INTRODUCTION
The process of skin penetration by the cercariae of Schistosoma mansoni is very rapid and involves a number of processes occurring almost simultaneously (Stirewalt, Reference Stirewalt1974). The cercariae attach to the skin surface and the post-acetabular glands evacuate their contents to allow surface adhesion and creeping exploratory movements. Arginine and ceramides are skin surface molecules which are important attractants to the cercariae (Haas et al. Reference Haas, Grabe, Geis, Pach, Stoll, Fuchs, Haberl and Loy2002, Reference Haas, Haeberlein, Behring and Zoppelli2008). Penetration also involves the evacuation of the pre-acetabular glands, and further migration has been found to be stimulated by linoleic acid and other defined molecules (Haas et al. Reference Haas, Haeberlein, Behring and Zoppelli2008; Haeberlein and Haas, Reference Haeberlein and Haas2008). After 1 h of penetration, both pre- and post-acetabular glands have been completely evacuated. The cercariae are now termed schistosomula. Progression involves violent movements of the head and body musculature as well as contraction and extension of the head and glandular ducts (Stirewalt, Reference Stirewalt1974), with a concomitant loss of the tail. In excised human skin, the tail is lost only after cercariae have made considerable inroads into the skin (Whitfield et al. Reference Whitfield, Bartlett, Khammo, Brain, Brown, Marriott and Clothier2003). A clear sequence of ultrastructural changes was described by Hockley and McLaren (Reference Hockley and McLaren1973). The glycocalyx and the cercarial membrane are lost and incorporation of multilayered vesicles into the surface occurs to form a very unusual double bilayered structure, thought to be an adaptation to existence in the bloodstream (McLaren and Hockley, Reference McLaren and Hockley1977). Many other changes occur to the surface membrane, such as an increase in membrane fluidity (Foley et al. Reference Foley, Kusel and Garland1988) and incorporation of glucose transporters (Skelly and Shoemaker, Reference Skelly and Shoemaker1996, Reference Skelly and Shoemaker2001).
Glycolipid and protein molecules can be incorporated into the surface membrane of the schistosomulum (Clegg et al. Reference Clegg, Smithers and Terry1971; McLaren et al. Reference McLaren, Clegg and Smithers1975; Smith and Kusel, Reference Smith and Kusel1979), but it has not been previously reported that macromolecules can be taken into the body of the parasite.
In the work described below we have shown using fluorescence microscopy that membrane-impermeant molecules enter the bodies of the parasites as they penetrate the skin and are transformed into schistosomula. The process has been examined further by using in vitro transformation procedures which, to a certain extent, reproduce the complex events which occur during skin penetration. Although the uptake mechanism is unclear, the implication is that there exists a new host-parasite interface.
MATERIALS AND METHODS
Origin of parasite material, skin penetration and in vitro culture
A Puerto Rican isolate (PR) of S. mansoni was maintained in our laboratory in Biomphalaria glabrata and TO mice.
Fluorescent chemicals used in the study
We were investigating whether membrane-impermeant molecules could enter the cercaria during skin penetration and we chose the following compounds to do this. Lucifer Yellow CH lithium and potassium salt (molecular weight 457 Da), Hoechst 33258 (molecular weight 624 Da) and propidium iodide (molecular weight 668 Da) were purchased from Invitrogen (UK); FITC-labelled dextrans of molecular weights 4, 10, 20 and 70 kDa were purchased from Sigma (UK) and Invitrogen. All fluorescent chemicals are water soluble and were dissolved (1 mg per ml) in RPMI 1640. Lucifer Yellow CH is a membrane-impermeant compound (Peracchia, Reference Peracchia1981), with a structure that enables it to be aldehyde-fixed into tissues with which it comes into contact (Heinrichs, Reference Heinrichs1985). Propidium iodide and Hoechst 33258 have been used as indicators of membrane integrity (Gillan et al. Reference Gillan, Evans and Maxwell2005). For ease of expression, these molecules are referred to below as ‘labelled compounds’.
Fluorescent dextrans are often used to assess membrane integrity and to examine membranes for the sizes of membrane pores (Shimizu and Kawazoe, Reference Shimizu and Kawazoe1996).
In vivo production of schistosomula
Skin schistosomula were obtained by allowing cercariae, concentrated by light, to penetrate into a shaved area of a recently euthanased mouse. Mice were euthanased by cervical dislocation and the undersides shaved with an electric shaver. Recently euthanased mice were used in these experiments since we wished to avoid any use of anaesthetic. This study involves possible changes in membrane permeability and anaesthetics needed for restraint of the mice during skin penetration have effects on cell membranes (Sweet and Schroeder, Reference Sweet and Schroeder1986). Intradermal injections were carried out (26 G needle) with 200 μl of 1 mg per ml fluorescent Lucifer Yellow containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml. Over this treated area, 1 ml of a cercarial suspension (containing approximately 1000 cercariae) was applied to the skin surface in a flanged tube (Clegg, Reference Clegg1965). After 15 min, the skin region exposed to the cercariae was removed with scissors, cut into small pieces and incubated in RPMI-1640 supplemented with 10% foetal calf serum (Sigma) at 37°C in a 5% CO2 incubator. Fifteen minutes after the beginning of this incubation, the medium was removed from the skin fragments to inspect any emerging schistosomula and to remove any fluorescent dye which had leaked from the fragments. Fresh medium (RPMI-1640 and foetal calf serum) was added and the incubation continued for up to 2 h. All schistosomula were viable since they showed strong muscular movements, excluded trypan blue and had active flame cells.
In vitro production of schistosomula
Syringe passage (Colley and Wikel, Reference Colley and Wikel1974) or vortex stress (Ramalho-Pinto et al. Reference Ramalho-Pinto, Gazzinelli, Howells, Mota-Santos, Figueiredo and Pellegrino1974) of cercariae with subsequent culture of schistosomula can reproduce the in vivo morphological and biochemical changes but, in vitro, such changes in the membrane and glands often need 24 h to complete (Stirewalt et al. Reference Stirewalt, Cousin and Dorsey1983; Cousin et al. Reference Cousin, Stirewalt, Dorsey and Watson1986).
