INTRODUCTION
The biological diversity of natural products and their ingredients renewed the interest in the use of natural compounds for medical applications and, more importantly, their role as a basis for drug development. During last decades, some promising advances had been achieved in understanding the chemistry and pharmacology of acyl phloroglucinol derivatives (APD), which are non-polar phenolic compounds. Their hydrophobicity increases with the number of attached prenyl functional groups (Kumar et al. Reference Kumar, Sharma and Chattopadhyay2013; Terrazas et al. Reference Terrazas, de Souza, Mariano, Cechinel-Filho, Niero, Andrade and Maistro2013). Acyl phloroglucinol derivatives are major intermediates in the biosynthetic pathway of xanthones and occur in plants of the Clusiaceae family. In scientific literature, APD have been recognized as interesting due to their biologically active secondary metabolites (aristophenones, bronianone, clusianone, garcicowin, garcimultiflorone, guttiferones, isogarcinol and xanthochymol) (Protiva et al. Reference Protiva, Hopkins, Baggett, Yang, Lipkin, Holt, Kennelly and Bernard2008; Kumar et al. Reference Kumar, Sharma and Chattopadhyay2013; Terrazas et al. Reference Terrazas, de Souza, Mariano, Cechinel-Filho, Niero, Andrade and Maistro2013). They exhibit significant cytotoxic, anti-microbial, anti-oxidant and anti-inflammatory activities (Diaz-Carballo et al. Reference Diaz-Carballo, Gustmann, Acikelli, Bardenheuer, Buehler, Jastrow, Ergun and Strumberg2012; Nunez-Figueredo et al. Reference Nunez-Figueredo, Garcia-Pupo, Ramirez-Sanchez, Alcantara-Isaac, Cuesta-Rubio, Hernandez, Naal, Curti and Pardo-Andreu2012; Kumar et al. Reference Kumar, Sharma and Chattopadhyay2013).
In recent years, two APD, nemorosone (Nem) and guttiferone A (GutA) have been subjected to pharmacological and toxicological studies for testing their anti-cancer (Cuesta-Rubio et al. Reference Cuesta-Rubio, Frontana-Uribe, Ramirez-Apan and Cardenas2002) and anti-microbial properties (Monzote et al. Reference Monzote, Cuesta-Rubio, Matheeussen, Van Assche, Maes and Cos2011). This research has demonstrated the anti-leishmanial activity of APD and their semi-synthetic derivatives (Lenta et al. Reference Lenta, Vonthron-Senecheau, Weniger, Devkota, Ngoupayo, Kaiser, Naz, Choudhary, Tsamo and Sewald2007; Monzote et al. Reference Monzote, Cuesta-Rubio, Matheeussen, Van Assche, Maes and Cos2011; Filho et al. Reference Filho, Meyre-Silva, Niero, Bolda Mariano, Gomes do Nascimento, Vicente, Gazoni, Dos Santos Silva, Gimenez, Gutierrez-Yapu, Salamanca and Malheiros2013; Fromentin et al. Reference Fromentin, Gaboriaud-Kolar, Lenta, Wansi, Buisson, Mouray, Grellier, Loiseau, Lallemand and Michel2013). Recently, the efforts have primarily been directed towards understanding the possible targets and mechanisms of action through which these compounds arrest cancer cells and inhibit the progression of the cell-cycle (Pardo-Andreu et al. Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti2011a , Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti b ). Although the potential involvement of mitochondria in the toxicity of GutA (Pardo-Andreu et al. Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti2011a ) and Nem (Pardo-Andreu et al. Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti2011b ) on (mammalian) cancer cells have been addressed, to our knowledge, no molecular studies on anti-microbial, in particular anti-leishmanial mechanisms of action have been reported. Therefore, the aim of this study was to explore the possible role of mitochondria in the anti-leishmanial activity of Nem and GutA in comparison with their action on mammalian mitochondria.
