- CLSM
confocal laser scanning microscopy
- CTK
cytoplasmatic tyrosine kinase
- Dia
diaphanous
- DRF
diaphanous-related formin
- EGFR
epidermal growth factor receptor
- Eps8
epidermal growth factor receptor pathway substrate 8
- FAK
focal adhesion kinase
- FKBP12
FK506 binding protein
- GCK
germinal center kinase
- GPC
gynaecophoric canal protein
- GPCRs
G-protein coupled receptors
- GVBD
germinal vesicle breakdown
- ITAM
immunoreceptor tyrosine-based activation motif
- LOK
lymphocyte oriented kinase
- MAPK
mitogen activated protein kinase
- Plk
polo-like kinase
- PTK
protein tyrosine kinase
- PZQ
praziquantel
- SER
Schistosoma mansoni EGFR
- SH
Src homology domain
- SLK
Ste20-like kinase
- RTK
receptor tyrosine kinase
- TK (domain)
tyrosine kinase (domain)
- TβRI/II
transmembrane serine/threonine receptor type I/II
- TRIKI
TGFβRI kinase inhibitor
- YTH
yeast two-hybrid
INTRODUCTION
Schistosomes are parasitic trematodes causing one of the most important parasitic infections for humans and animals, called schistosomiasis (Engels et al. Reference Engels, Chitsulo, Montresor and Savioli2002; Ross et al. Reference Ross, Bartley, Sleigh, Olds, Li, Williams and McManus2002; Chitsulo et al. Reference Chitsulo, Loverde and Engels2004; Quack et al. Reference Quack, Beckmann and Grevelding2006). Due to its world-wide importance different research programmes have been initiated by governmental or educational institutions, industry, or WHO programmes. Their aims are to elucidate the genome, the transcriptome, the proteome, and the glycome of schistosomes to identify new candidates for the development of a vaccine, or for the design of novel pharmaceuticals (Hu et al. Reference Hu, Brindley, McManus, Feng and Han2004; El-Sayed et al. Reference El-Sayed, Bartholomeu, Ivens, Johnston and LoVerde2004; Oliveira, Reference Oliveira2007; Wilson et al. Reference Wilson, Ashton, Braschi, Dillon, Berriman and Ivens2007; Haas et al. Reference Haas, Berriman, Hirai, Cerqueira, LoVerde and El-Sayed2007; van Hellemond et al. Reference van Hellemond, van Balkom and Tielens2007; Hokke et al. Reference Hokke, Deelder, Hoffmann and Wuhrer2007a, Reference Hokke, Fitzpatrick and Hoffmannb; Berriman et al. Reference Berriman, Haas, LoVerde, Wilson, Dillon, Cerqueira, Mashiyama, Al-Lazikani, Andrade, Ashton, Aslett, Bartholomeu, Blandin, Caffrey, Coghlan, Coulson, Day, Delcher, DeMarco, Djikeng, Eyre, Gamble, Ghedin, Gu, Hertz-Fowler, Hirai, Hirai, Houston, Ivens, Johnston, Lacerda, Macedo, McVeigh, Ning, Oliveira, Overington, Parkhill, Pertea, Pierce, Protasio, Quail, Rajandream, Rogers, Sajid, Salzberg, Stanke, Tivey, White, Williams, Wortman, Wu, Zamanian, Zerlotini, Fraser-Liggett, Barrell and El-Sayed2009; The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium, 2009). Treatment and control of schistosomiasis rely on praziquantel (PZQ), which is the only drug commonly used. Since PZQ is not able to prevent reinfection, and since there is a constant fear of emerging resistance against PZQ, the search for alternatives is an urgent and generally accepted requirement (Botros et al. Reference Botros, Sayed, Amer, El-Ghannam, Bennett and Day2005; Doenhoff et al. Reference Doenhoff, Cioli and Utzinger2008; Melman et al. Reference Melman, Steinauer, Cunningham, Kubatko, Mwangi, Wynn, Mutuku, Karanja, Colley, Black, Secor, Mkoji and Loker2009; Messerli et al. Reference Messerli, Kasinathan, Morgan, Spranger and Greenberg2009; Pica-Mattoccia et al. Reference Pica-Mattoccia, Doenhoff, Valle, Basso, Troiani, Liberti, Festucci, Guidi and Cioli2009). Although remarkable progress has been made to identify and characterize genes from this parasite during the last two decades, there are still many unresolved questions concerning the disease and the biology of its causative agent.
Schistosomes are the only trematodes that have evolved separate sexes. Another unusual phenomenon of schistosomes is the sexual development of the female which requires a constant pairing contact with the male (Kunz, Reference Kunz2001; Grevelding, Reference Grevelding2004; LoVerde et al. Reference LoVerde, Niles, Osman and Wu2004). Pairing induces mitogenic activity and differentiation processes in the female that lead to the final differentiation of the ovary and the vitellarium (Erasmus, Reference Erasmus1973; Den Hollander and Erasmus, Reference Den Hollander and Erasmus1985; Popiel, Reference Popiel1986; Kunz, Reference Kunz2001; Knobloch et al. Reference Knobloch, Kunz and Grevelding2002a). This process is accompanied by a remarkable increase in the size of the females. The differentiation of the female gonads is a prerequisite for egg production. This process is closely connected with the pathological consequences of the disease. Approximately half of the eggs penetrate the walls of the veins of the intestine or the urinary bladder to leave the body for continuation of the life cycle. However, about 50% of the eggs remain inside the body of a final host being trapped mainly in spleen and liver. This provokes hyperimmune reactions resulting in inflammatory processes and chronic progression of the disease that can be lethal (Ross et al. Reference Ross, Bartley, Sleigh, Olds, Li, Williams and McManus2002).
Viable eggs are exclusively produced by paired, mature females, but not by virgin-like, immature ones (Shaw, Reference Shaw1987). As in other trematodes, schistosome eggs consist of one oocyte synthesized within the ovary and 30–40 vitelline (nurse) cells synthesized within the vitellarium. Both cell types are combined within the ootype to form eggs. Since a paired female produces up to 300 eggs day-to-day (Cheever et al. Reference Cheever, Macedonia, Mosimann and Cheever1994), a remarkable production capacity of the vitellarium is required concurrently delivering more than 10,000 vitellocytes (Popiel, Reference Popiel1986). Hence, the vitellarium of mature females is the largest organ of schistosomes consisting of a great number of cells, which differ in their state of differentiation. In contrast, the vitellarium of immature females is not differentiated and consists only of precursor cells, which have stem-cell characteristics (Erasmus, Reference Erasmus1986; Shaw, Reference Shaw1987). According to detailed microscopical studies, 4 stages of vitelline cell development can be distinguished. In immature females, only precursor cells occur, called S1 cells (Erasmus, Reference Erasmus1973). Following pairing, cell division is initiated leading to two daughter cells, one maintains the stem-cell character, while the other one starts to differentiate into a S2 cell. The completely differentiated S4 cell-stage is reached via a S3 cell-stage which contains egg-shell precursor proteins needed for egg-shell synthesis. Furthermore, S4 cells fulfil nurse cell functions providing the embryo with yolk proteins, carbohydrates, and lipids (Erasmus, Reference Erasmus1975). Differentiation of vitelline cells can be reversed when mature females get separated from males. Within 72 hours of separation, cell proliferation decreases to the level found in immature females (Knobloch et al. Reference Knobloch, Kunz and Grevelding2006). After 35 days, separated females are similar to immature ones containing no S4 cells anymore (Popiel et al. Reference Popiel, Cioli and Erasmus1984). Upon re-pairing re-differentiation occurs.
In contrast to the vitellarium, less data are available concerning the consequences of pairing on the ovary. In 65-week-old immature females, the development of the vitellarium can be partly initiated, but it is restricted to the posterior end of the worm. However, the ovary remains completely undifferentiated (Shaw, Reference Shaw1987). In rare cases, immature females are able to lay few eggs (Shaw, Reference Shaw1987). But they are not viable and probably produced as a consequence of leaky control mechanisms, which can be accidentally activated. By carmine red-staining and confocal laser scanning microscopy (CLSM), morphological differences of the ovary of mature and immature females have been described recently (Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). It was shown that immature females possess small oocytes representing an immature stage without differentiation (oogonia), which may represent a stage prior to meiosis I. In contrast, the ovary of mature females is composed of oocytes of varying sizes, representing germ cells at different meiotic stages. Therefore, the influence of the male is to accelerate vitelline-cell proliferation and differentiation, and to induce mitotic and meiotic processes in the ovary.
Male schistosomes deliver sperm stored in the sperm vesicle (reservoir) to the female via a vas efferens. Here, sperm are stored in the seminal receptacle, which is located near the posterior part of the ovary and directly connected to the oviduct to assure the fertilisation of oocytes (Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). Earlier studies had shown that the male-induced maturation of females is independent of sperm transfer (Armstrong, Reference Armstrong1965; Basch and Basch, Reference Basch1984). In contrast to females, there is no evidence yet that pairing has similar dramatic consequences for males. Unpaired males are equal in size to their paired counterparts. They possess fully differentiated testes, which produce large amounts of sperm (Armstrong, Reference Armstrong1965). Again, use of carmine red-staining and CLSM Neves et al. (Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005) provided the first evidence for a morphological difference in the diameter of the testes of paired males, which was slightly reduced compared to that of unpaired males.
Although many efforts have been made to identify the male stimulus, its nature still remains unclear. Hormones, growth factors or lipids have been proposed to be secreted from the male to induce the initial events in female reproductive development (Shaw et al. Reference Shaw, Marshall and Erasmus1977; Popiel, Reference Popiel1986; Silveira et al. Reference Silveira, Friche and Rumjanek1986; Kunz, Reference Kunz2001). An 86 kDa protein was isolated and found to be widely distributed over the surface of mature females. In males, its expression seemed to be limited to the gynaecophoric canal, in which the female resides during the continuous pairing contact. Both results led to the hypothesis that the gynaecophoric canal protein (SmGCP) is involved in male-female interaction (Bostic and Strand, Reference Bostic and Strand1996). SmGCP transcription peaked in 28-day-old worms, a time that coincides with worm pairing (Aronstein and Strand, Reference Aronstein and Strand1985). At the sequence level, SmGCP exhibits homology to the cell adhesion molecule fasciclin-I, and its expression is regulated by the TGFβ pathway (Osman et al. Reference Osman, Niles, Verjovski-Almeida and LoVerde2006). Attempts to silence the S. japonicum homologue SjGCP provided preliminary evidence for an influence of this protein on the initial steps of pairing and pairing stability (Cheng et al. Reference Cheng, Fu, Lin, Shi, Zhou, Jin and Cai2009). Other appropriate candidate molecules for the male stimulus have not been identified yet.
