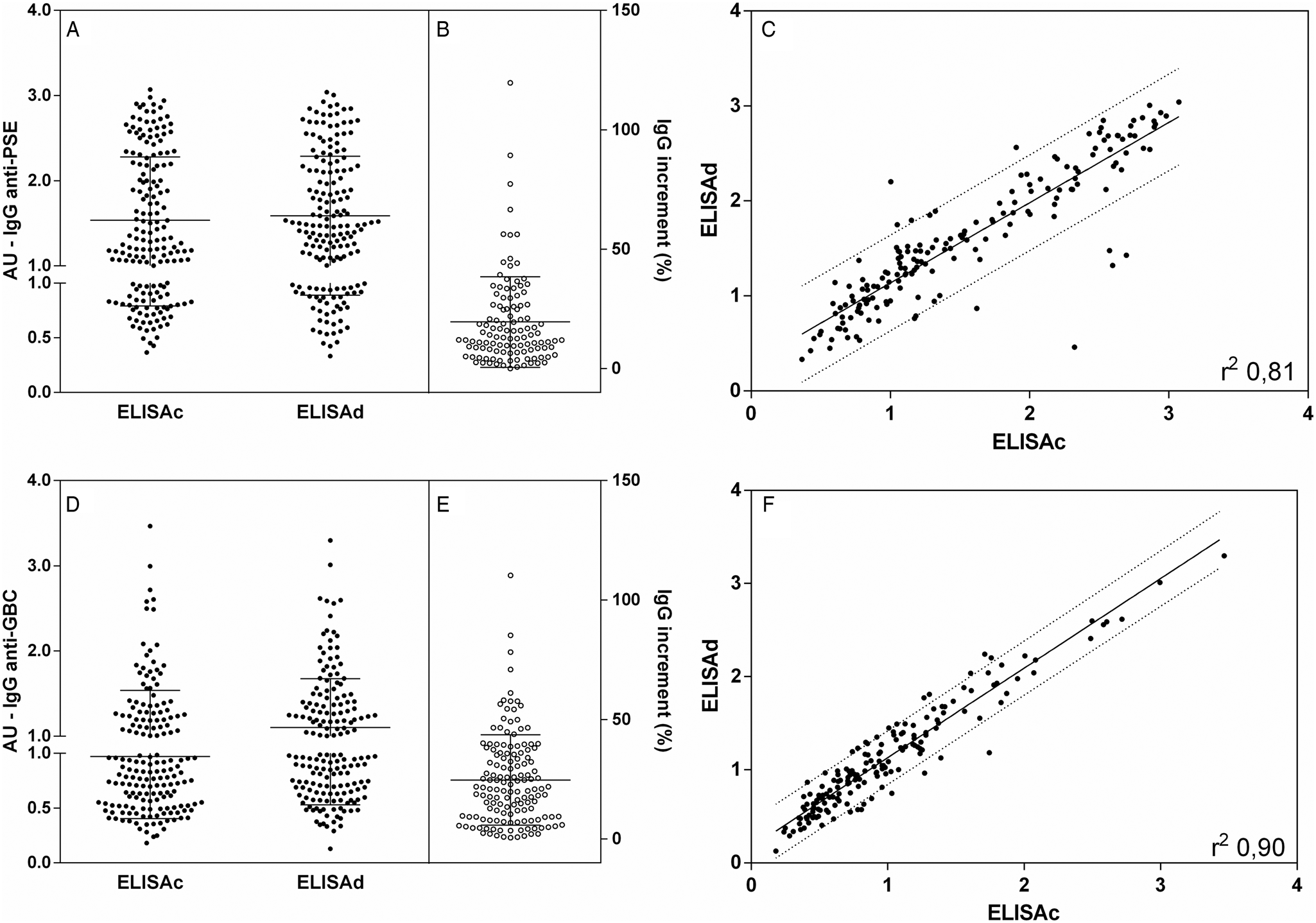
Fig. 1. IgG anti-leishmania antibodies in dog serum samples. Closed points represent samples tested by cELISA and dELISA with PSE (A) and GBC (D). Interruption in the y-axis represents the reactivity threshold of the calculated test (95% CI), differentiating negative and positive samples. The proportional increment IgG (%) in dELISA as related to cELISA for anti-PSE are shown in B and for anti-GBC in E. Individual distribution and correlation between IgG cELISA and dELISA individual values are shown in C for anti-PSE and in F for anti-GBC. Lines represent the linear regression line and 90% prediction band. Pearson correlation analyses were performed, and when significant, they were represented by Pearson r 2.