MATERIALS AND METHODS
Chemicals
Diethylenetriaminepentaacetic acid (DTPA), ethylenediaminetetraacetic acid (EDTA), glucose, KCN, K2HPO4, KH2PO4, Na2HPO4, NaCl, KCl, HCl, NaN3, succinate, sucrose and TRIS were obtained from Merck (Germany). Bovine serum albumin (BSA), cytochrome c3+ (cyt c3+), decylubiquinone (dUQ), heat-inactivated fetal bovine serum (HFBS), hemin, 2,6-dichlorophenol–indophenol (DCPIP), 3-carboxy-proxyl (CP•), reduced β-nicotinamide adenine dinucleotide (NADH), penicillin–streptomycin solution, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol–carbocyanine iodide (JC-1) and resazurin were purchased from Sigma-Aldrich (Germany). Pentamidine was obtained from Richet (Buenos Aires, Argentina). Dimethyl sulfoxide (DMSO), desferal (DFO) and triethanolamine (TEA) were from Roth (Germany), Novartis Pharma (Germany) and Fluka (Germany), respectively; while 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-HCl (CMH) and 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH) were from Noxygen (Germany). Yeast extract powder was supplied by Amresco (USA). Idebenone (IQ) was obtained from Takeda (Japan). 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) was from Sigma (USA). Decylubiquinol (dUQH2) was prepared from dUQ as described previously (Gille et al. Reference Gille, Staniek and Nohl2001).
Studied APD compounds
Nemorosone and GutA (Fig. 1) were purified from flowers of Clusia rosea and fresh fruit of Garcinia aristata, respectively (Monzote et al. Reference Monzote, Cuesta-Rubio, Matheeussen, Van Assche, Maes and Cos2011). The purity of both compounds was >98%. Compounds were dissolved in DMSO.
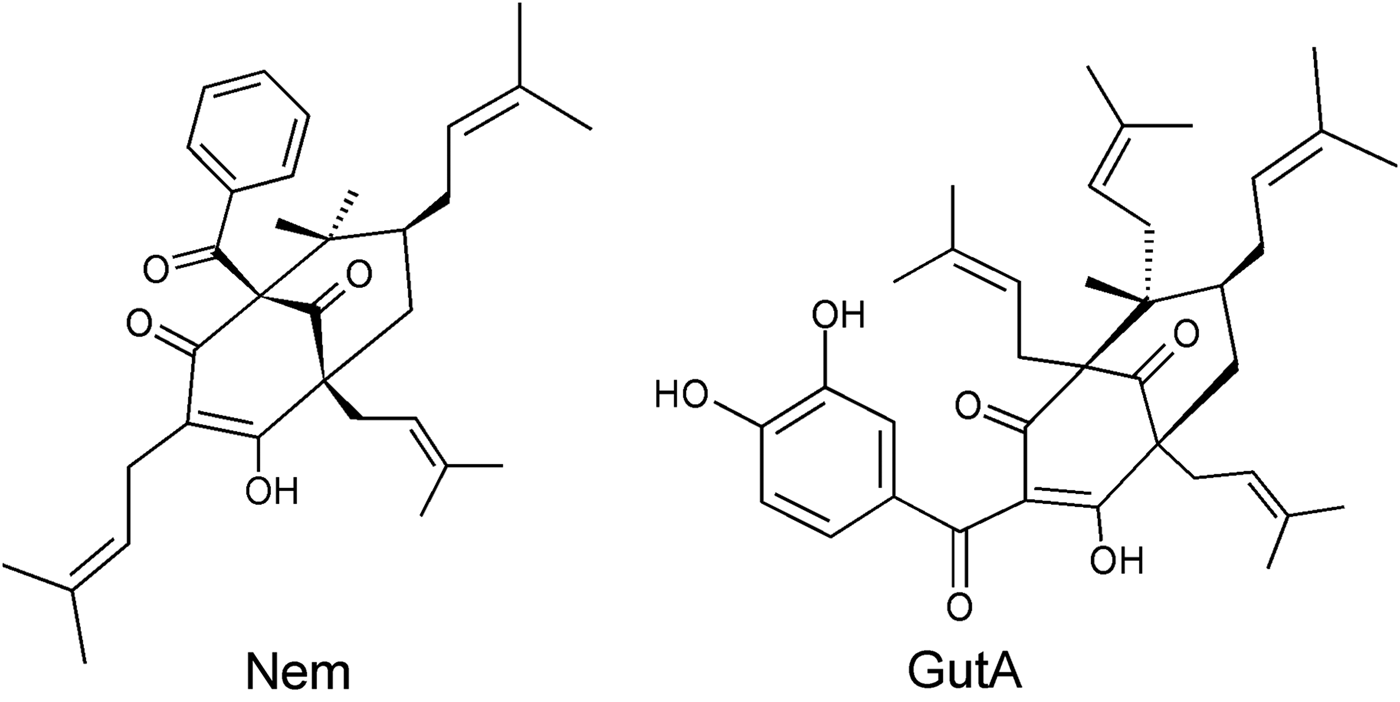
Fig. 1. Chemical structures of studied acyl phloroglucinol derivatives nemorosone (Nem) and guttiferone A (Gut A).
Macrophages
Peritoneal macrophages from mouse (PMM) were isolated at moment of use from normal female BALB/c mice in ice-cold RPMI 1640 medium (SIGMA, St. Louis, Mo, USA) supplemented with antibiotics (100 μg streptomycin mL−1 and 100 U penicillin mL−1).
Leishmania
Leishmania tarentolae promastigotes (LtP) strain P10 from Jena Bioscience were used. Parasites were cultivated at 26 °C in the yeast extract medium (YEM) consisting of 20·7 g L−1 yeast extract powder, 1·2 g L−1 K2HPO4 0·2 g L−1 KH2PO4, 2·9 g L−1 glucose, 5 mg L−1 hemin and 50·000 U L−1 penicillin – 50 mg L−1 streptomycin in 50 mL Saarstedt tubes with gas-permeable caps and agitation in a tube shaker (0·05 s−1).