In some laboratories including our own, efforts have been made to identify genes, whose protein products play regulating roles in the male-female interaction of schistosomes. Previously, genes characterized as female specific and expressed in the reproductive organs had been isolated from subtractive cDNA libraries (Bobek et al. Reference Bobek, Rekosh, van and LoVerde1986; Koster et al. Reference Koster, Dargatz, Schroder, Hirzmann, Haarmann, Symmons and Kunz1988; Chen et al. Reference Chen, Rekosh and LoVerde1992; Kunz, Reference Kunz2001; Michel et al. Reference Michel, Knobloch and Kunz2003). To be able to investigate whether such genes are pairing-dependently expressed, an in vitro culture system was established based on previous work (Basch and Rhine, Reference Basch and Rhine1983), which allowed the maintenance of adult schistosomes for many weeks after recovery from the host (Grevelding et al. Reference Grevelding, Sommer and Kunz1997; Knobloch et al. Reference Knobloch, Kunz and Grevelding2006). It was shown that in schistosomes paired under the conditions described, de novo RNA synthesis occurred and the females produced eggs during the first two weeks of culture. Based on pairing experiments in this culture system, it was demonstrated that males influence the transcription of such female-specifically expressed genes whose expression had been localised to the reproductive organs, e.g. the egg-shell precursor protein gene p14 in the vitellarium (Koster et al. Reference Koster, Dargatz, Schroder, Hirzmann, Haarmann, Symmons and Kunz1988; Grevelding et al. Reference Grevelding, Sommer and Kunz1997). This was in agreement with findings of other research groups (Chen et al. Reference Chen, Rekosh and LoVerde1992; Michel et al. Reference Michel, Knobloch and Kunz2003) and one of the first molecular proofs that the male controls transcriptional processes in the female. Along with the great amount of genome and transcriptome data available today, novel approaches have been performed recently to come closer to the molecular secrets of male-female interaction (Hoffmann, Reference Hoffmann2004). Comparison of the transcriptomes of mature versus immature females by microarray analyses revealed that immature females transcribe a broader spectrum of genes compared to mature females. About 180 genes were expressed more abundantly in immature females. About three times less genes were up-regulated after pairing in mature females. Among these were genes involved in egg formation or red blood cell consumption (Fitzpatrick and Hoffmann, Reference Fitzpatrick and Hoffmann2006). From these data it was hypothesised that the contact with the male streamlines the gene transcription profile of females on reproductive activities such as egg production.
Recently, compelling evidence has been obtained for conserved signal transduction cascades that are responsible for managing the pairing-dependent developmental processes in the female (LoVerde et al. Reference LoVerde, Osman and Hinck2007; Knobloch et al. Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007; Dissous et al. Reference Dissous, Ahier and Khayath2007). These are characterized by the induction of mitogenic activity of S1 cells and the subsequent differentiation of daughter cells. Proliferation and differentiation are biological processes that have been intensively studied in invertebrate and vertebrate models. These processes are regulated by complex signalling networks, which are conserved at the functional level, but also at the levels of the authenticity and the sequence structure of the molecules involved in these networks (Krauss, Reference Krauss2008). On the basis of these levels of conservation many signal transduction molecules have been identified in schistosomes (reviewed in LoVerde et al. Reference LoVerde, Niles, Osman and Wu2004, Reference LoVerde, Osman and Hinck2007; Vermeire et al. Reference Vermeire, Humphries and Yoshino2005; Dissous et al. Reference Dissous, Khayath, Vicogne and Capron2006; Yoshino, Reference Yoshino, Maule and Marks2006; Knobloch et al. Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007; Quack et al. Reference Quack, Knobloch, Beckmann, Vicogne, Dissous and Grevelding2009). Their characterization revealed a high degree of structural but also functional conservation, which was confirmed by replacing endogenous signalling molecules with their schistosome homologues in yeast, Xenopus oocytes, or mammalian cell-culture systems (Osman et al. Reference Osman, Niles and LoVerde2001, Reference Osman, Niles and LoVerde2004, Reference Osman, Niles, Verjovski-Almeida and LoVerde2006; Rossi et al. Reference Rossi, Pica-Mattoccia, Cioli and Klinkert2002; Santos et al. Reference Santos, Machado, Franco and Pena2002; Kapp et al. Reference Kapp, Schussler, Kunz and Grevelding2001, Reference Kapp, Knobloch, Schussler, Sroka, Lammers, Kunz and Grevelding2004; Vicogne et al. Reference Vicogne, Cailliau, Tulasne, Browaeys, Yan, Fafeur, Vilain, Legrand, Trolet and Dissous2004).
This review will summarise what is known about the occurrence of signalling molecules in the gonads of schistosomes. We will focus on the reproductive organs of females concentrating on the ovary, which has been a neglected organ with respect to its meaning as a target of pairing-induced regulatory processes initiating cell proliferation processes. In addition to a synopsis of the molecular and biochemical analyses of genes and their encoded products in the gonads, we will demonstrate the power of combining existing techniques towards the functional analyses of these genes or encoded enzymes in adult worms cultured in vitro. Among the assembled methods is a modified application of CLSM established by Machado-Silva et al. (Reference Machado-Silva, Pelajo-Machado, Lenzi and Gomes1998), which allowed us for the first time to identify a morphological structure in immature adult females that seems to obstruct the passage to the ootype. This structure is not present in mature adult females. The same technique is particularly suitable for analyzing the morphological consequences of inhibiting enzyme activities by chemical inhibitors, or gene expression by RNA interference, both methods being of increasing importance in the dawn of the post-genomic era of schistosomes. Finally, we propose the first model for signalling transduction cascades in the ovary that affect mitogenic activity and differentiation. Some of the identified molecules and networks are conserved in nature, although they may have different functions in organs or cell types of other organisms. Among the molecules described are candidates for ovary- and testes-specific expression. These are especially interesting with respect to the development of novel anti-schistosomal strategies since a subset of these molecules represent potential targets for existing pharmaceuticals.
MORPHOLOGICAL DIFFERENCES BETWEEN IMMATURE AND MATURE S. MANSONI FEMALES AND MALES
Former and recent morphological studies have revealed significant differences in the ovary and vitellarium between immature and mature schistosome females (Erasmus, Reference Erasmus1973; Popiel and Basch, Reference Basch1984; Popiel et al. Reference Popiel, Cioli and Erasmus1984; Shaw, Reference Shaw1987; Kunz, Reference Kunz2001; Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). In contrast to mature females, immature females have an underdeveloped reproductive system. Their ovary is small containing a low number of oocytes, which are in an undifferentiated stage of development (Fig. 1A, B) (Erasmus, Reference Erasmus1973; Popiel et al. Reference Popiel, Cioli and Erasmus1984; Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). Furthermore, the ootype is smaller compared to the ootype of mature females, it contains no eggs and is lined with Mehlis' gland cells (Fig. 1A, C). When we applied the CLSM-method established by Machado-Silva et al. (Reference Machado-Silva, Pelajo-Machado, Lenzi and Gomes1998) to investigate the reproductive organs of adult schistosomes, we discovered a filamentous structure which is located at the posterior end of the ootype, where oviduct and vitelloduct end (Fig. 1C). This structure has not been described before, and we have not found it in mature females. It seems to seal the ootype of immature females probably preventing undifferentiated vitellocytes or oocytes to permeate the ootype. In this sense the filamentous structure fulfils a hymen-like function. Our finding corresponds to the observation that females from unisexual infections do not produce eggs. As a consequence of pairing, the hymen presumably dispersed induced directly by male signals, or indirectly by contact with mature vitellocytes and/or oocytes. In females, which were separated from males for 7 days and started to de-differentiate to an immature state, the hymen did not exist. In separated females, immature vitellocytes and oocytes are able to permeate the ootype since the hymen was removed during a former pairing contact. This leads to the production of abnormal eggs, which has already been described before (Shaw and Erasmus, Reference Shaw and Erasmus1981; Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005).
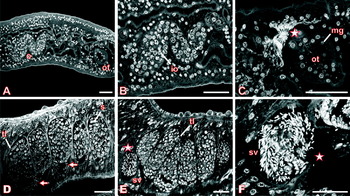
Fig. 1. Confocal laser scanning microscope (Leica TSC SP2) images of immature S. mansoni females (A–C) and pairing-unexperienced males (D–F) from unisexual infections stained with carmine red and analyzed by reflection mode as described elsewhere in detail (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). A: ovary (o) and ootype (ot), B: ovary with immature oocytes (io), C: ootype with Mehlis' glands (mg) (asterisk: “hymen”), D: testicular lobes (tl) with spermatocytes (s) (arrow: mature sperms), E: testicular lobes (tl) and sperm vesicle (sv, asterisk), F: sperm vesicle (sv, asterisk) filled with mature sperms [scale bar: 30 μm].
Whereas immature females differ considerably from mature females, unpaired males do not show similar significant morphological differences from paired males in bisexual infections. Although slightly reduced in size (Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005), their testicular lobes are completely developed containing many spermatocytes in different stages of maturation. In the ventral part of the lobes many elongated sperms are located, and which enter the vas deferens (Fig. 1D arrows). The sperm vesicle is located anterior of the testicular lobes and it is full of mature, elongated spermatozoa (Fig. 1E, F asterisks).
The ovary of mature S. mansoni females is composed of oocytes at different stages of maturation – small, immature oogonial cells in the anterior part and bigger germ cells in the posterior part (Fig. 2 A–C). The latter are presumably primary oocytes, since it has been described for trematodes that oogonial cells divide mitotically to form primary oocytes, which enlarge filling the centre and the posterior portion of the ovary (Gresson, Reference Gresson1964; Nollen, Reference Nollen, Fried and Graczyk1997). In some trematode species, early stages of meiosis are completed as the oocytes move into the oviduct, and fertilization finally stimulates the further meiotic progression. In other species meiosis is not initiated until fertilization has happened (Nollen, Reference Nollen, Fried and Graczyk1997). The primary oocytes of S. mansoni have a hexagonal form and contain a large amount of cytoplasm. They represent the biggest cells found in the ovary, and they also occur within the oviduct where fertilisation takes place (Fig. 2E). Upon carmine red staining, the large central nucleus appears dark and the nucleolus bright (Fig. 2A–E). The differentiation of the oocytes starts with the mitotic proliferation of oogonial stem cells in the anterior part of the ovary leading to small immature oocytes, which mature to large primary oocytes passing on to meiosis. In the posterior part, the primary oocytes leave the ovary and enter into the oviduct. Shortly behind the ovary the oviduct forms the receptaculum seminis by outpocketing its wall. Within this reservoir mature spermatozoa are stored (Fig. 2A, C, E) ready to fertilise primary oocytes (Fig. 2E arrow), which presumably complete their meiotic development thereafter. The oviduct, which lies anterior of the ovary, leads to the ootype (Fig. 2D). This organ is lined with big elongated cells representing the Mehlis' glands (Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). Here composite eggs are formed, which consist of one mature oocyte and 30–40 vitellocytes, delivered from the vitellarium (Fig. 2C, Fig. 3B) via the vitelloduct. Following egg-shell synthesis, the eggs are transferred to the uterus, which ends in a genital pore below the ventral sucker where the eggs are released.