Looking for IgA antibodies in Fig. 2A, cELISA and dELISA PSE showed greater differences in both qualitative and quantitative reactivities. There were clear differences between the cELISA and dELISA, with dELISA being more sensitive for IgA detection (P < 0.001). The IgA anti-PSE dELISA increment was quite expressive both in number (73% of the samples) and in a percentage increase in reactivity, with several samples becoming positive or showing an increase in IgA above 100% (Fig. 2B). The comparison between the results of cELISA and dELISA for IgA anti-PSE (Fig. 2C) showed a moderate correlation (r 2 0.42) with a large dispersion of values, especially with increased IgA reactions. The assays for the detection of IgA anti-GBC antibodies by cELISA and dELISA (Fig. 2D) revealed similar results, with better detection (P < 0.0001) and several-fold increases in reactivity, found in 75.9% of the samples (Fig. 2E). The correlation between cELISA and dELISA for GBC showed a moderate impact (r 2 of 0.55) and huge dispersion of values, especially in the upper left quadrant seroconversion area. For both PSE and GBC IgA dELISAs, the increment was high, with an increase of up to 600%, suggesting the presence of blocked antibodies.
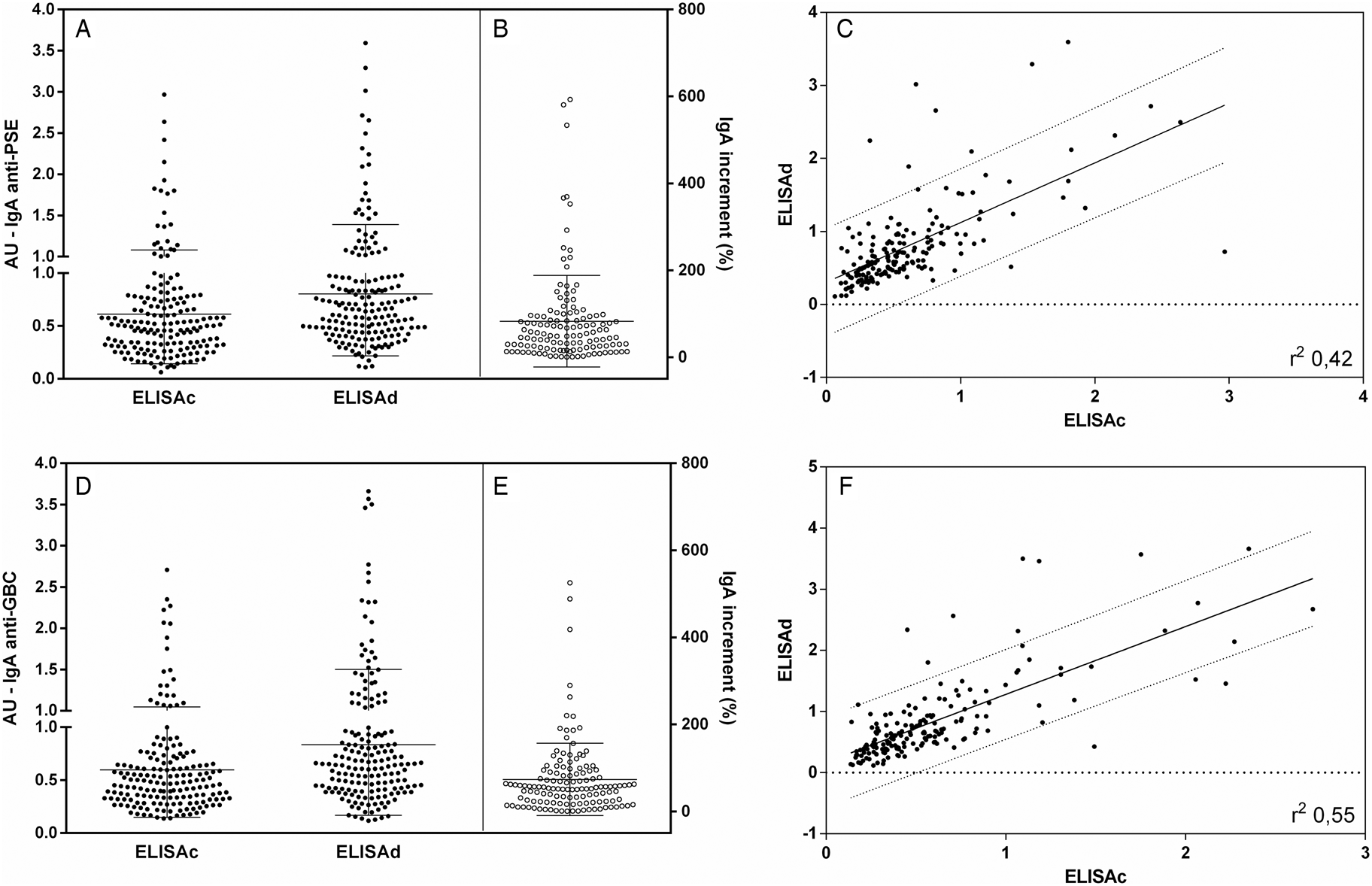
Fig. 2. IgA anti-leishmania antibodies in dog serum samples. Closed points represent samples tested by cELISA and dELISA with PSE (A) and GBC (D). Interruption in the y-axis represents the reactivity threshold of the calculated test (95% CI), differentiating negative and positive samples. The proportional increment IgA (%) in dELISA as related to cELISA for anti-PSE are shown in (B) and for anti-GBC in (E). Individual distribution and correlation between IgA cELISA and dELISA individual values are shown in (C) for anti-PSE and in (F) for anti-GBC. Lines represent the linear regression line and 90% prediction band. Pearson correlation analyses were performed, and when significant, they were represented by Pearson r 2.