(a) Cercariae were exposed to the fluorescent compounds during syringe transformation or vortex as follows. They were concentrated on ice for 30 min and reduced to a volume of 0·5 ml. Added to this suspension were 1·5 ml of RPMI-1640 medium and 100 μl of 1 mg per ml fluorescent Lucifer Yellow containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml. Syringe transformation, with a 21 G needle, was carried out at 4°C and the mixture incubated at 4°C or at 37°C, washed in RPMI-1640 and further incubated in RPMI-1640 in the presence of 10% foetal calf serum for 2 h at 37°C.
(b) Lucifer Yellow, propidium iodide and Hoechst 33258 were also added to in vitro-transformed schistosomula after transformation had been completed. The freshly transformed schistosomula were washed and kept in RPMI-1640 in 10% foetal calf serum for up to 2 h at 37°C. During this incubation period, the schistosomula were washed 3 times in RPMI-1640 to remove the foetal calf serum and then they were exposed to 100 μl of 1 mg per ml fluorescent Lucifer Yellow containing Hoechst 33258 and propidium iodide added to a final concentration of 10 μg per ml for 15 min at 37°C.
In both experimental situations (transformation in the presence of labelled compounds (a) or transformation and then addition of labelled compounds (b), all parasite samples were washed 3 times in RPMI-1640 before observation under the fluorescence microscope.
Fixation of schistosomula
Labelled schistosomula were fixed at 4°C for 4 h in 4% paraformaldehyde. The 4% paraformaldehyde was prepared by dissolving the solid (Sigma) in RPMI-1640 at 60°C.
Examination of parasites by fluorescence microscopy
All labelled parasites were viewed and photographed using a Leitz Orthoplan Laborlux S microscope. This allowed the variability of the labelling of the parasite population to be observed, counted and recorded. Photographs were taken after observation of the parasites under the 40× or 50× objectives. Further detail in the distribution of the labelled compounds was sought using a Zeiss microscope (Axioplan 2).
The Laborlux and Zeiss microscopes are both fluorescence microscopes used with an X40 objective. They both have similar optics but the Zeiss software can take optical sections and display online images to improve resolution within the multicellular schistosomulum. All specimens in Laborlux and Zeiss microscopes were unfixed. All specimens examined in the confocal microscope were fixed in 4% paraformaldehyde. Confocal microscopy was carried out with the Leica TCS NT AOBS SP2 confocal microscope. A Delta vision deconvolution microscope (Delta Vision, UK) was used to verify the localization of the labelled compounds within the living parasite.
Carmine staining of cercariae
Cercariae of S. mansoni (LE strain) were obtained by exposing infected Biomphalaria glabrata snails to artificial light and shedding into filtered water. Cercariae were processed using a modified protocol adapted from adult worms (Machado-Silva et al. Reference Machado-Silva, Pelajo-Machado, Lenzi and Gomes1998). They were fixed with ethanol 70° GL, stained with carmine diluted in ethanol 70° GL, washed in 2 baths of ethanol 70° GL, dehydrated (ethanol 70°–100° GL) and clarified with beechwood creosote. Each step was performed using conical tubes, with the cercarial solution centrifuged at 1000 g for 1 min. Whole mounts of the sedimented, stained and clarified cercariae were obtained on slides under a cover-slip and preserved in Damar gum solution. Whole mounts were analysed by CLSM (LSM 410, Zeiss), in reflected mode, using a 543 He/Ne laser, with LP 570 filter. Tomographic images of whole cercariae, including the anterior organ or oral sucker, body segment, body/tail junction and tail stem and furcus, were obtained.
RESULTS
Carmine staining of cercariae followed by confocal microscopy
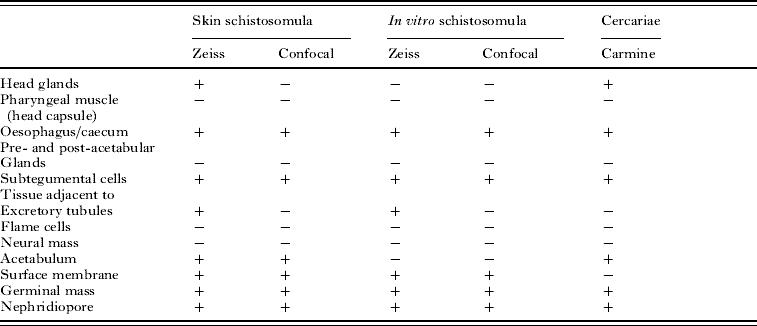
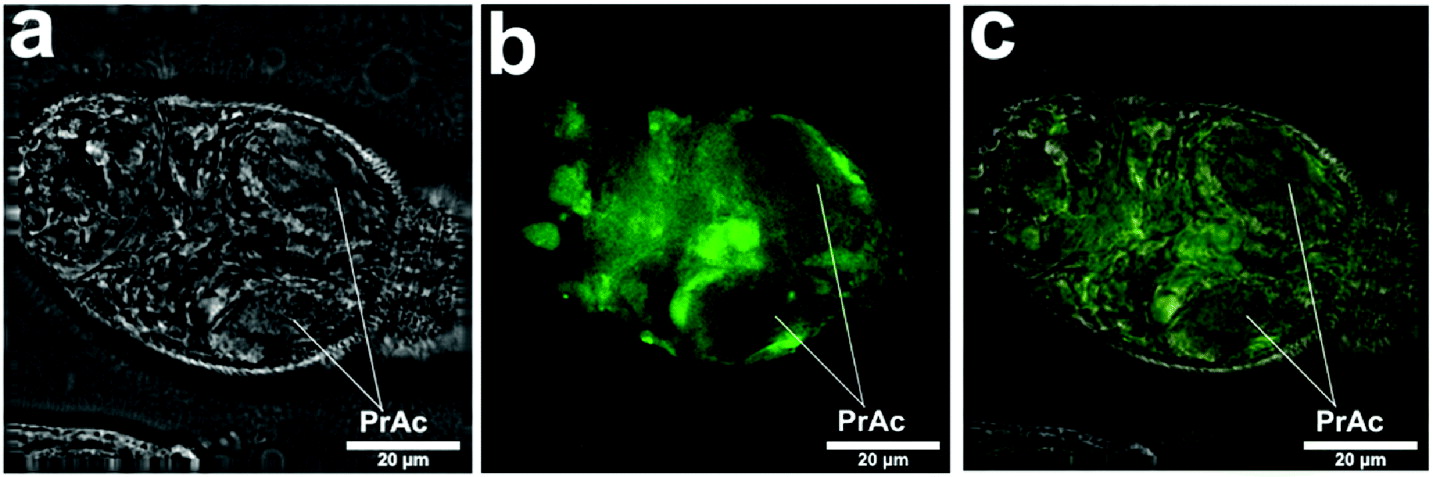
This method revealed the distribution of many organs and cells (Fig. 1) in a manner which aided our description of the labelling of the schistosomula by Lucifer Yellow and other fluorescent compounds (summarized in Table 1). In Fig. 1b, the labelling of the region where the oesophagus penetrates the pharyngeal muscle (or head capsule) can be matched with the labelling with Lucifer Yellow. The cells of the germinal mass are very evident in Fig. 1d. The position of the nephridiopore is clear (Fig. 1d).