Fig. 2. Confocal laser scanning microscope images of S. mansoni couples from a mixed infection. A: overview, B: ovary of the female and “pseudo ovary” of the male (asterisk), C: ovary with immature and mature oocytes, D: ootype with egg, E: mature oocyte in the oviduct, F: testicular lobes and sperm vesicle, G: vas deferens, H: cirrus [abbreviations: a: anterior, c: cirrus, cg: canalis gynaecophorus, e: egg, io: immature oocyte, mo: mature oocyte, mg: Mehlis' glands, o: ovary, od: oviduct, ot: ootype, p: posterior, rs: receptaculum seminis, sv: sperm vesicle, t: testes, te: tegument, tu: tubercle, v: vitelline cell, vg: vitelline gland; scale bar: 60 μm].

Fig. 3. Confocal laser scanning microscope images of the reproductive organs of S. mansoni couples treated with Herbimycin A (A, D: testes of the male; B, E: vitellarium of the female; C, F: ovary of the female). A–C: control worms, treated for two days with DMSO, the solvent of the inhibitor; D–F: worms treated for two days with 4·5 μM Herbimycin A [abbreviations: io: immature oocyte, mo: mature oocyte, v: vitellarium; arrow: holes in the vitellarium; asterisk: sperm vesicle; scale bar: 40 μm].
The testes of paired S. mansoni males are composed of several testicular lobes enclosed by a thin albuginea-like capsule, containing germ cells at different stages of maturation. Here, the bigger cells are less mature than the smaller ones (Fig. 2A, F). In the dorsal part of the lobes, the round spermatogonial cells are located. They proliferate mitotically leading to primary spermatocytes that enter meiosis to become primary and secondary spermatocytes (meiose I) and finally spermatids (meiose II). These round cells differentiate to mature, elongated spermatozoa located in the ventral part of the testicular lobes (Fig. 2F arrows). In the ventral part the lobes are interconnected allowing the transport of sperm via the vas deferens to the anterior seminal vesicle (Machado-Silva et al. Reference Machado-Silva, Pelajo-Machado, Lenzi and Gomes1998), which contains a high number of elongated, mature sperm (Fig. 2F asterisk). The vesicle is connected to the ejaculatory duct ending in a cirrus posterior of the ventral sucker (Fig. 2G, H; arrows). From here sperm are continuously released into the gynaecophoric canal during pairing (Kitajima et al. Reference Kitajima, Paraense and Correa1976).
In evolutionary terms, schistosomes have evolved from hermaphroditic blood flukes to dioecious trematodes with a distinct gender dimorphism of the adults (Basch, Reference Basch1990). However, a complete functional separation of the sexes is debatable, because female worms still need the constant pairing contact with the male to reach sexual maturation. This is reminiscent of hermaphroditism since here the development of the male reproductive organs precedes that of the female gonads. Furthermore, some S. mansoni males can possess a rudimentary ovary (Hulstijn et al. Reference Hulstijn, Barros, Neves, Moura, Gomes and Machado-Silva2006). In earlier studies it was detected as a round structure posterior of the testicular lobes and discussed to be a supernumerary testicular lobe in an abnormal position (Vogel, Reference Vogel1947; Najim, Reference Najim1951; Saoud, Reference Saoud1966). However, by CLSM it was shown that it contains fully developed oocytes instead of testicular cells (Hulstijn et al. Reference Hulstijn, Barros, Neves, Moura, Gomes and Machado-Silva2006) (Fig. 2B, asterisk). Number and sizes of oocytes vary inside this organ (Fig. 2B) indicating ongoing proliferation and maturation processes. Since no efferent duct could be observed in these feminized males (Hulstijn et al. Reference Hulstijn, Barros, Neves, Moura, Gomes and Machado-Silva2006) the oocytes cannot leave this organ and are probably not functional. The occurrence of a rudimentary ovary or vitelline follicles (Hulstijn et al. Reference Hulstijn, Barros, Neves, Moura, Gomes and Machado-Silva2006) in some males corresponds to data showing a leaky expression of some female-specific genes in the male (Wippersteg, unpublished). It seems likely that the expression of genes specific for female germinal cells is still somehow fixed in the male. The oocyte-containing lobes in the male appear to be remnants from hermaphroditic ancestors (atavism). In rare cases also females were found which exhibited a small follicle anterior to the normal ovary resembling the supernumerary lobes in the males. This follicle is not connected with the ovary or the oviduct, and it contains in its centre more developed oocytes, whereas smaller oocytes are locates in the periphery (Hulstijn et al. Reference Hulstijn, Barros, Neves, Moura, Gomes and Machado-Silva2006).
SIGNALLING MOLECULES INVOLVED IN THE MATURATION OF THE VITELLARIUM
One of the best characterized signalling pathways in schistosomes is the TGFβ/Smad pathway, which is initiated by transmembrane serine/threonine kinase receptors of type I (TβRI) and II (TβRII) playing a key role in cell growth and proliferation (Massague, Reference Massague1998; Krauss, Reference Krauss2008). Upon TGFβ-ligand binding the constitutively active TβRII recruits TβRI and promotes its phosphorylation. The activated TβRI subsequently phosphorylates and activates receptor-regulated Smads (R-Smads). Activated R-Smads in turn bind to Co-Smads (Smad4) forming heterodimers, which enter the nucleus to regulate gene transcription (Massague and Wotton, Reference Massague and Wotton2000; Massague et al. Reference Massague, Seoane and Wotton2005). TβRI, TβRII, R-Smad, Co-Smad as well as the TβRI-regulating protein FKBP12 as pivotal TGFβ-pathway members have been identified in schistosomes (Davies et al. Reference Davies, Shoemaker and Pearce1998; Beall et al. Reference Beall, McGonigle and Pearce2000; Osman et al. Reference Osman, Niles and LoVerde2001, Reference Osman, Niles and LoVerde2004, Reference Osman, Niles, Verjovski-Almeida and LoVerde2006; Knobloch et al. Reference Knobloch, Rossi, Osman, LoVerde, Klinkert and Grevelding2004). Their expression has been detected amongst other tissues in the vitellarium by in situ-hybridisation and/or immunolocalisation suggesting a role in vitellarium development. With the exception of Smad4, Smad2, and TβRII the expression of all other TGFβ pathway members has been detected in the ovary and testes (see Table 1).
Table 1. Overview about the identified signalling molecules and the gonad tissues of S. mansoni in which expression products were localised [+=present; −=absent; ?=not yet known]

To unravel the biological role of the TGFβ/Smad signalling pathway for the development of the vitellarium, experiments were performed employing the synthetic TGFβRI kinase inhibitor (TRIKI), which specifically abolishes mammalian TβRI kinase activity (Sawyer et al. Reference Sawyer, Anderson, Beight, Campbell, Jones, Herron, Lampe, McCowan, McMillen, Mort, Parsons, Smith, Vieth, Weir, Yan, Zhang and Yingling2003). The treatment of in vitro-cultured schistosome pairs with 300 nM TRIKI significantly reduced mitotic activity in females about 46%, and in agreement with its anti-proliferative properties also egg production about 28% (Knobloch et al. Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007). Interestingly, higher concentrations up to 450 nM of TRIKI did not enhance these effects. This strongly argues for the existence of alternative signalling pathways involved in the development of the vitellarium.
An analogous pharmacological approach employing Herbimycin A as a Src-kinase specific inhibitor in a concentration of 4·5 μM reduced male-induced mitotic activity to 27% in paired females cultured in vitro. Consistently, egg production was reduced to about 14% under the same conditions (Knobloch et al. Reference Knobloch, Kunz and Grevelding2006, Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007). Cytoplasmic tyrosine kinases (CTKs), besides being receptor tyrosine kinases (RTKs) and are members of the protein tyrosine kinase (PTK) superfamily, function as cytoplasmic transducer molecules in signal-transduction cascades and forward incoming signals from activated receptors to a variety of downstream targets within the cell, thereby regulating cell proliferation or cytoskeletal rearrangement (Tatosyan and Mizenina, Reference Tatosyan and Mizenina2000). The subfamily of CTKs comprise eight classes, which are grouped by the presence and the number of different conserved functional domains as well as their structural arrangement (Thomas and Brugge, Reference Thomas and Brugge1997; Hubbard and Till, Reference Hubbard and Till2000; Bromann et al. Reference Bromann, Korkaya and Courtneidge2004). In schistosomes, several CTKs have been isolated and characterized. Among these are a Fes kinase (Bahia et al. Reference Bahia, Mortara, Kusel, Andrade, Ludolf, Kuser, Avelar, Trolet, Dissous, Pierce and Oliveira2007), the Src kinases SmTK3 (Kapp et al. Reference Kapp, Knobloch, Schussler, Sroka, Lammers, Kunz and Grevelding2004) and SmTK6 (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010; Beckmann et al., unpublished), the Fyn-type Src kinase SmTK5 (Kapp et al. Reference Kapp, Schussler, Kunz and Grevelding2001; Knobloch et al. Reference Knobloch, Winnen, Quack, Kunz and Grevelding2002b), and SmTK4 (Knobloch et al. Reference Knobloch, Winnen, Quack, Kunz and Grevelding2002b; Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010), which belongs to the class of Syk kinases. Except Fes, which is predominantly expressed in cercariae and newly-transformed schistosomula suggesting its role in penetration processes, all other schistosomal CTKs were localised in the gonads of both sexes probably being involved in differentiation processes here. However, SmTK4 and SmTK6 transcripts were only detected in the testes and the ovary, but not in the vitellarium (see Table 1).