The reactivity of IgM antibodies was poor in both assays and antigens. IgM anti-PSE antibody detection (Fig. 3A) showed low dispersion and homogeneous results. Considering quantitative and qualitative data by cELISA and dELISA, there was no statistical significance. An IgM anti-PSE increment (Fig. 3B) was identified in fewer samples (37.6%). The comparative analysis between the results of cELISA and dELISA (Fig. 3C) showed a high correlation (r 2 of 0.82) and some samples outside the confidence limits, but without a clear direction. IgM anti-GBC antibodies detected by cELISA and dELISA (Fig. 3D) were qualitatively and quantitatively similar to most of the negative samples, as well as in PSE. An IgM anti-GBC increment (Fig. 3E) was found in the fewest samples (25.9%), despite the good correlation (r 2 of 0.85), forced by the large negative concordant fraction (Fig. 3F).
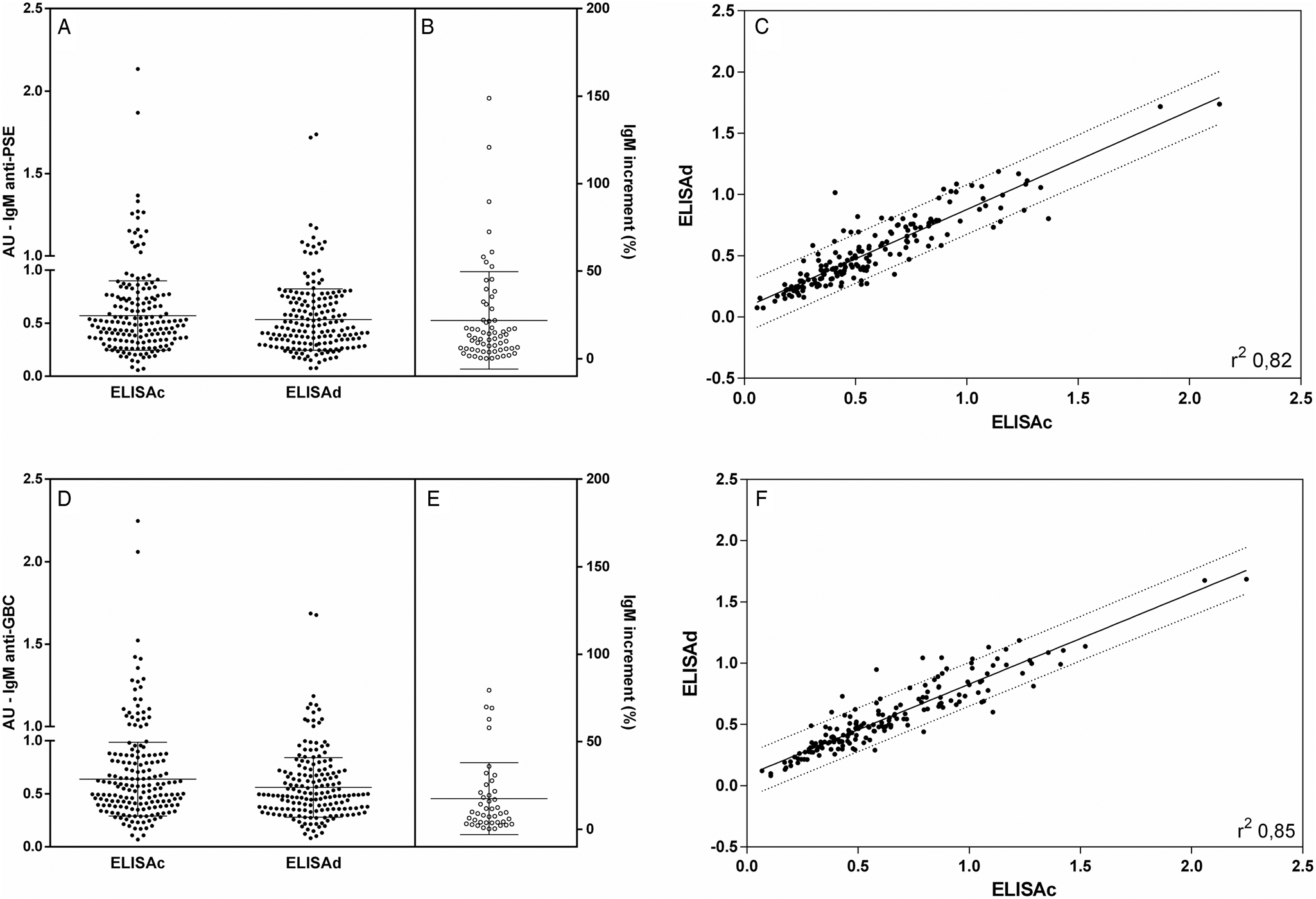
Fig. 3. IgM anti-leishmania antibodies in dog serum samples. Closed points represent samples tested by cELISA and dELISA with PSE (A) and GBC (D). Interruption in the y-axis represents the reactivity threshold of the calculated test (95% CI), differentiating negative and positive samples. The proportional increment IgM (%) in dELISA as related to cELISA for anti-PSE are shown in (B) and for anti-GBC in (E). Individual distribution and correlation between IgM cELISA and dELISA individual values are shown in (C) for anti-PSE and in (F) for anti-GBC. Lines represent the linear regression line and 90% prediction band. Pearson correlation analyses were performed, and when significant, they were represented by Pearson r 2.