Fig. 1. Cercaria labelled with carmine to show cellular and other structures (confocal). The positions of the pre-acetabular glands (PrAc) and post-acetabular glands (PtAc) are indicated and the arrangement of germinal cells (GM) in the region of the excretory bladder is shown. Ac, acetabulum; Es, region where oesophagus traverses pharyngeal mass; ET, excretory tubule; HG, head region with glands; N, nephridiopore; NM, neural mass; Np, neuropile; PM, pharyngeal muscles (head capsule); T, tail.
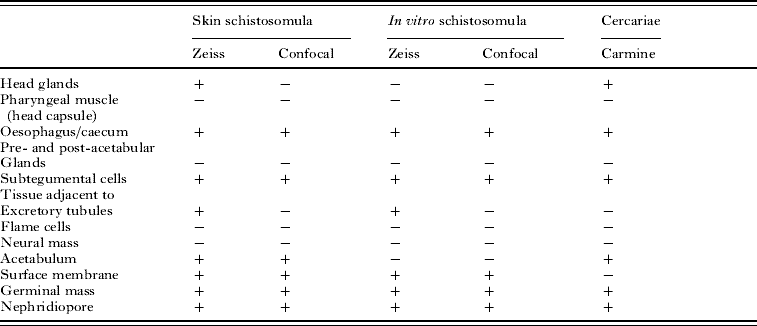
Table 1. The tissues and cells labelled with Lucifer Yellow after passage of cercariae through skin or transformed in vitro through a syringe
(A summary of the regions of the schistosomula labelled with Lucifer Yellow after skin penetration or in vitro transformation with a syringe, as observed by 2 different microscopic techniques, the Zeiss fluorescence microscope (Fig. 2b and c) and the Leica confocal microscope (Figs 2d–j and 4c–g). A comparison with the labelling with carmine (Fig. 1) confirms some of these assignments. Specimens examined in the Zeiss microscope were unfixed and living; those in the confocal microscope were fixed in paraformaldehyde.)
The images give guidance to the interpretation of the labelling seen in Figs 2 and 3 after labelling with fluorescent compounds. The pre- and post-acetabular glands (Fig. 1a–d) are unlabelled and the acetabulum is well stained (Fig. 1a).

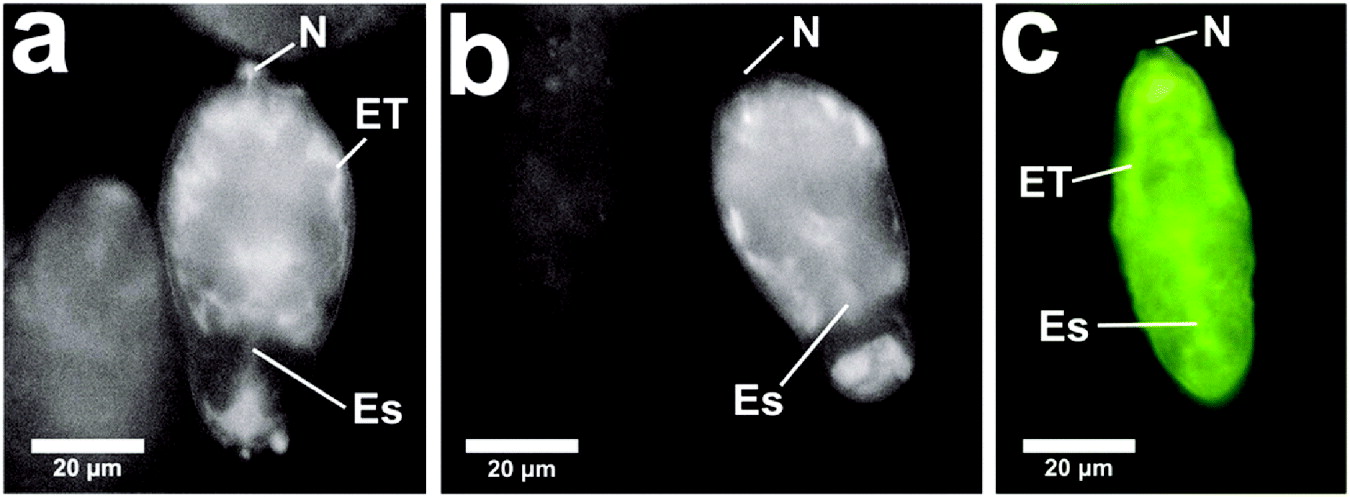
Fig. 2. Skin schistosomula. (a) Fluorescence micrograph (Laborlux) of Lucifer Yellow incorporated into skin schistosomula during penetration of skin injected with Lucifer Yellow and propidium iodide. The schistosomula were recovered after culture of skin fragments for 2 h. The pre- and post-acetabular glands have been evacuated in most larvae. The dye cannot be localized in this micrograph and seems to be in contact with all internal regions and to have stained the surface membrane. (b) and (c) Fluorescence micrographs (Zeiss) from similar preparation to (a). The Lucifer Yellow shows exclusion from pharyngeal muscles (PM) (or head capsule) and remnants of the pre-acetabular glands (PrAc), and some concentration in the rear subtegumental region and that of the excretory tubules. The nephridiopore is indicated (N) and carries some fluorescence. (d) Shows Lucifer Yellow entry into body cells. (e)–(j) Show 2 specimens labelled with Lucifer Yellow and propidium iodide and merged images. (e) Lucifer Yellow has entered cells in most body regions. (f) Propidium iodide has entered some of these cells. (g) Lucifer Yellow and propidium iodide merged to show coincidence of labelling of some internal cells. (h–j) Fluorescence micrographs (confocal) from same sample as (a) showing labelled cells within the larva but in a pattern which suggests some aggregation of Lucifer Yellow into large bodies similar to those shown in Fig. 3(k). This schistosomulum may have developed to a later stage than (e–j). (e) Shows Lucifer Yellow in large bodies and elsewhere in cells. (f) Shows propidium iodide having entered some cells (g), the 2 fluorescent compounds merged. Some of the large bodies are nuclei. (k) A schistosomulum incubated for a further 2 h after recovery from skin to show viability and localization of Lucifer Yellow in large bodies. Ac, acetabulum; Es, oesophagus, and region in the PM where the oesophagus lies in the muscle mass; GM, germinal mass; Np, neuropile.