Influence of APD on cell viability
To analyse the activity of APD in LtP, experiments were carried out to determine the viability of parasites after treatment. Leishmania tarentolae promastigotes in YEM were distributed in 96-well plates at 8 × 106 cells mL−1. Compounds were added and five 1:3 serial dilutions were performed. Control rows with YEM (no activity) and with untreated (vehicle) LtP (100% activity) were loaded. Then, 50 μL of resazurin stock solution was added in each well (20 μ m final conc.) and the plate was incubated at 26 °C during 48 h. Finally, images of the plates were recorded with a Canon EOS 300D camera and reduction levels of resazurin were quantified by the PLReader Software (0·3·8, 2014) using the absorption of the red channel minus the absorption in the green channel in analogy to (Borra et al. Reference Borra, Lotufo, Gagioti, Barros and Andrade2009). In parallel, the cytotoxicity of the compounds in PMM was studied. Macrophages were harvested and distributed to 96-well plates (1–6 × 105 PMM mL−1) and incubated at 37 °C under an atmosphere of 5% CO2 for 2 h. Non-adherent cells were removed and 2 μL of APD stock solutions were added to 98 μL of RPMI medium containing 10% HFBS and antibiotics (100 μg of streptomycin mL−1 and 100 U penicillin mL−1). Following, 1:2 serial dilutions were performed and macrophages treated with 2 μL DMSO were included as controls. The plates were incubated for 72 h at 37 °C and 5% CO2. Afterwards the cytotoxicity was determined using the colorimetric assay based on MTT reduction. 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide solutions were prepared at 5 mg mL−1 in saline solution, sterile-filtered immediately before use, and 15 μL were added to each well. After incubation for additional 4 h, supernatant was eliminated and the accumulated formazan crystals were dissolved by addition of 100 μL DMSO per well. The optical density was determined using an EMS Reader MF Version 2·4–0, at a wavelength of 560 nm using 630 nm as reference wavelength. In both cases, pentamidine was used as reference drug.