Our results were analysed qualitatively using cut-off values for each test based on negative control samples. Negative results agreement in cELISA and dELISA with PSE or GBC for each class of immunoglobulin was evaluated, excluding positive samples and with relative seroconversion. The frequency of agreement for specific IgG-negative samples was 22.4% and 51.7% for PSE and GBC, respectively. Specific IgA antibodies presented high frequency numbers and agreement for negative samples, 75.3% for PSE and 75.9% for GBC. Specific IgM antibodies were less frequent in all assays, with 87.4% negative samples for PSE and 83.3% negative samples for GBC. According to our data, specific anti-Leishmania IgG antibodies are more frequent in serum during CVL compared to other classes of immunoglobulins. Reactivity for specific antibodies is a more complex evaluation, as relative seroconversion could occur by discrepancy in the assays for the same antigen and immunoglobulin class. As presented in Table 1, the relative seroconversion frequency was clear in dissociative assays for IgG and IgA. IgM assays provide an inverse effect with loss of reactivity in dissociative assays. This index allowed the comparison and statistical analysis of the results, using the number of positive samples and the relative seroconverted samples for each assay. Using this statistical approach, it was possible to define that IgM dissociative assays presented higher negative sample numbers. For the main class of immunoglobulin, specific IgG, it was clear that the dissociative assays had a low number of seroconversion samples clearly found.