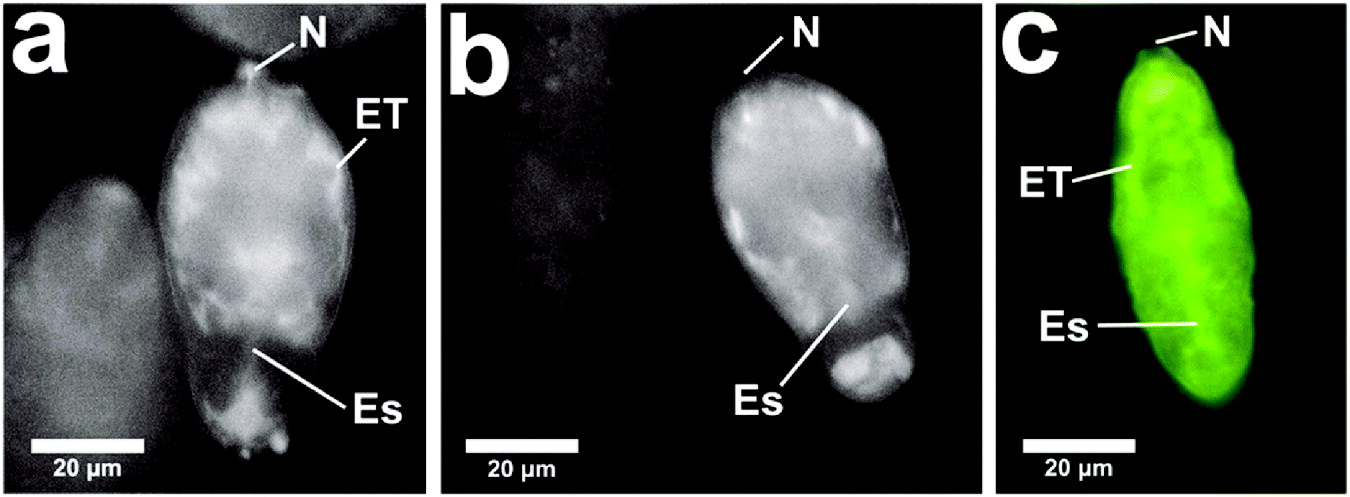
Fig. 3. Cercariae were transformed by the syringe or vortex method in RPMI-1640 at 4°C in the presence of fluorescent dextrans (FITC dextran 20 kDa and TRITC dextran 10 kDa). The schistosomula were kept at 4°C for 3 min and then washed 3 times in RPMI-1640 to remove fluorescent molecules from the schistosomula. Schistosomula show (a) influx of 20 kDa FITC dextran, and (b) influx of 10 kDa FITC dextran. This is compared (c) to an image of a schistosomulum showing influx of Lucifer Yellow. These images were captured in the Laborlux microscope and were from unfixed specimens. N, nephridiopore; Es, region of the oesophagous; ET, tissue region close to the excretory tubules.
In vivo labelling during penetration of fluorescently-infiltrated mouse skin
Experiments leading to the initial observation of an uptake of macromolecules were carried out with recently euthanased mice, whose skin had been injected with fluorescent dyes (Lucifer Yellow, Hoechst 33258 and propidium iodide) after which cercariae were applied to the skin in the injected area. Schistosomula recovered from skin after 15 min showed influx of labelled molecules. Fig. 2a–d clearly shows the presence of fluorescent label (Lucifer Yellow) within the parasite. Some parasites displayed an indeterminate staining pattern with Lucifer Yellow (Fig. 2a). These images were displayed in the Laborlux microscope which, although localization is indistinct, had great value in facilitating counting those parasites which were heavily labelled in the population. In Fig. 2a there is some variability among parasites, but all (100%) in the course of 10 experiments were brightly labelled (compare these results with the in vitro results below).