Src kinases are known to be involved in signalling pathways regulating cell proliferation and differentiation by controlling cell cycle progression by the transcriptional activation of mitotic genes, as well as cytokinesis by the regulation of cytoskeleton rearrangements (Barone and Courtneidge, Reference Barone and Courtneidge1995; Thomas and Brugge, Reference Thomas and Brugge1997; Tatosyan and Mizenina, Reference Tatosyan and Mizenina2000; Frame, Reference Frame2002; Ishizawar and Parsons, Reference Ishizawar and Parsons2004). Therefore, with respect to their expression in the vitellarium, SmTK3 and SmTK5 seem to fulfil essential functions in the development of the vitellarium. Among the other pathways that Src kinases are involved in, is the Ras/MAPK-pathway (Downward, Reference Downward1997; Dunn et al. Reference Dunn, Espino, Drobic, He and Davie2005; Qi and Elion, Reference Qi and Elion2005) mainly elicited by plasma membrane RTKs upon their activation by growth factors. SH2 domain-containing signalling proteins such as Src kinases, as well as the adapter proteins Shc or Grb2, bind to the cytoplasmic part of the activated receptor. The interplay of these proteins leads to the recruitment of the guanine-nucleotide exchange factor (GEF) SOS promoting the activation of the membrane-associated small GTPase Ras by accelerating the exchange of GDP for GTP. GTP-bound Ras induces the downstream located MAP kinase (MAPK) cascade, finally leading to the transcription of genes promoting cell division and differentiation (Downward, Reference Downward1997; Qi and Elion, Reference Qi and Elion2005). In parallel, activated Src phosphorylates and blocks the GTPase-activating protein (GAP), thereby prolonging the activated conformation of Ras. Both events synergistically result in an activation of Ras signalling (Giglione et al. Reference Giglione, Gonfloni and Parmeggiani2001; Wennerberg et al. Reference Wennerberg, Rossman and Der2005).
There is evidence for a cross-talk between the MAPK pathway and the TGFβ signalling pathway, since activated Erk as a Ras/MAPK-pathway member is able to phosphorylate Smad2, promoting TGFβ-mediated signalling (Hayashida et al. Reference Hayashida, Decaestecker and Schnaper2003). This cross-talk may occur in the vitellarium of schistosome females, too. In vitro culture experiments simultaneously employing both the TGFβRI kinase-inhibitor TRIKI and the Src kinase-inhibitor Herbimycin A for the treatment of schistosome pairs led to a synergistic effect reducing mitotic activity in females to 16%, and egg production to about 5%, which is less than with TRIKI or Herbimycin A alone (Knobloch et al. Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007).
Following treatment with these inhibitors, we observed by CLSM varying phenotypes in adult schistosomes maintained in vitro (Fig. 3). Although no clear phenotypes were obtained in the reproductive organs when pairs had been treated with TRIKI, morphological abnormalities were detected in the vitellarium of the female and in the testes of the male following Herbimycin A treatment. Compared to control worms, the vitellarium of treated females revealed a poriferous structure and a lower number of vitelline cells (Fig. 3E). Within the ovary, in some females the number of mature oocytes was increased, and these large cells were distributed widely throughout this organ (Fig. 3F). In treated males, a significantly reduced number of sperms was observed in the ventral part of the testicular lobes as well as in the seminal vesicle (Fig. 3D) compared to control males, which contained much sperm. This supports the biochemical and physiological results obtained before with regard to the treatment of schistosome pairs with Herbimycin A and supports the conclusion that this inhibitor influences mitogenic activity, but may also have an influence on differentiation.
Aside from RTKs, Src kinases can also be activated by integrins via the focal adhesion kinase (FAK), a further CTK member (see below; Delon and Brown, Reference Delon and Brown2007), as well as via G-protein coupled receptors (GPCRs) (Thomas and Brugge, Reference Thomas and Brugge1997; Tatosyan and Mizenina, Reference Tatosyan and Mizenina2000). GPCRs, also denoted as seven transmembrane (7TM) receptors, serve as ligand-activated GEFs for the class of heterotrimeric G-proteins inducing GDP/GTP exchange to control the activity of enzymatic effectors. GPCRs also co-operate with RTKs in the regulation of cell proliferation and differentiation. Whereas RTKs respond to growth factors and then activate Src kinase pathways, GPCRs respond to ligands such as α-thrombin and lysophosphatidic acid (LPA) to activate Rho-GTPases (Luttrell et al. Reference Luttrell, la Rocca, van, Luttrell and Lefkowitz1997; Parsons and Parsons, Reference Parsons and Parsons2004). Both pathways bridge at diaphanous (Dia), a signalling protein interacting with Src kinases and Rho-GTPases. The schistosome homologue of diaphanous (SmDia) was recently identified as a strong interaction partner of SmTK3 by screening a yeast two-hybrid (YTH) cDNA library of adult (mixed-sex) S. mansoni (Quack et al. Reference Quack, Knobloch, Beckmann, Vicogne, Dissous and Grevelding2009). From the literature it is known that Dia proteins are involved in actin-mediated processes controlling cell and tissue architecture playing important roles in cell polarity, cell-cell interactions, gastrulation, or cytokinesis (Wallar and Alberts, Reference Wallar and Alberts2003; Olson, Reference Olson2003; Faix and Grosse, Reference Faix and Grosse2006). This also applies to members of the Rho-family, which were shown to be involved in the regulation of cell adhesion, and cell cycle progression (Krebs et al. Reference Krebs, Rothkegel, Klar and Jockusch2001; Wennerberg and Der, Reference Wennerberg and Der2004). Binding experiments with the known schistosome Rho-GTPase homologue SmRho1 (Santos et al. Reference Santos, Machado, Franco and Pena2002; Vermeire et al. Reference Vermeire, Osman, LoVerde and Williams2003) demonstrated the GTP-dependent binding of SmRho1 to the Rho-binding domain of SmDia (Quack et al. Reference Quack, Knobloch, Beckmann, Vicogne, Dissous and Grevelding2009). In situ hybridisation experiments finally confirmed SmDia as well as SmRho1 transcription in the gonads of both sexes. This perfectly coincided with localisation data obtained for SmTK3. Therefore, a functional trimeric complex of SmRho1, SmTK3 and SmDia was postulated, integrating RTK- and GPCR-mediated signals to influence actin cytoskeleton reorganisation, which is fundamental for differentiation processes within the reproductive organs.
In this context it was of interest to identify binding partners controlling SmTK3. Since the SH2 domain of Src kinases is known to bind to upstream partners in a signalling hierarchy, the SmTK3-SH2 domain was used as a probe to screen the YTH-library. The vast majority of prey clones obtained represented a schistosome homologue of the epidermal growth factor receptor pathway substrate 8 (SmEps8; Burmeister et al., unpublished). Eps8 proteins are known to fulfil diverse functions in signalling pathways leading to cell proliferation, differentiation and cytoskeleton remodelling. Originally, Eps8 had been identified as a substrate of the EGFR (Fazioli et al. Reference Fazioli, Minichiello, Matoska, Castagnino, Miki, Wong and Di Fiore1993). But it was shown later that Eps8 may also interact with integrin receptors (Calderwood et al. Reference Calderwood, Fujioka, de Pereda, Garcia-Alvarez, Nakamoto, Margolis, McGlade, Liddington and Ginsberg2003). Interaction with EGFR leads to Eps8 phosphorylation, which can be alternatively accomplished by other RTKs, or by Src kinases (Gallo et al. Reference Gallo, Provenzano, Carbone, Di Fiore, Castellani, Falcone and Alema1997; Maa et al. Reference Maa, Lai, Lin and Leu1999). Ectopic expression of Eps8 enhances EGF-induced mitogenic signals (Fazioli et al. Reference Fazioli, Minichiello, Matoska, Castagnino, Miki, Wong and Di Fiore1993; Xu et al. Reference Xu, Shorts-Cary, Knox, Kleinsmidt-DeMasters, Lillehei and Wierman2009), tumorgenesis (Matoskova et al. Reference Matoskova, Wong, Salcini, Pelicci and Di Fiore1995; Wang et al. Reference Wang, Patel, Miyazaki, Gutkind and Yeudall2009), serum-induced activation of the extracellular responsive kinase (ERK; Maa et al. Reference Maa, Hsieh and Leu2001; Xu et al. Reference Xu, Shorts-Cary, Knox, Kleinsmidt-DeMasters, Lillehei and Wierman2009), as well as the expression and activation of the focal adhesion kinase (FAK) (Maa et al. Reference Maa, Lee, Chen, Chen, Lee, Wang, Huang, Chow and Leu2007). In addition, Eps8 is involved in receptor endocytosis by binding to RN-tre, a Rab5 GTPase-activating protein that regulates EGF receptor internalisation (Lanzetti et al. Reference Lanzetti, Rybin, Malabarba, Christoforidis, Scita, Zerial and Di Fiore2000). As a regulator of Ras/Rac signalling, Eps8 was also shown to be involved in cytoskeleton remodelling (Scita et al. Reference Scita, Nordstrom, Carbone, Tenca, Giardina, Gutkind, Bjarnegard, Betsholtz and Di Fiore1999; Innocenti et al. Reference Innocenti, Frittoli, Ponzanelli, Falck, Brachmann, Di Fiore and Scita2003; Offenhauser et al. Reference Offenhauser, Borgonovo, Disanza, Romano, Ponzanelli, Iannolo, Di Fiore and Scita2004; Croce et al. Reference Croce, Cassata, Disanza, Gagliani, Tacchetti, Malabarba, Carlier, Scita, Baumeister and Di Fiore2004).
Molecular analyses of SmEps8 demonstrated that it is transcribed in schistosome males and females, but not in larval stages. By in situ hybridisation SmEps8 transcripts were localised in the vitellarium and the ovary as well as in the testes (Burmeister et al., unpublished). This transcription profile corresponded to the tissue-specific activity of SmTK3 (Kapp et al. Reference Kapp, Knobloch, Schussler, Sroka, Lammers, Kunz and Grevelding2004). Four receptor tyrosine kinases have been characterized in S. mansoni, of which one belongs to the EGFR family, SER (Shoemaker et al. Reference Shoemaker, Ramachandran, Landa, dos Reis and Stein1992; Vicogne et al. Reference Vicogne, Pin, Lardans, Capron, Noel and Dissous2003, Reference Vicogne, Cailliau, Tulasne, Browaeys, Yan, Fafeur, Vilain, Legrand, Trolet and Dissous2004). This receptor was immunolocalised in the muscles of adult schistosomes, where it may influence developmental processes (Ramachandran et al. Reference Ramachandran, Skelly and Shoemaker1996). In situ hybridisation experiments recently performed in our laboratory indicated that SER is also transcribed in the vitellarium and ovary of schistosome females. However, the initial YTH analyses to investigate a putative binding of SmEps8 to the cytoplasmic part of SER did not provide evidence for interaction (Burmeister et al., unpublished). Although the proper receptor acting upstream of SmEps8 has still to be identified, it seems likely that SmEps8 represents a protein bridging a membrane receptor and SmTK3 in signalling pathways controlling mitogenic activity and differentiation in the vitellarium, but also in other reproductive organs of schistosomes.