Table 1. Qualitative results of the comparison of samples tested for the detection of IgG, IgA and IgM anti-PSE and anti-GBC antibodies
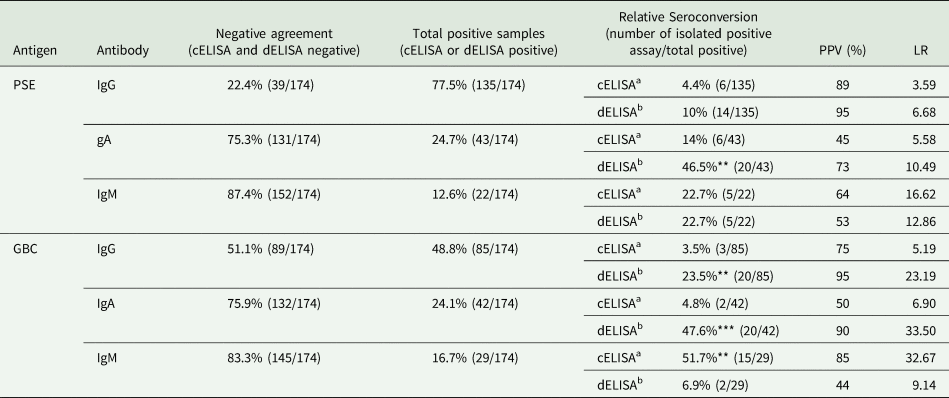
Negative agreement, agreement of negative results; Positive samples, agreement positive samples and samples with relative seroconversion in cELISA or dELISA; Relative seroconversion, seroconverted samples to cELISA and dELISA; PPV, Positive predictive value and LR, Likelihood ratio. Statistical difference between cELISA and dELISA in the detection of positive samples: P < 0.01 (**) and P < 0.001 (***).
a cELISA as experimental assay and dELISA as control assay.
b dELISA as experimental assay and cELISA as control assay.
For IgA detection, there was a large proportion of relative seroconversion samples, despite the relatively low frequency of IgA-positive samples. In fact, looking for cELISA IgA, there is a low reactivity for this class, with more than 83% of negative samples. dELISA for IgA promoted an important increase in this frequency, almost doubling the number of positive samples (P < 0.001), unrespected of the antigen. This is a puzzling aspect, but the concordance in IgA sample reactivity increase with both antigens suggests that most circulating IgA could be antigen blocked and freed by dissociative assay. These findings are confirmed by the increment of estimated serological parameters to the results shown by clear PPV and LR for IgA dELISA with PSE or GBC. The LR shows that IgA dELISA is at least ten times more efficient than cELISA for this detection of PSE and more than 30 times more efficient for GBC. The fact that the two assays presented similar results suggests that the blocking antigen would be similar to the antigens that were used to construct GBCs.
Discussion
Our results showed that IgA and IgG ICs composed mostly of glycan antigens are present in CVL and interfere with conventional serology. Serology proved to be an essential tool for the diagnosis of CVL in suspected dogs, since most samples showed antibodies in the cELISA. IgG anti-PSE and anti-GBC levels were similar between cELISA and dELISA and showed a high frequency of positive samples, a profile related to the biological characteristics of this class of immunoglobulin, maintaining itself for long periods after infection. Anti-Leishmania IgG antibody detection is an important tool for diagnosis, especially when associated with clinical information of infected or vaccinated dogs (Pereira et al., Reference Pereira, Silva, Menegati, Pinheiro, Assunção, de Lourdes, Araújo, Abass, Duthie, Steinhoff and Teixeira2020). ICs formed by anti-Leishmania IgG revealed by dELISA had significantly high specificity for PSE and GBC. Our results demonstrated a greater blockade of antibodies caused by hapten glycans (GBCs) than the blockade caused by varied antigens (PSEs). The potential for the formation of ICs by glycoconjugate antigens from Leishmania sp. is described for human samples, in which detection has been associated with active disease (Jaiswal et al., Reference Jaiswal, Datta, Sardar, Chaudhuri, Maji, Ghosh, Saha and Mukhopadhyay2018). Most likely, the high presence of circulating ICs composed of anti-PSE and anti-GBC IgG revealed in our results is related to a deficit in the clearance of these complexes caused by the negative regulation of the immune response, as exemplified in systemic lupus erythematosus (Macedo and Isaac, Reference Macedo and Isaac2016).