Influence of APD on oxygen consumption
To assay the effect of compounds on oxygen consumption of LtP, a Clark-type oxygen electrode (Hansatech, Germany) and software MCREC were used. Leishmania tarentolae promastigotes at 108 cells mL−1 in YEM were added and treated with increasing concentrations of test compounds between 25 and 400 μ m. Each concentration was assayed in quintuplicates and the results were expressed as percentage of oxygen consumption in comparison with the control LtP treated with the solvent (DMSO).
Influence of APD on mitochondrial complexes
Isolation of the mitochondrial fraction from LtP
To isolate the mitochondrial fraction (LtP-Mit) from LtP, a culture of 2700 mL of LtP was centrifuged for 10 min at 1900 g and 20 °C and the supernatant was discarded. The cell pellet was resuspended in buffer (10 mm TRIS–HCl, 0·3 m sucrose, 0·2 mm EDTA, 0·2% BSA, pH 7·4) and washed in two extra centrifugation steps for 10 min at 1900 g and 20 °C. The washed cell pellet was incubated in lysis buffer (5 mm TRIS–HCl, pH 7·4) for 10 min at 20 °C and subsequently homogenized in a dounce homogenizer. Cell debris was removed by centrifugation for 10 min at 1900 g and 4 °C. The supernatant was centrifuged for 20 min at 13 200 g and 4 °C to sediment the crude mitochondrial fraction. Mitochondrial fractions from LtP were resuspended in 1 mL buffer (250 mm sucrose, 50 mm KH2PO4, 0·2 mm EDTA, pH 7·2) and stored in liquid nitrogen until use.
Isolation of submitochondrial particles from bovine heart
Bovine heart submitochondrial particles (BH-SMP) were obtained from bovine heart mitochondrial suspensions (Nohl and Hegner, Reference Nohl and Hegner1978). Mitochondrial suspensions were sonicated ten times for 30 s with 1 min interruption at 4 °C in buffer (0·25 m sucrose, 10 mm TRIS, 1 mm EDTA, pH 9·0) using a Branson Sonifier set to 40 W energy output. Remaining mitochondria were removed by a centrifugation at 6500 g for 10 min at 4 °C and afterwards BH-SMP were sedimented from the supernatant by centrifugation at 95 000 g for 30 min. Pellets were resuspended in buffer (0·25 m sucrose, 10 mm TRIS, 1 mm EDTA, pH 7·4) and stored in liquid nitrogen until use. Protein was assayed by the Biuret method using BSA as standard (Gornall et al. Reference Gornall, Bardawill and David1949).
Inhibition of mitochondrial electron transfer activities
Reduced β-nicotinamide adenine dinucleotide:ubiquinone oxidoreductase (complex I) and succinate:ubiquinone oxidoreductase (complex II) activities were measured in 96-well plates using DCPIP as terminal electron acceptor. In each well, 200 μL of pre-mix containing buffer (250 mm sucrose, 20 mm TEA and 1 mm EDTA, pH 7·4), DCPIP (75 μ m), KCN (1 mm), BSA (4·3 mg mL−1 for complex I and 1·25 mg mL−1 for complex II), IQ (62·5 μ m) and LtP-Mit (170 μg protein mL−1) or BH-SMP (10 μg protein mL−1) were added. In addition, in the first row, 97 μL of pre-mix and 3 μL of compounds were added. Subsequently, six 1:3 serial dilutions were carried out, transferring always 100 μL. The reaction was started by adding 50 μL of start-mix per well with NADH (1·5 mm final conc.) for complex I or succinate (4 mm final conc.) for complex II, dissolved in the same buffer. After start of the reaction, images of the plates were recorded with a Canon EOS 300D camera in 2 min intervals (20 absorbance measurements). Reduction of DCPIP (ε600 nm = 19·1 mm −1 cm−1) as function of time was quantified by PLReader software using the absorption of the red channel minus the absorption of the green channel. For each individual concentration triplicates were measured and two evaluations were carried out.