SIGNALLING MOLECULES INVOLVED IN THE MATURATION OF THE OVARY
For a long time it has been known that the pairing contact with the male is essential not only for the development of the vitellarium of the female, but also for the development of the ovary. But at the functional level less is known about signalling processes regulating proliferation and differentiation of oocytes. Most signalling molecules expressed in the vitellarium of the female and supposed to be involved in the differentiation of this reproductive organ were also found to be expressed in the ovary (Knobloch et al. Reference Knobloch, Beckmann, Burmeister, Quack and Grevelding2007; see Table 1). Among these are Src kinases, SmDia, SmRho1, SER, SmEps8 and TGFβ pathway members. Ontogenetically, vitelline cells and oocytes arise from common mother cells (Kunz, Reference Kunz2001), and thus they are closely related. This includes similar proliferation and differentiation processes, in which similar sets of molecules are involved. But differences have to exist considering the distinct functions of the cells generated in the vitellarium or the ovary. In this respect it was no surprise to find molecules to be exclusively expressed in one of both organs such as the CTKs SmTK4 (Knobloch et al. Reference Knobloch, Winnen, Quack, Kunz and Grevelding2002b) and SmTK6 (see below; Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010; Beckmann et al., unpublished), or the receptor tyrosine kinase SmVKR (Vicogne et al. Reference Vicogne, Pin, Lardans, Capron, Noel and Dissous2003). Their transcripts were detected in oocytes but not in vitelline cells.
A CTK OF THE SYK TYPE AND ITS INTERACTION PARTNERS
SmTK4 belongs to the Syk family of CTKs containing two N-terminal SH2 domains in a tandem formation, a linker region, and a C-terminal catalytic tyrosine-kinase domain (Knobloch et al. Reference Knobloch, Winnen, Quack, Kunz and Grevelding2002b). The presence of a member of the Syk family in schistosomes was unexpected, since Syk kinases are well known from mammals, but they are absent in Caenorhabditis elegans, and in Drosophila melanogaster only the related kinase Shark (SH2 domain ankyrin repeat kinase; Ferrante et al. Reference Ferrante, Reinke and Stanley1995) is present. This suggested a recent evolutionary origin of kinases of the Syk family. In addition, the function of Syk kinases in mammals seemed to be highly specialised and restricted to cells of the hematopoietic system. Recently, evidence has accumulated for further functions of Syk kinases in the proliferation, differentiation or morphogenesis of non-hematopoietic cells like epithelial, endothelial and neuronal cells (Fluck et al. Reference Fluck, Zurcher, Andres and Ziemiecki1995; Coopman et al. Reference Coopman, Do, Barth, Bowden, Hayes, Basyuk, Blancato, Vezza, McLeskey, Mangeat and Mueller2000; Inatome et al. Reference Inatome, Yanagi, Takano and Yamamura2001; Tsujimura et al. Reference Tsujimura, Yanagi, Inatome, Takano, Ishihara, Mitsui, Takahashi and Yamamura2001). In the hematopoietic system, Syk kinases interact with a large set of immune and antigen receptors lacking intrinsic catalytical activity (Geahlen, Reference Geahlen2007). Upon receptor activation, each SH2 domain of the Syk kinase binds to one tyrosine-phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) in the intracellular part of the receptor (Ottinger et al. Reference Ottinger, Botfield and Shoelson1998) leading to a conformational change of Syk accompanied by an increase in its enzymatic activity (Pawson and Kofler, Reference Pawson and Kofler2009). In SmTK4 the conserved sequence within the SH2 domains responsible for this binding is absent, suggesting that this Syk kinase interacts with receptors without ITAMs. Such Syk-receptor interactions have been described in mammals, e.g. for Syk and integrin receptors, but the binding mechanisms are still not fully understood. However, binding stimulates the autophosphorylation of Syk on tyrosine residues (Carsetti et al. Reference Carsetti, Laurenti, Gobessi, Longo, Leone and Efremov2009), which occurs on multiple tyrosine residues. Some of these modify the activity of the kinase directly by conformational changes, and others serve additionally as docking sites for proteins that contain SH2 domains (Geahlen, Reference Geahlen2009). The phosphorylation of Syk can be enhanced by Src tyrosine kinases, which directly interact with Syk kinases to phosphorylate them (Geahlen, Reference Geahlen2007). Thus, tyrosine residues within the linker region of Syk kinases are phosphorylated creating docking sites for downstream-acting signalling molecules of which many, but not all, are also substrates of Syk kinases (Geahlen, Reference Geahlen2007).
To discover signalling cascades in which SmTK4 is involved, YTH screenings were performed with the aim to identify molecules acting upstream and downstream of this Syk kinase in a signalling hierarchy (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). For identifying upstream interaction partners the tandem SH2-domain of SmTK4 was used, because this domain binds to phosphorylated tyrosine residues of upstream-acting signalling molecules (Marengere and Pawson, Reference Marengere and Pawson1994). Among other proteins, a new schistosome CTK, named SmTK6 was identified as the strongest upstream binding partner of SmTK4. In silico analyses showed that SmTK6 belongs to the Src family of CTKs. In situ hybridisation experiments confirmed co-localisation of SmTK6 and SmTK4 in the ovary of the female and the testes of the male (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). The same screening approach led to the identification of SmTK3 as additional interaction partner, which is also expressed in the ovary and the testes (Kapp et al. Reference Kapp, Knobloch, Schussler, Sroka, Lammers, Kunz and Grevelding2004; see Table 1).
To further unravel the signalling cascade that SmTK4 and its upstream interaction partner SmTK6 are involved in, another YTH screening was performed to identify molecules acting upstream of SmTK6 (Beckmann et al., unpublished). With the SH2 domain of SmTK6 as probe, SmTK3 was identified as the strongest interaction partner. Since SmTK3 also interacted with the tandem SH2-domain of SmTK4, a multi-kinase complex in cells of the ovary was postulated, in which SmTK4, SmTK3 and SmTK6 co-operate (Beckmann et al., unpublished).
For the identification of molecules acting downstream of SmTK4, the complete linker region together with the tyrosine kinase domain was used for library screening (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). Downstream partners of Syk kinases are known to bind to amino acids within the linker region, or within the tyrosine kinase domain of a Syk kinase (Moon et al. Reference Moon, Post, Durden, Zhou, De, Harrison and Geahlen2005). This can be influenced by tyrosine residues within the TK domain, which are phosphorylated in trans by Src kinases, for example (Geahlen, Reference Geahlen2007). Therefore, the TK domain of the Src kinase SmTK3 was co-expressed (yeast three-hybrid approach) to ensure tyrosine phosphorylation of the bait. Among the identified molecules were schistosome homologues of a MAP kinase-activating protein (PM20/PM21) and mapmodulin (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). By in situ hybridisation, transcripts of both molecules were detected in the ovary of the female and in the testes of the male, which perfectly corresponded to the localisation of SmTK4 (see Table 1).
MAPK (mitogen activated protein kinase) pathways control major cell fate decisions such as proliferation, differentiation and apoptosis, mainly by inducing alterations in gene expression (Bluthgen and Legewie, Reference Bluthgen and Legewie2008). MAPK signalling has been described as initiated by integrin-mediated signalling (Giancotti and Ruoslahti, Reference Giancotti and Ruoslahti1999; Guo and Giancotti, Reference Guo and Giancotti2004), or by a cross-talk between integrin and RTK-signalling cascades (Wu et al. Reference Wu, Wu and Hu2008). In mammalian cells, it was demonstrated that downstream of integrin receptors Src and FAK get activated triggering via further signalling molecules the activation of MAPK. Therefore, it may be possible that a MAPK-containing pathway gets activated downstream of integrin receptors in schistosome females (see section below). In this scenario, SmTK6 and/or SmTK3 and SmTK4 may be mediators to forward the inducing signal to MAPK controlling cytoskeleton organisation and thus proliferation processes of the oocytes. However, no direct interactions between Syk kinases and MAP kinase-activating proteins of the PM20/PM21 type have been described yet.
Mapmodulin is a leucine-rich acidic nuclear phosphoprotein (Opal et al. Reference Opal, Garcia, Propst, Matilla, Orr and Zoghbi2003), which is predominantly cytosolic, but it can also be associated with the endoplasmic reticulum or Golgi membranes of mammalian cells (Ulitzur et al. Reference Ulitzur, Humbert and Pfeffer1997). It occurs as a trimer in the cytosol, and phosphorylation is required for its microtubule-associated protein-binding activity. Mapmodulin interacts with free microtubule-associated proteins, and via these with microtubules (Ulitzur et al. Reference Ulitzur, Humbert and Pfeffer1997). So far, no interaction between a Syk kinases and mapmodulin has been described. Since the phosphorylation of mapmodulin is necessary for binding of microtubule-associated proteins, it is possible that mapmodulin is a substrate of a Syk kinase. Therefore, it is tempting to speculate that the Syk kinase SmTK4 may phosphorylate mapmodulin to influence the reorganisation of the microtubule-based cytoskeleton in oocytes from S. mansoni.