The presence of anti-PSE and anti-GBC IgA antibodies was similar, but the discrimination of positive samples was greater for specific anti-GBC antibodies. An increase in IgA antibodies revealed by dELISA showed a high frequency of circulating ICs in the samples for both PSE and GBC. Specific IgA antibodies in CVL are poorly explored in diagnosis due to the evolutionary characteristics of the disease and the variables period of onset and systemic involvement. The correlation between the presence of high levels of IgA and high parasitism in dogs (Batista et al., Reference Batista, Torrecilha, Silva, Utsunomiya, Silva, Tomokane, Pacheco, Bosco, Paulan, Rossi, Costa, Marcondes, Ciarlini, Nunes, Matta and Laurenti2020) confirms the predictive potential of the detection of symptomatic CVL by this class of immunoglobulins (Reis et al., Reference Reis, Teixeira-Carvalho, Vale, Marques, Giunchetti, Mayrink, Guerra, Andrade, Corrêa-Oliveira and Martins-Filho2006). Studies related to the presence and formation of IgA ICs are aimed at understanding the mechanisms involved in the development and evolution of kidney disease, involving the glomerular deposition of these complexes (Yeo et al., Reference Yeo, Cheung and Barratt2018). Secreted IgA is the main immunoglobulin involved in mucosal immunology and can be used to study the humoral immune response and in the diagnosis of CVL (Cantos-Barreda et al., Reference Cantos-Barreda, Escribano, Egui, Thomas, López, Tecles, Bernal, Cerón and Martínez-Subiela2019). This immunoglobulin may be present in several biological samples, and high levels of anti-Leishmania IgA detected in the mucosa during CVL may be associated with intestinal parasitism (Rodríguez-Cortés et al., Reference Rodríguez-Cortés, Fernández-Bellón, Ramis, Ferrer, Alberola and Solano-Gallego2007). Although frequently reported in VL humoral immune response studies, IgA antibodies and IgA ICs are poorly studied compared to IgG antibodies.
IgM antibodies are the first immunoglobulin produced during the immune response. Anti-PSE and anti-GBC IgM antibody reactivity was similar and predominantly negative in our results. Our results showed that the reactivity of IgM anti-PSE and anti-GBC antibodies was similar and predominantly negative, as observed for the presence of IgM ICs, with a greater increase detected for PSE. In CVL, specific IgM antibodies are detected from 1 month postinfection, followed by IgG (Martinez-Subiela et al., Reference Martinez-Subiela, Strauss-Ayali, Cerón and Baneth2011). Considering an acute phase antibody, the period of infection can influence its detection. IgM antibodies appear in the acute phase at the beginning of the disease, and this characteristic can influence its detection. High concentrations of IgG antibodies, followed to a lesser extent by the presence of IgA antibodies, detected in our results may have influenced the detection of IgM antibodies due to antigen binding competition. The antibody–glycan interaction has specificities, such as competition for binding to antigenic epitopes; this characteristic can interfere with detection tests. High levels of circulating anti-glycan IgG and/or IgA antibodies may inhibit the detection of IgM antibodies due to competition for the antigen-binding site in solid support, as elsewhere reported (Muthana et al., Reference Muthana, Xia, Campbell, Zhang and Gildersleeve2015). In our assays, conditions of vast antigen excess were used to avoid competition between antibodies, such as IgG interference in less concentrated IgM and IgA assays.