To measure the inhibition of ubiquinol:cytochrome c oxidoreductase (complex III) dUQH2 (75 μ m) and cyt c3+ (100 μ m) were used as substrates. The reduction of cyt c3+ in the presence of dUQH2 was monitored at 550 nm using 540 nm as reference in buffer (250 mm sucrose, 50 mm KH2PO4, 0·2 mm EDTA, pH 7·2, 2 mm KCN, 4 mm NaN3) (Müllebner et al. Reference Müllebner, Patel, Stamberg, Staniek, Rosenau, Netscher and Gille2010). Test compounds were added at different concentrations 120 s prior to addition of dUQH2 starting the enzymatic reaction. In the presence of test compounds, the residual dUQH2:cyt c3+ oxidoreductase activity of BH-SMP (3·2 μg protein mL−1) and LtP-Mit (40 μg protein mL−1) was obtained and expressed in percentage of the non-inhibited rate (100%). For all test compounds the absence of significant reactions with dUQH2 and cyt c3+ was verified in the absence of mitochondrial preparations. All compound concentrations were tested in triplicate and the reduction rates for cyt c3+ were calculated using the extinction coefficient from the time trace of the absorption difference at 550 nm minus 540 nm (ε550−540 nm = 19 mm −1 cm−1). The specific activities in the absence of xenobiotics were: complex I (NADH:DCPIP) in BH-SMP 2541 nmol min−1 mg−1 and LtP-Mit 97 nmol min−1 mg−1, complex II (Succinate:DCPIP) in BH-SMP 2784 nmol min−1 mg−1 and LtP-Mit 87 nmol min−1 mg−1 and complex III (dUQH2:cyt c3+) in BH-SMP 4906 nmol min−1 mg−1 and in LtP-Mit 87 nmol min−1 mg−1.
Influence of APD on O2 •– production
Oxidation of CMH or CPH to stable nitroxyl radicals detectable by electron spin resonance (ESR) spectroscopy was used to measure O2 •– production. Measurements were performed in PBS (136 mm NaCl, 1·15 mm KH2PO4, 14 mm Na2HPO4, 2·7 mm KCl, pH 7·4) containing 100 μ m DFO and 25 μ m DTPA. Before measurements of LtP cell suspensions, cells were washed twice with PBS to remove the YEM. Bovine heart submitochondrial particles (1·08 mg mL−1), LtP-Mit (0·85 mg mL−1) or LtP (5×108 cells mL−1) were suspended in the PBS. Samples with LtP cells were supplemented with 15 mm glucose. For samples with mitochondria 12 mm succinate was added as substrate. Subsequently APD stock solutions in DMSO were added. Prior to the ESR measurement 400 μM CPH (for mitochondria) or 400 μ m CMH (for cells) were added. For ESR measurements 17 μL of the suspension were aspirated in a gas permeable Teflon tube (ID 0·7 mm). This capillary tube was placed in the resonator of the ESR instrument [Bruker EMX (Germany), split ring MD5] and ten sequential measurements were performed. Following instrument settings were used: microwave frequency 9·682 GHz, modulation frequency 100 kHz, modulation amplitude 1 G, time constant 0·082 s, centre field 3446 G, scan rate 71 G min−1, sweep width 100 G, scan time 84 s and attenuation CPH: 2 × 104 CMH: 7·96 × 103. From the ESR spectra the middle peak-to-peak intensity was retrieved and concentrations of oxidized CPH and CMH were obtained by comparison with a standard curve prepared from CP· solutions with defined concentrations.