To elucidate potential functions of SmTK4 in the gonads of adults, S. mansoni pairs were treated with Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene). This Syk kinase-specific inhibitor blocks the activity of Syk kinases in cell culture with a IC50 value of 10 μM (Willeke et al. Reference Willeke, Schymeinsky, Prange, Zahler and Walzog2003) and is used for tissues in high concentrations of 100–200 μM (Oliver et al. Reference Oliver, Burg, Wilson, McLaughlin and Geahlen1994). Treatment of S. mansoni pairs in vitro with 70 μM Piceatannol for six days resulted in significant physiological and morphological changes. First, Piceatannol reduced egg production of paired females within 7 days down to 51% compared to a DMSO control. Second, Piceatannol led to morphological changes of the ovary (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010). The number of large primary oocytes was significantly increased compared to untreated control worms (see Fig. 4). We concluded that due to the Syk inhibition a dysfunction in the proliferation of the oogonial cells occurred leading to a reduced supply of primary oocytes. In oocytes of marine nemertean worms (Cerebratulus sp.), inhibitor studies provided evidence for a role of Syk and Src kinases during oocyte maturation (Stricker and Smythe, Reference Stricker and Smythe2006). Using the non-specific TK inhibitor Genistein, or the Syk kinase-specific inhibitor Piceatannol, a decrease in the maturation of oocytes was observed (indicated by germinal vesicle breakdown; GVBD) as well as reduced MAPK activity. With Src kinase-specific inhibitors, no reduction in GVBD occurred, but in MAPK elevated activity was observed. It was concluded that in oocytes of nemertean worms, a signal is transduced from receptor tyrosine kinases to Syk kinases followed by Src kinases and ending in a MAPK signalling pathway leading to the activation of MPF (maturation-promoting factor) and GVBD (Stricker and Smythe, Reference Stricker and Smythe2006). The inhibition data of this study correspond to the data of our study with respect to dysfunctions in oocyte maturation with Piceatannol treatment. But in S. mansoni oocytes, the signal is probably transduced from a receptor to a Src kinase first leading to the activation of Syk, which in turn activates a MAPK cascade. Furthermore, using YTH analyses we have obtained direct evidence for interactions of a Syk kinase (SmTK4) with Src kinases (SmTK6, SmTK3) as well as with a MAP kinase-activating protein. Also interactions of SmTK4 with a receptor tyrosine kinase (SmVKR) were observed (Beckmann et al., unpublished).

Fig. 4. Confocal laser scanning microscope images of S. mansoni pairs treated with Piceatannol (A–C: testes of the male; D–F: ovary of the female). A, D: untreated control worms; B, E: worms treated for six days with 70 μM Piceatannol; C, F: control worms treated for six days with DMSO, the solvent of the inhibitor [abbreviations: e: egg, io: immature oocyte, mo: mature oocyte, od: oviduct, rs: receptaculum seminis, vd: vitelloduct; arrow: mature sperms, asterisk: sperm vesicle; scale bar: 40 μm].
Piceatannol treatment also results in clear morphological aberrations in the testes of males (see Fig. 4). This effect was expected, since SmTK4 is also transcribed in this organ. In treated males, the number of spermatocytes in the testes was decreased, and in the ventral part of the testicular lobes or in the anterior sperm vesicle significantly less mature elongated sperms were detected.
To pinpoint the observed phenotype to SmTK4, RNA interference (RNAi) experiments were performed following established protocols (Ndegwa et al. Reference Ndegwa, Krautz-Peterson and Skelly2007). After electroporation with SmTK4-specific dsRNAs, a significant reduction in the transcript level of this gene was observed compared to control worms. At the morphological level, treated worms exhibited phenotypes qualitatively similar to the phenotypes observed after Piceatannol treatment. The effect of this inhibitor was therefore specifically attributed to the inhibition of SmTK4, which is the only known Syk kinase in schistosomes (Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010).
INTERACTIONS BETWEEN INTEGRIN RECEPTORS AND KINASES
Integrins are α/β heterodimeric glycoprotein receptors that bind components of the extracellular matrix or cell surface molecules. Thus they transmit signals regulating cellular processes such as proliferation, differentiation, migration, cytoskeletal organisation or apoptosis (Hynes, Reference Hynes2002; Giancotti and Ruoslahti, Reference Giancotti and Ruoslahti1999; van der Flier and Sonnenberg, Reference van der Flier and Sonnenberg2001; LaFlamme et al. Reference LaFlamme, Nieves, Colello and Reverte2008). Upon ligand binding to integrin receptors, conformational changes occur within their intracellular domains, which lack catalytic activity but interact with a variety of intracellular signalling and/or adaptor molecules (Zaidel-Bar et al. Reference Zaidel-Bar, Itzkovitz, Ma'ayan, Iyengar and Geiger2007). Among these are Syk and/or Src kinases (Schlaepfer and Hunter, Reference Schlaepfer and Hunter1998; van der Flier and Sonnenberg, Reference van der Flier and Sonnenberg2001; Woodside et al. Reference Woodside, Obergfell, Talapatra, Calderwood, Shattil and Ginsberg2002; Arias-Salgado et al. Reference Arias-Salgado, Lizano, Sarkar, Brugge, Ginsberg and Shattil2003; Harrison, Reference Harrison2003; Zaidel-Bar et al. Reference Zaidel-Bar, Itzkovitz, Ma'ayan, Iyengar and Geiger2007). Binding studies had demonstrated that the SH2 domains of Syk kinases mediate interaction. To this end the intracellular part of a β integrin receptor can be contacted either by the tandem SH2 domain (Woodside et al. Reference Woodside, Obergfell, Talapatra, Calderwood, Shattil and Ginsberg2002; Arias-Salgado et al. Reference Arias-Salgado, Lizano, Sarkar, Brugge, Ginsberg and Shattil2003), or by the N-terminal SH2 domain, whereas the C-terminal SH2 domain simultaneously binds to phosphotyrosines of ITAM-containing receptors (e.g. growth factor receptors, receptor tyrosine kinases) in the same complex (Gao et al. Reference Gao, Zoller, Ginsberg, Brugge and Shattil1997; Guo and Giancotti, Reference Guo and Giancotti2004; Jakus et al. Reference Jakus, Fodor, Abram, Lowell and Mocsai2007; Zou et al. Reference Zou, Kitaura, Reeve, Long, Tybulewicz, Shattil, Ginsberg, Ross and Teitelbaum2007). Hence, Syk kinases have the potential to link receptors, and accordingly co-operating pathways. In this complex scenario, Src kinases are considered to phosphorylate Syk for activation (Arias-Salgado et al. Reference Arias-Salgado, Lizano, Sarkar, Brugge, Ginsberg and Shattil2003; Arnaout et al. Reference Arnaout, Goodman and Xiong2007; Zou et al. Reference Zou, Kitaura, Reeve, Long, Tybulewicz, Shattil, Ginsberg, Ross and Teitelbaum2007). In light of this information from mammals, we speculated about potential interactions of the schistosome Syk kinase SmTK4 or the Src kinases SmTK3/ SmTK6 with integrin receptors, or RTKs. Using the S. mansoni genome database (Berriman et al. Reference Berriman, Haas, LoVerde, Wilson, Dillon, Cerqueira, Mashiyama, Al-Lazikani, Andrade, Ashton, Aslett, Bartholomeu, Blandin, Caffrey, Coghlan, Coulson, Day, Delcher, DeMarco, Djikeng, Eyre, Gamble, Ghedin, Gu, Hertz-Fowler, Hirai, Hirai, Houston, Ivens, Johnston, Lacerda, Macedo, McVeigh, Ning, Oliveira, Overington, Parkhill, Pertea, Pierce, Protasio, Quail, Rajandream, Rogers, Sajid, Salzberg, Stanke, Tivey, White, Williams, Wortman, Wu, Zamanian, Zerlotini, Fraser-Liggett, Barrell and El-Sayed2009), one α and one β integrin receptor were identified and cloned. In silico analyses of the cloned sequences showed that the S. mansoni integrin receptors (Smα-Int1, Smβ-Int1) possess all hallmark domains of this receptor class (Beckmann et al., unpublished). Using in situ hybridisations in our laboratory, transcripts of both receptors were detected in the ovary, ootype and vitellarium of the female as well as in the testes of the male (see Table 1). Accordingly, these transcripts co-localise with SmTK4, SmTK3 and SmTK6 among other tissues in the ovary of the female. Direct interactions between these molecules were identified by binding studies in the YTH system. Here, SmTK4 was able to bind by its tandem SH2-domain to the C-terminus of the β integrin receptor, which was also bound by the SH3 domains of the Src kinases SmTK3 and SmTK6. This is in agreement with the literature (Li et al. Reference Li, Zhang and Wu1999; Arias-Salgado et al. Reference Arias-Salgado, Lizano, Sarkar, Brugge, Ginsberg and Shattil2003; Harrison, Reference Harrison2003; Arnaout et al. Reference Arnaout, Goodman and Xiong2007) suggesting the following scenario. Upon ligand binding, clustering of the integrin receptors Smα-Int1, Smβ-Int1 occurs, which is intracellulary accompanied by an increase in the local Src concentration. Binding to the β integrin receptor and phosphorylations in trans result in a full activation of SmTK6 or SmTK3, a prerequisite for the subsequent SmTK4 activation.
In signalling cascades downstream of integrin receptors, focal adhesion kinases (FAKs) may also play a role in association with Syk and Src kinases (Schlaepfer and Hunter, Reference Schlaepfer and Hunter1998; Mitra and Schlaepfer, Reference Mitra and Schlaepfer2006). The β integrin receptor facilitates FAK activation by undefined mechanisms leading to FAK autophosphorylation. This creates a binding site for the SH2 domain of a Src kinase, which gets activated by FAK. Thus, there are two theoretical ways for the activation of a Src kinase downstream of an integrin receptor. First, FAK becomes activated by the integrin receptor leading to Src activation by forming a FAK/Src complex. Second, Src is activated independently from the FAK by direct interaction with the β integrin receptor (Mitra and Schlaepfer, Reference Mitra and Schlaepfer2006). We identified a FAK homologue from schistosomes (SmFAK) in the S. mansoni genome database (Smp_137610) and cloned its cDNA, which contained the linker region, the kinase domain, the proline-rich region, and the C-terminal FAT domain. An N-terminal FERM domain could not be identified, neither in the database nor by RT-PCR (Burmeister et al., unpublished), which otherwise is characteristic for FAK kinases from mammals (Zhao and Guan, Reference Zhao and Guan2009). Preliminary in situ hybridisation studies in our laboratory have localised SmFAK transcripts in the ovary, the ootype and the vitellarium of the female. No transcripts could be detected in males. Initial binding studies in the YTH system have shown that the combined SH2/SH3 domains of SmTK3 interact with the C-terminal part of SmFAK covering part of the TK domain, the proline-rich region and the FAT domain. In contrast to SmTK3, neither SmTK6 nor SmTK4 were able to bind SmFAK. In the same binding studies, an interaction of SmFAK with the intracellular C-terminus of Smβ-Int1 was shown (Beckmann et al., unpublished). From the literature it is known that truncated FAK variants without N-terminal FERM domain also occur that are involved in integrin signalling (Jacamo and Rozengurt, Reference Jacamo and Rozengurt2005). Thus the FERM-less schistosome FAK may be involved in integrin pathways.