Using Zeiss and confocal microscopy, Fig. 2b–d shows label in the tissues adjacent to excretory tubules and subtegumental cells, the head gland region and the area adjacent to the neural mass. Fig. 2d–g shows in confocal that a number of body cells become labelled, showing both propidium iodide and Lucifer Yellow. Fig. 2d shows Lucifer Yellow entry into body cells. Fig. 2e–j shows 2 specimens labelled with Lucifer Yellow and propidium iodide and merged images. Fig. 2e shows that Lucifer Yellow has entered cells in most body regions, in Fig. 2f, propidium iodide has entered some of these cells and in Fig. 2g, Lucifer Yellow and propidium iodide have merged to show coincidence of labelling of some internal cells. Fig. 2h–j are fluorescence micrographs (confocal) from the same sample as (a) showing cells within the larva labelled but in a pattern different from (d–g) which suggests some aggregation of Lucifer Yellow into large bodies similar to those shown in Fig. 2k. This schistosomulum may have developed to a later stage than Fig. 2e–j. Fig. 2e shows Lucifer Yellow in large bodies and elsewhere in cells, (f) shows propidium iodide having entered some cells, and (g) shows that the 2 fluorescent compounds have merged. Some of the large bodies are nuclei shown by coincident labelling of Lucifer Yellow and propidium iodide. Fig. 2k is a schistosomulum incubated for a further 2 h after recovery from skin showing localization of Lucifer Yellow in large bodies.
Both the nephridiopore and the surface membrane are clearly labelled (Fig. 2c).These specimens examined in the confocal microscope have been fixed in 4% paraformaldehyde. Thus the extensive staining of the interior of cells may be due to redistribution of dye during the fixation process.
In some unfixed specimens examined under the Laborlux and Zeiss microscopes, Lucifer Yellow and propidium iodide can be seen to stain the interior of cells and cell nuclei. Thus the permeability properties of internal tissues may increase during transformation and are currently unknown. These observations are summarized in Table 1 and should be compared to the localization of carmine, used in fixed cercariae to reveal the distribution of organs and tissue cells (Fig. 1).
To examine whether other membrane-impermeant molecules can enter the body of the parasites, fluorescent dextrans of molecular weights between 3 and 20 kDa were injected into the skin and exposed to cercariae. It was observed that with all dextrans up to and including those of 20 kDa, the schistosomula became labelled in a pattern very similar to that found with Lucifer Yellow. Dextrans of molecular weight >20 kDa did not show influx into the parasite. The appearance of fluorescent dextrans after influx is very similar to that of other molecules experiencing influx. To illustrate this we present 2 images from in vitro influx in Fig. 3 with a comparison with a distribution of Lucifer Yellow, as observed in the Laborlux microscope.
In vitro labelling during transformation of cercariae in the presence of the membrane-impermeant molecules
Cercariae concentrated on ice were syringe or vortex transformed in RPMI-1640 in the presence of the labelled membrane-impermeant molecules. Fig. 4 shows that the pattern of labelling was similar to that obtained after skin penetration. The nephridiopore and the surface membrane were labelled, while the pre- and post-acetabular glands were not labelled. The region associated with the oesophagus within the pharyngeal muscle (or head capsule) clearly contained the fluorescent molecules, as did the subtegumental region and the germinal mass.

Fig. 4. Cercariae were transformed by the syringe or vortex method in RPMI-1640 at 4°C in the presence of propidium iodide and Lucifer Yellow. The schistosomula were kept at 4°C for 3 min and then washed 3 times in RPMI-1640 to remove Lucifer Yellow from the schistosomula. (a) and (b) (Laborlux) show localization of Lucifer Yellow in the rear of the parasite in the subtegumental region. The nephridiopore (N) contains fluorescent material. (c–h) Schistosomula prepared in vitro by syringe transformation as above, but incubated further at 4°C for 10 min showing localization of both Lucifer Yellow and propidium iodide in the confocal microscope. The nephridiopore contains fluorescent Lucifer Yellow (c) and propidium iodide (d) has diffused into cells close to the nephridiopore. Some of these cells are the germinal cells (GM). The pre- and post-acetabular glands are not labelled under these conditions. (e) Lucifer Yellow and propidium iodide merged. (f–h) Both sets of acetabular glands are unlabelled (f) and (g) (confocal) and (h) (Laborlux). Es, region where oesophagus traverses pharyngeal mass; PrAc, pre-acetabular glands.
As described above (in vivo influx), in living unfixed specimens examined under the Laborlux or Zeiss microscopes, it is unclear whether the Lucifer Yellow and dextrans entered the cells of the schistosomula or occupied the tissue spaces only. In some specimens cells had been entered by propidium iodide. In paraformaldehyde-fixed specimens used for confocal microscopy, the molecules had entered the cells as shown in the images where propidium iodide is merged with the Lucifer Yellow. The propidium iodide is specific for nuclei, while the Lucifer Yellow is observed in the cytoplasm (Fig. 4c–g).
Differences between influx during in vivo skin penetration and influx during in vitro transformation
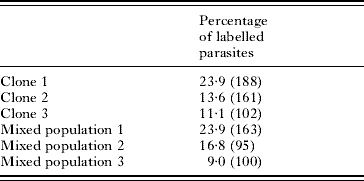
The pattern of in vitro labelling was very similar to that found in the schistosomula from the skin for Lucifer Yellow and all dextrans up to and including those of 20 kDa molecular weight. One major difference was observed in the results of exposing the labelled compounds to schistosomula derived from skin penetration and to those produced from cercariae in vitro. The skin forms were all very brightly labelled (Fig. 2a) (100% brightly labelled in 10 experiments), while the in vitro forms showed considerable variability (range 9–30% brightly labelled (Fig. 4h) from 25 experiments). An example of results from in vitro labelling can be seen in Table 2.
Table 2. Variability seen within and between clones (monomiracidial infections) and mixed populations of cercariae
(Schistosomula were transformed by syringe passage in the presence of Lucifer Yellow. The parasites were washed and, in up to 10 microscope fields, the number of labelled parasites was counted. The total number is in parentheses. The figures are for 3 mixed populations and 3 clones (Al Adhami et al. Reference Al Adhami, Doenhoff, Thornhill, Akhkha, White and Kusel2001).)

The rate of influx in vitro is very rapid: syringe transformation in the presence of membrane-impermeant Lucifer Yellow, dextrans, Hoechst 33258 and propidium iodide at +4°C
At +4°C, membranes are considered to be at their lowest permeability state (Hidalgo and Latorre, Reference Hidalgo and Latorre1970) and lipid-soluble dyes do not readily penetrate the non-fluid lipid phase (Kusel et al. Reference Kusel, Stones and Harnett1982).