Influence of APD on mitochondrial membrane potential
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol–carbocyanine iodide (JC-1) was used to investigate the changes in mitochondrial membrane potential of LtP. From a stock solution of JC-1 (6·1 mm) in DMSO a diluted solution (15 μ m JC-1) in PBS (pH 7·4) and 15 mm glucose was prepared. The cell density of LtP was adjusted to approximately 5 × 107 LtP cells mL−1. Leishmania tarentolae promastigotes cultured in YEM were centrifuged in a sterile 50 mL tube for 10 min at 2000 g at 20–25 °C. After the centrifugation step, the supernatant was discarded and the cell pellet was resuspended in a corresponding volume of the diluted JC-1 solution. To load cells with JC-1 they had to be incubated for 30 min under dark conditions in the incubator (26 °C). Then a washing step with 10 min centrifugation at 2000 g at 20–25 °C was included. The supernatant was removed and the pellet resuspended in a PBS–glucose solution (without JC-1). Subsequently, 200 μL cell suspension loaded with JC-1 were placed in each well of a black 96-well culture plate. In the third row 100 μL extra suspension were added and in the respective wells Nem (200 μ m final conc.) or GutA (200 μ m final conc.) were placed. Afterwards, a serial dilution (1:3) into the rows below was performed (200–0·82 μ m).
Fluorescence measurements (Perkin Elmer Enspire) were started after 4 h of incubation using an excitation wavelength of 488 nm and two emission wavelengths: 530 nm for JC-1 monomers and 590 nm for JC-1 dimers. The fluorescence intensity ratio (F 590 nm/F 530 nm) of control cells (including DMSO) were set to 100% and ratios of other samples were expressed in relation to this value. All samples were measured in triplicates.
Statistical analyses
Percentages of residual activity in relation to control experiments (100%) were calculated at each concentration of APD. Resulting activity–concentration plots were used to determine the half maximal inhibitory concentration (IC50) values by a non-linear regression according to a four parameter logistic model (4PL, Hill-Slope model) (Müllebner et al. Reference Müllebner, Patel, Stamberg, Staniek, Rosenau, Netscher and Gille2010) using Origin 6·1 (OriginLab Corporation). The results were expressed as mean ± s.d. In case of PMM, the IC50 value was obtained by fitting a sigmoidal E max model to dose–response curves (Bodley et al. Reference Bodley, McGarry and Shapiro1995). Selectivity indices were calculated by dividing the IC50 values for PMM by the IC50 values for LtP.
RESULTS
Influence of APD on cells viability
Both APD inhibited the growth of LtP and caused partial cytotoxicity in PMM (Table 1). Higher and more selective anti-leishmanial activity (P < 0·05) was observed by Nem; whereas GutA was less specific for LtP and showed similar IC50 values in PMM (P > 0·05). In addition, Nem displayed higher activity and selectivity (P < 0·05) compared with pentamidine, a clinically used drug.
Table 1. Effect of nemorosone (Nem) and guttiferone A (GutA) on the viability of L. tarentolae promastigotes and peritoneal macrophages from BALB/c mice

a IC50: Concentration that caused 50% of mortality.
b s.d.: Standard deviation.
c Selectivity index: IC50 in macrophages/IC50 in promastigotes.
d Pentamidine was included as reference drug.