CROSS-TALK BETWEEN RTK AND INTEGRIN PATHWAYS
RTKs comprise a large family of membrane receptors that regulate various cellular processes in cell biology. RTKs represent single-pass membrane proteins consisting of an extracellular ligand-binding domain and an intracellular kinase domain. In S. mansoni, four RTKs (belonging to the EGF-R and IR families) have been characterized (Dissous et al. Reference Dissous, Khayath, Vicogne and Capron2006, Reference Dissous, Ahier and Khayath2007). Among these, SmRTK-1 (or SmVKR) was described as an unusual receptor composed of an extracellular Venus Flytrap module (VFT; a ligand-binding domain in many GPCRs) linked by a single transmembrane domain to a TK domain, which is similar to that of insulin receptors. High levels of SmRTK-1 transcripts were detected in mature oocytes of female schistosomes (Vicogne et al. Reference Vicogne, Pin, Lardans, Capron, Noel and Dissous2003). Recently, it was shown that SmRTK-1 is a member of a new family of RTKs found in various invertebrates, particularly insects. Therefore, this new family was named VKR for Venus Kinase Receptor (Ahier et al. Reference Ahier, Rondard, Gouignard and Khayath2009). Insect VKRs are, as in schistosomes, mainly expressed in female gonads, indicating a putative function of these receptors in reproduction and/or development. Structural and phylogenetic studies performed on VFT and TK domains showed that VKR sequences formed monophyletic groups, the VFT group being close to that of GABAB receptors, and the TK one being close to that of insulin receptors. Recombinant VKRs were able to autophosphorylate tyrosine residues, and they were activated by L-amino-acids (Ahier et al. Reference Ahier, Rondard, Gouignard and Khayath2009). This result is in agreement with the high degree of conservation of the alpha amino-acid-binding residues found in various amino acid-binding VFTs (Alioto and Ngai, Reference Alioto and Ngai2005; Wellendorph et al. Reference Wellendorph, Hansen, Balsgaard, Greenwood, Egebjerg and Bräuner-Osborne2005). The exact nature of the ligand(s) specific for SmVKR as well as signalling pathways induced by activated SmVKR in parasite oocytes remain to be determined. It is tempting to speculate that SmVKR might be involved in the recognition of a male pheromone signal necessary for the development and maturation of the ovary since it possesses a VFT module, also present in mammalian pheromone receptors (Herrada and Dulac, Reference Herrada and Dulac1997; Matsunami and Buck, Reference Matsunami and Buck1997; Vicogne et al. Reference Vicogne, Pin, Lardans, Capron, Noel and Dissous2003). The expression of SmVKR in the female ovary (Vicogne et al. Reference Vicogne, Pin, Lardans, Capron, Noel and Dissous2003) co-localises with the expression of SmTK4, SmTK6, SmTK3, Smα-Int1, and Smβ-Int1. RTK and integrin signalling pathways can cooperate (Giancotti and Ruoslahti, Reference Giancotti and Ruoslahti1999; Jakus et al. Reference Jakus, Fodor, Abram, Lowell and Mocsai2007; Wu et al. Reference Wu, Wu and Hu2008) involving Syk and Src kinases that interact with ITAM-containing receptor tyrosine kinases (Geahlen and Burg, Reference Geahlen and Burg1994; Thomas and Brugge, Reference Thomas and Brugge1997; Bromann et al. Reference Bromann, Korkaya and Courtneidge2004; Geahlen, Reference Geahlen2007). Therefore, we investigated the potential of the schistosome CTKs to interact with SmVKR, since we identified an ITAM-related motif in its intracellular part by in silico analyses. Indeed, YTH analyses confirmed interactions of the intracellular part of SmVKR to the tandem SH2-domain of SmTK4 and even stronger to the SH3 and SH2 domains of SmTK6 and SmTK3 (Beckmann et al., unpublished). Since these schistosome CTKs are also able to interact with Smβ-Int1, we suggest a linkage between the Smβ-Int1 and the SmVKR pathways.
Cross-talks between integrin and RTK pathways are well known, and it has been discussed that this co-operation potentiates downstream-signalling events including the activation of MAPK pathways (Giancotti and Ruoslahti, Reference Giancotti and Ruoslahti1999; Wu et al. Reference Wu, Wu and Hu2008). Direct interactions between integrins and RTKs may also be assisted by the integrin-linked kinase (ILK; Li et al. Reference Li, Zhang and Wu1999). In the S. mansoni genome data-base a potential ILK2 was identified (Smp_079760.1) and cloned (Beckmann et al., unpublished). Using the full length sequence of SmILK2 an interaction with the cytoplasmic part of Smβ-Int1 was shown in the YTH system. In mammals, an involvement of a Src/FAK complex in linking integrin and RTK pathways is also discussed (Wu et al. Reference Wu, Wu and Hu2008). Since all these molecules were identified in schistosomes, and since their transcripts co-localise in oocytes (see Table 1; no localisation data for SmILK2 yet), a co-operation of Smβ-Int1 and SmVKR pathways via Src kinases (SmTK3 and SmTK6) and SmFAK (or SmILK2) is suggested.
POLO-LIKE AND STE20-LIKE KINASES
Polo-like kinases (Plks) are cell-cycle regulators essential for progression through mitoses in all species from yeast to mammals. Plx1, a Xenopus Plk homologous to human Plk1, was initially shown to be implicated in the activation of Cdc25C phosphatase, and therefore in Cdc2 activation (Kumagai and Dunphy, Reference Kumagai and Dunphy1996) at the initiation of mitosis. Numerous studies then showed that Plk1 co-ordinated the functional maturation of centrosomes with Cdc2 activation, playing an essential role in early mitotic events (Lane and Nigg, Reference Lane and Nigg1997). Expression of Plk1 is cell-cycle regulated and peaks during late G2 phase (Hamanaka et al. Reference Hamanaka, Smith, O'Connor, Maloid, Mihalic, Spivak, Longo and Ferris1995). This kinase is aberrantly expressed in tumours constituting an important target for human cancer intervention.
A member of the Plk family has been recently characterized in S. mansoni that showed extensive homology with human Plk1 (Golsteyn et al. Reference Golsteyn, Lane, Mundt, Arnaud and Nigg1996). Quantitative RT-PCR analyses indicated that SmPlk-1 was mainly transcribed in sporocysts and in female worms, thus in two stages where intense cell divisions occur. This indicated a potential role of this kinase in mitosis and/or meiosis in schistosomes. In situ hybridisation experiments demonstrated the presence of large amounts of SmPlk-1 transcripts in vitelline cells and oocytes of female schistosomes (Long et al., unpublished). Moreover, we could demonstrate that incubation of adult worm pairs with a specific inhibitor of human Plk1, BI 2536 (Steegmaier et al. Reference Steegmaier, Hoffmann, Baum, Lenart, Petronczki, Krssak, Gurtler, Garin-Chesa, Lieb, Quant, Grauert, Adolf, Kraut, Peters and Rettig2007), resulted in morphological aberrations in male and female gonads (Long et al., unpublished). After treatment of S. mansoni pairs in vitro with BI 2536, male parasites showed a considerable decrease in the size of testicular lobes and in the number of spermatocytes. In female worms, the number of large mature oocytes increased and, in addition, the morphology of the small immature oocytes was significantly modified exhibiting a more elongated form with a loose arrangement (Long et al., unpublished). These phenotypes were similar to those obtained for Piceatannol-treated worms indicating that BI 2536 as described before (Steegmaier et al. Reference Steegmaier, Hoffmann, Baum, Lenart, Petronczki, Krssak, Gurtler, Garin-Chesa, Lieb, Quant, Grauert, Adolf, Kraut, Peters and Rettig2007) presumably led to mitotic arrests in gonadal cells inducing apoptosis, which stops the supply of spermatogonia or oogonia, respectively. These results substantiate the importance of SmPlk-1 in reproductive organ functions and thus its potential as a new target for anti-schistosomal strategies.
Activation of Plk1 requires its phosphorylation, but the identity of the activating kinase still remains elusive. A protein kinase xPlkk1 (polo-like kinase kinase) that belongs to the large family of Ste20-like kinases (SLK) has been demonstrated to phosphorylate and activate Plx1 in Xenopus oocytes, inducing the transition from G2 to M phase of the cell cycle (Qian et al. Reference Qian, Erikson and Maller1998). In schistosomes, a serine/threonine kinase very similar to xPlkk1, and to other related SLKs, has been characterized (Yan et al. Reference Yan, Tulasne, Browaeys, Cailliau, Khayath, Pierce, Trolet, Fafeur, Ben and Dissous2007). This kinase SmSLK is present in all schistosome stages, and its gene is abundantly transcribed in female schistosomes, particularly in the ovary. Recent data have shown that similar to xPlkk1, activated forms of SmSLK could induce GVBD and maturation of Xenopus oocytes following phosphorylation of Plx1, suggesting that SmSLK could also regulate cell division and multiplication in schistosomes by phosphorylating SmPlk-1 (Dissous and Long, unpublished).
SmSLK belongs to the Germinal Center Kinase (GCK)-subfamily of the Ste20 group, and it is homologous to mouse and human LOK (Lymphocyte Oriented Kinases) (Yan et al. Reference Yan, Tulasne, Browaeys, Cailliau, Khayath, Pierce, Trolet, Fafeur, Ben and Dissous2007). LOKs are known to regulate cell shape, motility and cortical reorganisation by phosphorylating ERM (erzin-radixin-moesin) proteins, whose major function is to link cortical actin filaments to the plasma membrane (Belkina et al. Reference Belkina, Liu, Hao, Karasuyama and Shaw2009). Therefore, besides its potential role in initiating mitosis by a direct action on SmPlk-1, SmSLK could be additionally involved in various cytoskeletal rearrangements associated with reproductive organ development and function.
SIGNALLING MOLECULES INVOLVED IN CELL PROLIFERATION IN THE TESTES
In contrast to the female, there is no evidence from the literature that pairing significantly influences maturation processes in the male. Unpaired males already have fully developed testes and produce mature elongated sperms, although the diameter of their testicular lobes may be reduced (Neves et al. Reference Neves, de Lamare, Machado-Silva, Carvalho, Branquinho, Lenzi, Hulstijn and Gomes2005). Thus, the proliferation and differentiation of spermatocytes seem to be independent of the pairing contact (Armstrong, Reference Armstrong1965; Basch and Basch, Reference Basch1984). At the molecular level, nearly nothing is known about the mechanisms regulating these processes. Spermatocytes are continuously produced in large amounts, thus, cell proliferation in the testes seems to be a pairing-independent, continuous process.