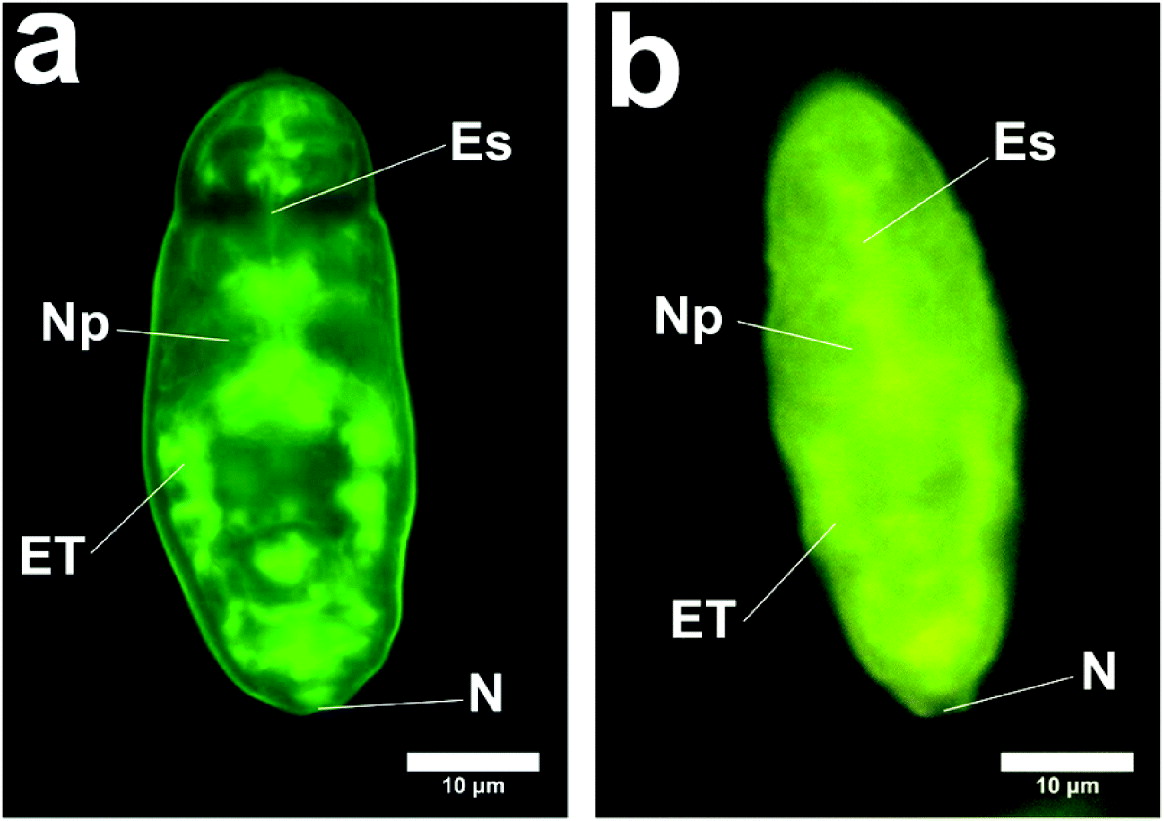
Cercariae were subjected to syringe passage or vortex stress, as described in the Materials and Methods section. The schistosomula were examined with the fluorescence and confocal microscope at a number of intervals after transformation. The results are shown in Fig. 4a and b, which demonstrate the distribution of the fluorescent compounds 3 min after the syringe transformation. These figures demonstrate a very rapid influx even at 4°C. After further incubation for 10 min at 4°C, the schistosomula appear as in Fig. 4c–h. Fig. 4a, b shows the region close to the excretory tubules and subtegumental cells. This is also seen clearly in Fig. 5. We do not know how much the excretory tubules are involved in the uptake of labelled molecules.
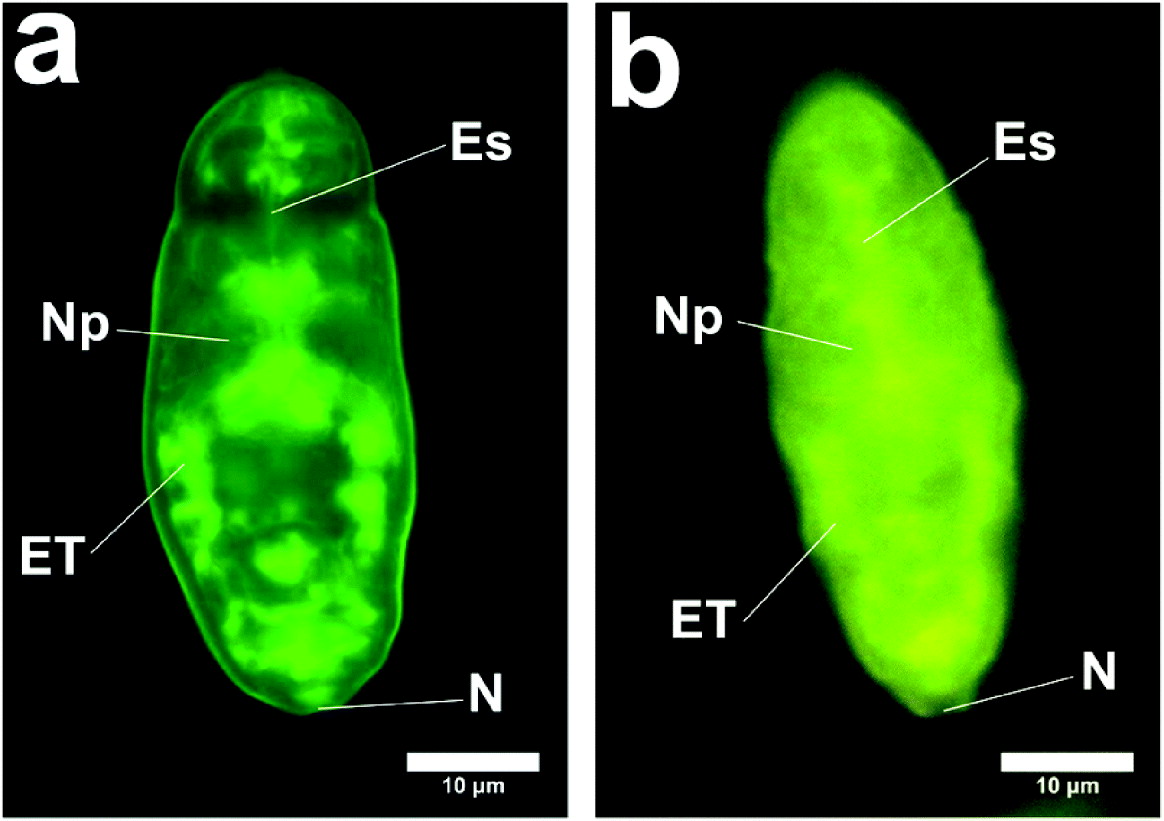
Fig. 5. Schistosomula showing labelling of excretory region and subtegumental region. (a) Schistosomulum produced in vitro (method (a) with incubation for 1 h at 37°C) (Zeiss). ET, regions of excretory tubules and subtegumental cells; N, position of nephridiopore; Es, region of oesophagus; Np, Neuropile. (b) Schistosomulum produced in vitro (method (a) with incubation for 10 min at 4°C) (Laborlux). ET, regions of excretory tubules and subtegumental cells; N, position of nephridiopore; Es, region of oesophagus; Np, Neuropile.