Influence of APD on L. tarentolae oxygen consumption
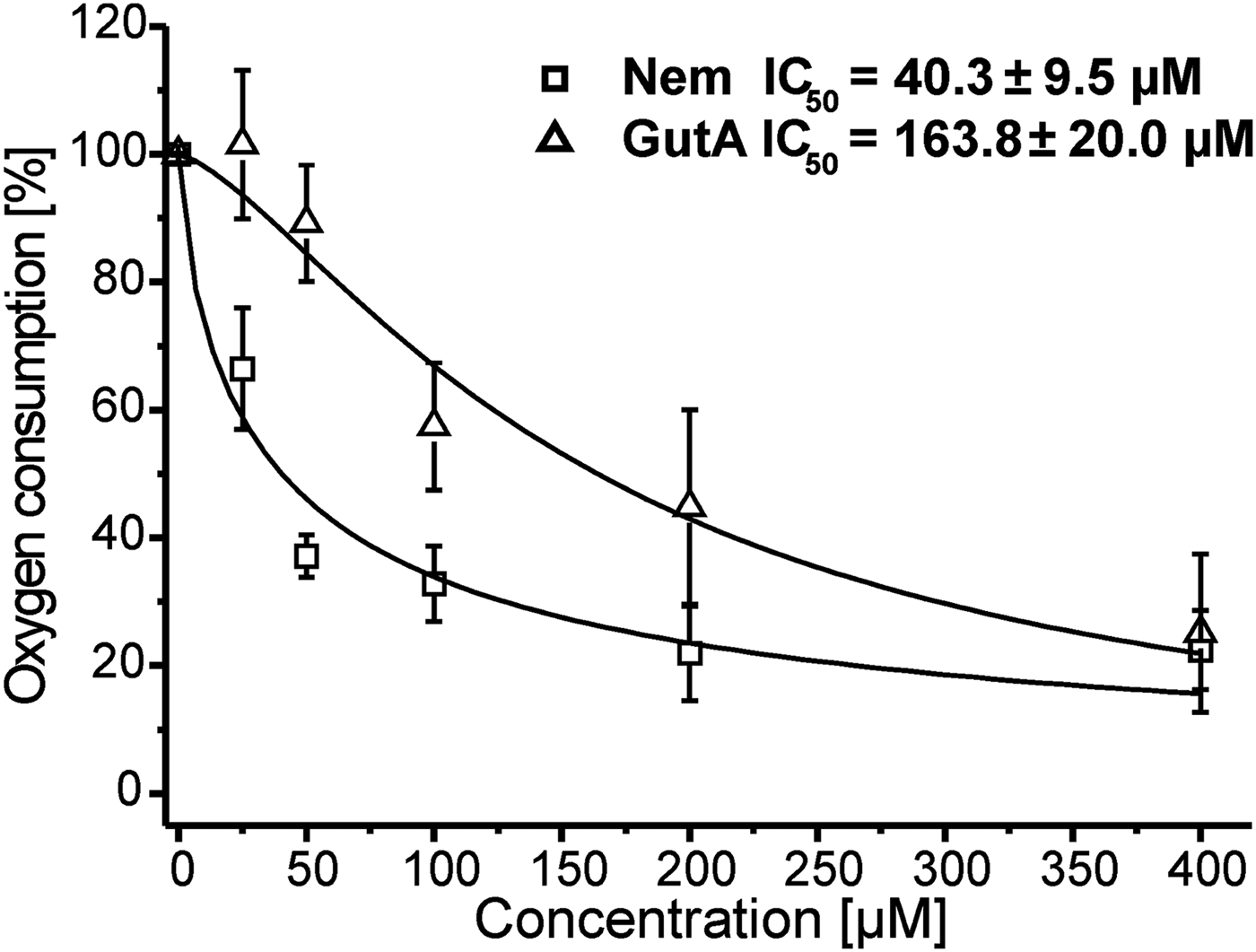
To elucidate the role of mitochondria in the anti-leishmanial activity of Nem and GutA we studied the effect of these APD on the oxygen consumption of LtP cells. Inhibitions of oxygen consumption caused by Nem and GutA with respect to cultures treated with solvent are shown in Fig. 2. The derived IC50 values demonstrated that Nem strongly inhibited LtP respiration; while only a moderate reduction of oxygen consumption was observed at GutA concentrations >50 μ m. The solvent DMSO caused an inhibition lower than 1% with respect to untreated control cultures.