The Syk kinase SmTK4 is tissue-specifically expressed in the ovary of the female, but also in the testes of the male. Taken together with molecular analyses showing a higher abundance of SmTK4 transcription in males than in females (Knobloch et al. Reference Knobloch, Winnen, Quack, Kunz and Grevelding2002b), this allows us to speculate about the function of SmTK4 during maturation of male germ cells. We hypothesise similar pathways, involving SmTK4 and upstream receptors such as integrins and RTKs as well as further molecules such as the Src kinases SmTK6 and SmTK3 to be involved not only in the proliferation of oogonial cells, but also in the proliferation of spermatogonial cells in the male. This hypothesis is supported by localisation data demonstrating that nearly all molecules localised in the ovary of the female were also transcribed in the testes of the male. This indicates a function in both genders of S. mansoni, and both gonadal cell types (oocytes, spermatocytes). With respect to the evolutionary ancestry from hermaphroditic blood flukes, it is conceivable that both genders still use similar pathways regulating proliferation processes in the gonads. According to this assumption, all inhibitors tested (Herbimycin A, BI 2536, Piceatannol) showed morphological effects not only in the female, but also in the testes of the male (see Fig. 3, 4; result not shown). These inhibitors led to a reduced number of spermatocytes in the testicular lobes and a lack of mature, elongated sperms in the ventral part of the lobes and in the anterior sperm vesicle. It is tempting to speculate that the inhibition of Src, Plk or Syk influenced the proliferation of the spermatogonial cells in the dorsal part of the testes. Since the proliferation of the spermatogonial cells is necessary for the initiation of spermatogenesis and the continuous production of mature sperms (Braydich-Stolle et al. Reference Braydich-Stolle, Kostereva, Dym and Hofmann2007), a dysfunction of spermatogenesis at this early stage would lead to the absence of mature sperms. This coincides with studies from other organisms (mouse, human) showing that the proliferation of spermatogonial cells is activated by CTKs. Accordingly, Src-specific inhibitors or RNAi in these organisms led to dysfunctions in spermatogenesis (Braydich-Stolle et al., Reference Braydich-Stolle, Kostereva, Dym and Hofmann2007). For CTKs of the Src family and also for Plk1, an involvement in spermatogenesis is well known, and this has also been hypothesised for Lyn- or Fyn-family kinases (Herrmann et al. Reference Herrmann, Amorim and Sunkel1998; Kierzenbaum, Reference Kierzenbaum2006; Braydich-Stolle et al. Reference Braydich-Stolle, Kostereva, Dym and Hofmann2007). In contrast, an involvement of a Syk in spermatogenesis has not been described before in eukaryotes.
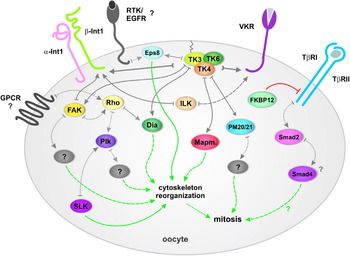
Fig. 5. Schematic model of signalling cascades in the oocytes regulating cytoskeleton rearrangements and mitotic activity [continuous lines starting with a bar: direct interactions confirmed by YTH analyses; continuous lines starting with a cramp: interactions of several molecules as confirmed by YTH analyses; dotted lines: direct interactions known from other organisms, not proven for schistosomes yet; grey dashed lines: indirect interactions via adapter molecules known from other organisms; green dashed lines: activating function; red lines: inhibitory function].
CONCLUSION
For parasitic trematodes, Schistosoma mansoni represents a well advanced model. Starting with the fundamental work of Paul Basch nearly 30 years ago the in vitro culture of adult and larval schistosomes has been established and improved over the years (Basch and Rhine, Reference Basch and Rhine1983; Irie et al. Reference Irie, Basch and Beach1983; Basch, Reference Basch1984; Yoshino and Laursen, Reference Yoshino and Laursen1995; Ivanchenko et al. Reference Ivanchenko, Lerner, McCormick, Toumadje, Allen, Fischer, Hedstrom, Helmrich, Barnes and Bayne1999; Kapp et al. Reference Kapp, Coustau, Wippersteg, Jourdane, Kunz and Grevelding2003). However, maintaining schistosomes in vitro still suffers from limitations. Among these are the reduced life span of adults, which in vitro survive for weeks to months and in exceptional cases up to a year (Quack et al. unpublished) or the limited period of the egg-laying capacity, which ceases after two weeks in culture. Although more work has to be invested to overcome these hurdles, the in vitro culture allows us today to investigate at least some part of schistosome biology outside the intermediate or final hosts. Additionally, comprehensive genome information has been generated for schistosomes during the last years (Berriman et al. Reference Berriman, Haas, LoVerde, Wilson, Dillon, Cerqueira, Mashiyama, Al-Lazikani, Andrade, Ashton, Aslett, Bartholomeu, Blandin, Caffrey, Coghlan, Coulson, Day, Delcher, DeMarco, Djikeng, Eyre, Gamble, Ghedin, Gu, Hertz-Fowler, Hirai, Hirai, Houston, Ivens, Johnston, Lacerda, Macedo, McVeigh, Ning, Oliveira, Overington, Parkhill, Pertea, Pierce, Protasio, Quail, Rajandream, Rogers, Sajid, Salzberg, Stanke, Tivey, White, Williams, Wortman, Wu, Zamanian, Zerlotini, Fraser-Liggett, Barrell and El-Sayed2009; The Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium, 2009), and today we are at the beginning of the post-genomic era, which requires us to exploit the sequence data. In this context it is important to establish technologies for functional genetics. Among these are YTH analyses to unravel protein networks influencing physiological processes (Quack et al. Reference Quack, Knobloch, Beckmann, Vicogne, Dissous and Grevelding2009), or methods for manipulating gene expression in schistosomes, either transiently or permanently based on biolistics, electroporation, viral- or transposon-mediated transformation, or RNA interference (RNAi) (Correnti and Pearce, Reference Correnti and Pearce2004; Yuan et al. Reference Yuan, Shen, Wang, Wu, Liu, Dong and Jiang2005; Grevelding, Reference Grevelding, Maule and Marks2006; Ndegwa et al. Reference Ndegwa, Krautz-Peterson and Skelly2007; Cheng and Davis, Reference Cheng and Davis2007; Kines et al. Reference Kines, Morales, Mann, Gobert and Brindley2008). Although in our hands RNAi did not work for every gene of our interest up to now, this method has significantly improved the situation for functional gene characterization in schistosomes in general (Boyle et al. Reference Boyle, Wu, Shoemaker and Yoshino2003; Correnti et al. Reference Correnti, Brindley and Pearce2005; Osman et al. Reference Osman, Niles, Verjovski-Almeida and LoVerde2006; Kuntz et al. Reference Kuntz, Davioud-Charvet, Sayed, Califf, Dessolin, Arnér and Williams2007; Krautz-Peterson et al. Reference Krautz-Peterson, Radwanska, Ndegwa, Shoemaker and Skelly2007; Nabhan et al. Reference Nabhan, El-Shehabi, Patocka and Ribeiro2007; Zhao et al. Reference Zhao, Lei, Liu, Zhu, Ren, Wang and Shen2008; Pearce and Freitas, Reference Pearce and Freitas2008; Pereira et al. Reference Pereira, Pascoal, Marchesini, Maia, Magalhaes, Zanotti-Magalhaes and Lopes-Cendes2008; Morales et al. Reference Morales, Rinaldi, Gobert, Kines, Tort and Brindley2008; Beckmann et al. Reference Beckmann, Buro, Hirzmann, Dissous and Grevelding2010; Cheng et al. Reference Cheng, Fu, Lin, Shi, Zhou, Jin and Cai2009; Rinaldi et al. Reference Rinaldi, Morales, Alrefaei, Cancela, Castillo, Dalton, Tort and Brindley2009). In combination, in vitro culture and RNAi are elementary building blocks for schistosomes as model trematodes since they offer the possibility to answer questions about the function of genes of interest. Furthermore, in vitro culture allows the analysis of the effects of chemical inhibitors such as testing potential anti-schistosomal compounds (Caffrey et al. Reference Caffrey, Rohwer, Oellien, Marhofer, Braschi, Oliveira, McKerrow and Selzer2009). Beyond that, the use of specific inhibitors may complement and verify RNAi results thus serving as an additional tool to study gene function. Finally, with the help of carmine red staining and CLSM, morphological effects can be studied without great effort but with considerbale value.
As summarised in this review, the combinatory application of these techniques allows functional analyses of signalling molecules of S. mansoni regulating reproductive biology, and the elucidation of signal transduction pathways these molecules are involved in. Among other data, the interplay of integrin, GPCR, RTK, and TGFβR pathways in co-operation with downstream signalling molecules is postulated, which presumably induces cytoskeletal changes and mitotic activity in oocytes of S. mansoni females. This model of communicating signalling pathways is supported by literature data of studies in other systems, but also by the first schistosome studies with TβRI (TRIKI)-, Src (Herbimycin A)-, Syk (Piceatannol)-, or Plk (BI 2536) – specific inhibitors, or RNAi experiments, which resulted in corresponding physiological effects and morphological phenotypes. The molecules identified and interactions postulated are summarized in Fig. 5. Along with the understanding of the cellular processes during gonad differentiation and with the increasing knowledge about the molecules controlling these processes, novel strategies to fight schistosomes can be designed. To this end, existing inhibitors with effects on key cellular processes can be identified serving as lead structures for drug design or vaccination. Since some inhibitors have already been proven as pharmaceuticals for human use, they may represent potential alternatives to Praziquantel quickly available for first anti-schistosomal tests in animal models and then humans.
Acknowledgements
At this point the senior authors (CGG and CD) would like to gratefully acknowledge the enthusiasm, engagement, and valuable inputs of all post-docs, PhD and Diploma students, and technical assistances, who have contributed to the great amount of data summarised in this review. The authors acknowledge the great contribution of the Welcome Trust Sanger Institute and TIGR for providing sequence data. Furthermore, the authors would like to point out the fruitful collaborations with Mo Klinkert, Alessandro Rossi, and Phil LoVerde concerning the analyses of FKBP12 and TβRI, Katia Cailliau and Edith Browaeys for functional analyses of Plk and SLK proteins in Xenopus oocytes, and they thank Anne Holz and Adriaan Dorresteijn for their support concerning CLSM. Financial support was obtained by grants of the Deutsche Forschungsgemeinschaft (CGG), and of the Institut National Sante Recherche Medicale and the French Ministere de l'Education Nationale et de la Recherche (CD).