Fig. 6 shows the appearance under the Delta vision, and confirms the interior location of the fluorescent compounds and lack of label in the pre- and post-acetabular glands.
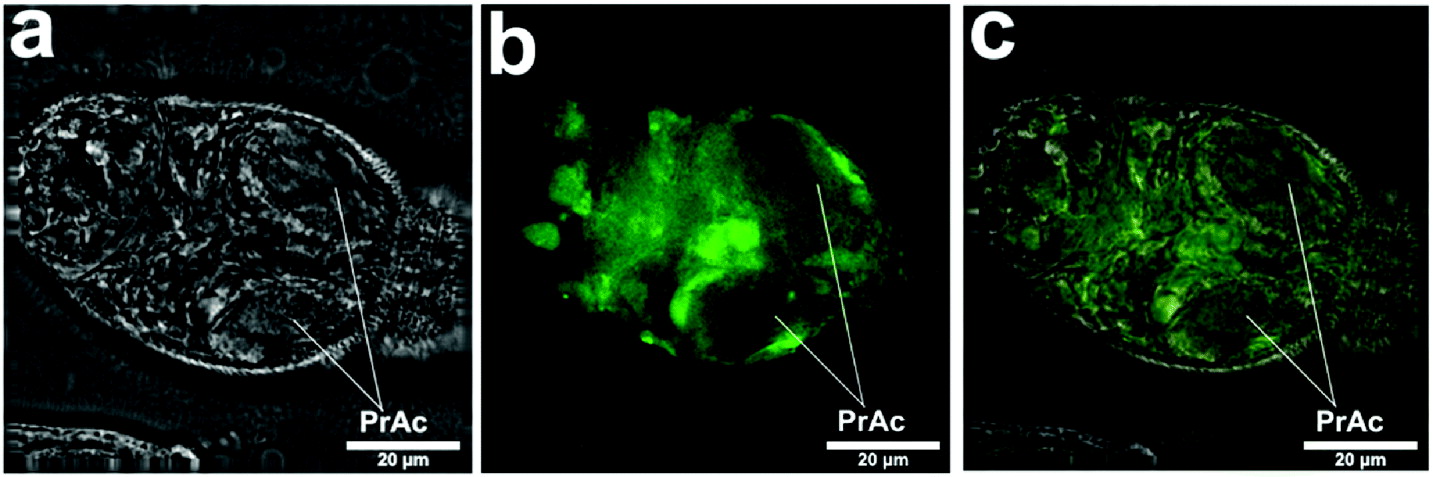
Fig. 6. Images captured in Delta vision of schistosomulum prepared in vitro by syringe transformation as above. (a) Bright field. (b) Lucifer Yellow. (c) Both bright field and Lucifer Yellow merged. Localization of Lucifer Yellow in some distinct regions (b) can be seen, but other localization is general (c). Pre-acetabular glands (PrAc) are not labelled with Lucifer Yellow.
DISCUSSION
Lucifer Yellow is a fluorescent compound of low molecular weight (457 Da), which is membrane impermeant. It has been used extensively in mammalian cells to study endocytosis, and pinocytosis (Swanson et al. Reference Swanson, Bushnell and Silverstein1987; Tassin et al. Reference Tassin, Lang, Antoine, Hellio and Ryter1990), the movement of molecules into cellular compartments through gap junctions (Dean et al. Reference Dean, Cooper and Stahl1988; Alves et al. Reference Alves, Coutinho-Silva, Persechini, Spray, Savino and Campos de Carvalho1996; Eugenin et al. Reference Eugenin, Branes, Berman and Saez2003; Fortes et al. Reference Fortes, Pecora, Persechini, Hurtado, Costa, Coutinho-Silva, Braga, Silva-Filho, Bisaggio, De Farias, Scemes, De Carvalho and Goldenberg2004) and by organic anion transporters (Steinberg et al. Reference Steinberg, Newman, Swanson and Silverstein1987; Cao et al. Reference Cao, Steinberg, Neu, Cohen, Horwitz, Hickman and Silverstein1993). Other membrane-impermeant compounds used in this study were propidium iodide, Hoechst 33258 and a variety of fluorescent dextrans of increasing molecular weights. We used Lucifer Yellow, fluorescent dextrans, propidium iodide and Hoechst 33258 (labelled compounds, injected intradermally) to show that, during penetration of skin, there is an influx of molecules into the body of the schistosomulum. Fluorescent dextrans of greater than 20 kDa molecular weight did not appear to enter the schstosomulum. Further work to examine the distribution of different molecular weight dextrans within the schistosomulum is required.