Qualitatively, our results showed high agreement for negative profiles of IgG, IgA and IgM anti-PSE and anti-GBC antibodies. Negative IgG samples showed greater agreement when tested against GBC, IgA- and IgM-negative samples and showed similar agreement with no difference between antigens. The greater reactivity associated with the use of PSE to detect IgG antibodies is probably related to a greater quantitative exposure to L. (L.) infantum antigenic epitopes, considering the variety of protein and glycan antigens. GBC has antigenic glycans of low molecular weight of <5 kDa (de Carvalho et al., Reference de Carvalho, Ferrão, de Freitas and de Andrade2019). These characteristics are compatible with glycoinositol phospholipid (GILP) molecules that play an important role in the modulation of the VL immune response (Cabezas et al., Reference Cabezas, Legentil, Robert-Gangneux, Daligault, Belaz, Nugier-Chauvin, Tranchimand, Tellier, Gangneux and Ferrières2015). As shown, there was a high similarity of negative, positive and relative seroconversion profiles in the samples tested. These concordances are probably justified due to the greater quantitative presence of GILPs in PSE, since this glycoconjugate is abundant in the membrane of Leishmania sp. (Valente et al., Reference Valente, Castillo-Acosta, Vidal and González-Pacanowska2019). Alternatively, to the use of protein antigens, the use of glycan antigens has been explored in the detection of antibodies in VL. Specific IgG antibody efficacy detected in VL by GILPs by ELISA has been reported and has performed well in detecting CVL with subclinical infection (Sampaio et al., Reference Sampaio, Soares, Barral, Passos, Fonseca, Meyer, Barrouin-Melo and Portela2021). Samples with relative seroconversion in cELISA and dELISA were detected in a lower proportion for IgG anti-PSE and anti-GBC antibodies, in a proportion similar to other studies (de Carvalho et al., Reference de Carvalho, Ferrão, de Freitas and de Andrade2019). Higher frequencies of relative seroconversion were found in the detection of IgA anti-PSE and anti-GBC antibodies in dELISA, a condition associated with the dissociation of ICs and an antibody increment, reaching approximately 50% of the samples that are possibly positive. IgM anti-PSE antibodies were less frequent, showing low reactivity, even in dELISA. Higher detection sensitivity was found for IgM anti-GBC in cELISA when compared to the results of dELISA. Speculatively, this condition may be associated with the specific affinity characteristics of IgM anti-GBC antibodies that are unable after acid dissociation to reestablish the ability to bind to the antigen bound to the solid support in ELISA.
Anti-Leishmania IgG has specific characteristics related to its production, quantitative availability, formation of ICs, maturation and affinity (de Carvalho et al., Reference de Carvalho, Ferrão, Cavalcante, de Freitas, Meireles and de Andrade Júnior2020), which makes the study of these biological markers a challenge. The presence of circulating ICs in serological samples from dogs is considered a CVL biomarker and can be used to monitor the disease (Parody et al., Reference Parody, Cacheiro-Llaguno, Osuna, Renshaw-Calderón, Alonso and Carnés2019), in addition to assisting in its diagnosis. IgM antibodies are unstable after acid dissociation, which affects their ability to bind and recognize the antigen, influencing the sensitivity of the test and its results. IgA monovalent ICs were more frequently found in sera from dogs with VL. Using parasite glycan antigen bound to support protein compared with soluble whole parasite extract in dELISA, we found a large fraction of IgA ICs, while IgG are blocked to a lesser extent, despite significant seroconversions, which also supports IgA findings, much more impressive.
We have demonstrated the existence of circulating ICs in CVL, with seroconversion and improvement of diagnostic tests, but we also showed a prominent role of ICs in VL pathogenesis and humoral immune response. In this work, the presence of circulating ICs composed of IgA antibodies and, to a lesser extent, IgG reinforces the need for studies related to specific antibodies and hypergammaglobulinemia, old aspects that are reappearing as important characteristics to be studied in this neglected disease.
Acknowledgements
The authors thank Flavia Regina Novais de Freitas and Bruno Márcio da Silva for scientific technical support.
Author contributions
All authors affirm that they have contributed to the development of the manuscript. de Carvalho CA collected data, performed analysis, evaluated study findings, discussed results and wrote the manuscript. Hiramoto RM and Meireles LR contributed to the availability of samples and manuscript review. Andrade Jr HF contributed to the study design, performed the analysis, and provided support in the writing and editing of the manuscript.
Financial support
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Processo: 2017/14675-0; 2017/26558-9) and Laboratório de Investigação Médica (LIM49) da Faculdade de Medicina da Universidade de São Paulo.
Conflict of interest
None.
Ethical standards
The Ethics Committee in Animal Experimentation of the Faculdade de Medicina da Universidade de São Paulo (no. 1437/2020) previously approved all procedures.