To enable us to identify the region of the schistosomulum which was labelled by these compounds, we have included images of cercariae labelled with carmine and observed by the confocal microscope. Comparison of these images with those stained by Lucifer Yellow shows that the regions of the germinal cells, the region occupied by the oesophagus, a region close to the neuropile, and the acetabulum contain the fluorescent molecules. Surface membrane, nephridiopore and the subtegumental region near excretory tubules are labelled.
Influx of labelled compounds from the skin is very rapid (it could be observed within 15 min). The process was examined in vitro by transforming cercariae by the syringe or vortex method. These two methods gave essentially the same results in labelling with the fluorescent compounds. Fig. 4a and b show that even if in vitro transformation occurs at 4°C, the influx of labelled molecules occurs after 3 min during syringe transformation.
A major difference between in vitro and in vivo influx of labelled molecules was found to be the variability in the extent of labelling with the fluorescent molecules. During in vitro labelling, a very variable intensity of labelling occurred. In contrast, the skin-derived parasites, although variable, always showed a high intensity of labelling. This difference may be due to the different stresses on the membrane found in the two methods, and is observed in situations of membrane stress with mammalian cells (Shimizu and Kawazoe, Reference Shimizu and Kawazoe1996). It also may be that exposure to compounds such as ceramide and linoleic acid (Haas et al. Reference Haas, Haeberlein, Behring and Zoppelli2008) is required for complete influx.
The influx of labelled molecules during syringe or vortex transformation gives a similar pattern to that seen during skin penetration, except that the distribution of the labelled compound seems less distinct than the in vitro-transformed schistosomula. This may reflect the very rapid changes which occur in vivo. Fig. 2h–k shows a distribution of Lucifer Yellow that suggests incorporation into distinct membranous bodies, yet to be identified.
To interpret these observations, we must consider the following sequence of events: when the cercariae penetrate through the host skin, the tail breaks away from the body revealing the nephridopore, and there are changes to the structure of the surface membrane. During transformation of the cercariae, concomitant changes are taking place in the surface membrane (Hockley and McLaren, Reference Hockley and McLaren1973). Vesicles fuse with the surface, areas of membrane are cast off, and there is a possibility of endocytosis (Ribeiro et al. Reference Ribeiro, Maldonado Júnior, D'Andrea, Vieira and Rey1998). As a consequence, regions of increased surface membrane permeability could be exposed (Skelly et al. Reference Skelly, Da'dara and Harn2003). During these processes, molecules of up to 20 kDa might enter the parasite. However, entry of such molecules may occur through the nephridiopore or through other apertures such as those leading to the oesophagus and head gland. Further work is required to determine the route of uptake.
It is our hypothesis that, during skin penetration, influx into the body of molecules of up to 20 kDa molecular weight may stimulate organelle and surface membrane development in a manner which may be absent or delayed in development in in vitro-transformed schistosomula. These molecules taken up by the parasite may diffuse within the internal tissues and stimulate receptors. For example, growth factors present in the skin (e.g. epidermal growth factor) may enter the parasite and stimulate schistosome epidermal growth factor receptors (Shoemaker et al. Reference Shoemaker, Ramachandran, Landa, dos Reis and Stein1992; Ramachandran et al. Reference Ramachandran, Skelly and Shoemaker1996) presented on internal tissue cells.
We do not yet know the identity of the molecules which enter the parasite from the skin, but epidermal growth factor (6400 Da) (Schultz et al. Reference Schultz, Rotatori and Clark1991; Tanaka et al. Reference Tanaka, Nagate and Matsuda2005), small carbohydrate fragments (Taylor et al. Reference Taylor, Trowbridge, Rudisill, Termeer, Simon and Gallo2004), free amino acids (Scott and Harding, Reference Scott and Harding1986; Meyer et al. Reference Meyer, Poehling and Neurand1991; Takahashi and Tezuka, Reference Takahashi and Tezuka2004) and a variety of vitamins are present in the skin. Also, as the cercaria penetrates the skin, it will be immersed in an environment rich in parasite molecules from the pre- and post-acetabular glands and from the glycocalyx. Digestion of host tissue (McKerrow et al. Reference McKerrow, Keene, Jeong and Werb1983) will release peptides and small carbohydrate fragments, and there will be an influx of growth factors as a result of skin damage (Hansell et al. Reference Hansell, Braschi, Medzihradszky, Sajid, Debnath, Ingram, Lim and McKerrow2008).
The influx processes described in this paper should alert us to the importance of studying the morphological and molecular features of the parasite as it traverses the human skin, since this may reveal processes not seen during penetration of other mammalian skin or in vitro-transformed schistosomula. Differences between human and mouse skin have been shown already at a morphological level by the description of tail retention by the cercaria in excised human skin (Whitfield et al. Reference Whitfield, Bartlett, Khammo, Brain, Brown, Marriott and Clothier2003). Cercariae having delayed tail loss during the penetration of human skin may show altered responses, since any uptake by the nephridiopore may provide molecules from different regions of the epidermis. Signals perceived very early in the development of the parasite may determine its development and even resistance to drugs and immune damage in later stages of its growth.
In conclusion, it is our hypothesis that internal receptors stimulated by the influx of molecules are part of a new host-parasite interface. This interface, which is active in the skin, may be crucial for the normal development of the schistosome parasite. Further work to compare skin and in vitro-transformed schistosomula is urgently required.
We would like to thank Professor Paul Hagan for all his support for this work, Dr Andrew MacDonald and Professor Mike Doenhoff for the supply of essential parasite material, Thiago Branquinho for his assistance with confocal microscopy, and Professor Henrique Lenzi for great support.
FINANCIAL SUPPORT
This work was supported by The Leverhulme Trust (J. T. and J. R. K., grant number EM20252); John Robertson Bequest (J. T. and J. R. K., grant number JR08/06); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (A. D. J., grant number 105411/2002-7); Fundação de Apoio à Pesquisa de Minas Gerais/CNPq (P. M. Z. C., grant number FAPEMIG/CNPq Universal 484552/2006-6); and the National Institutes of Health (P.M. and A.M., grant number R01 AI049162).