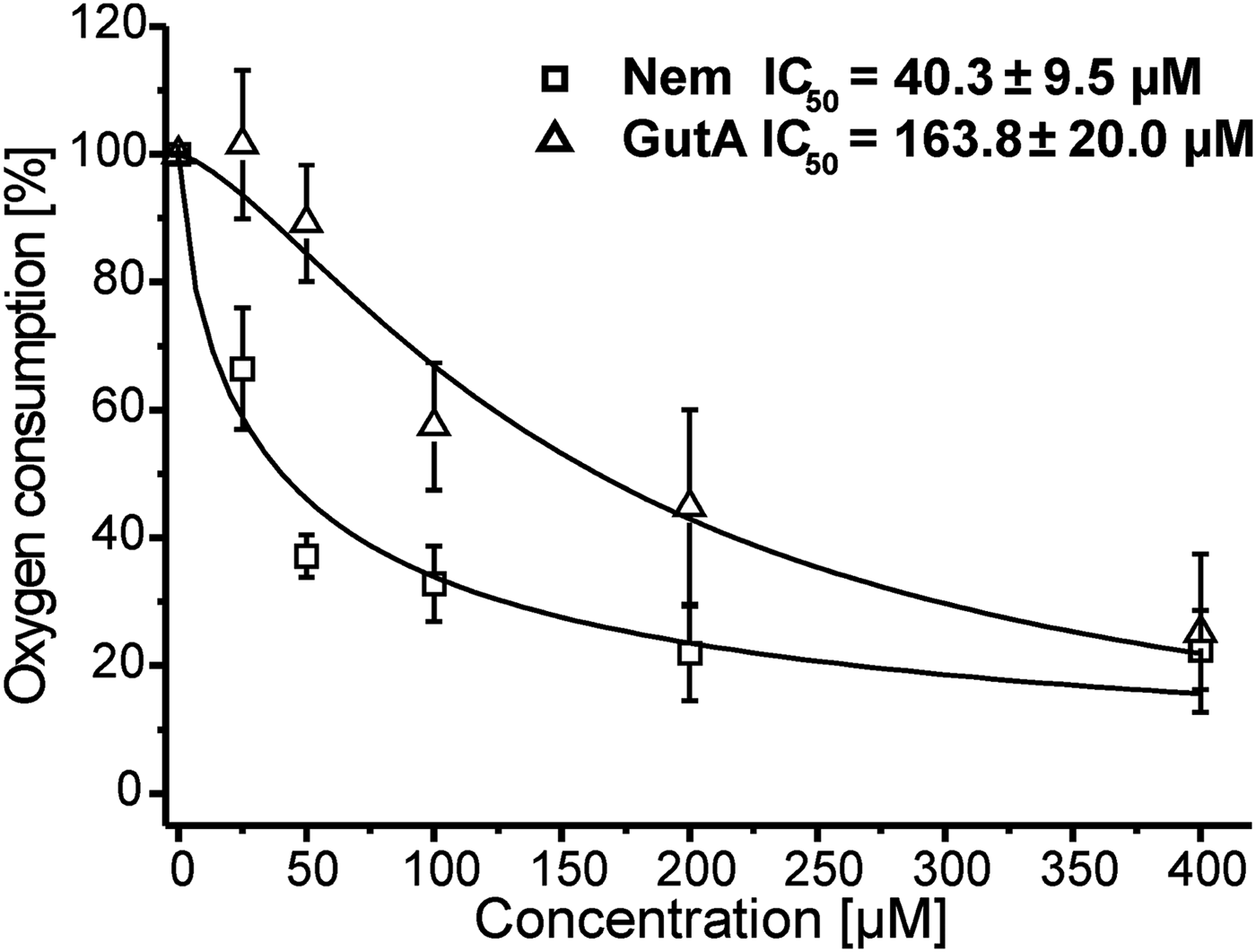
Fig. 2. Influence of nemorosone (Nem) and guttiferone A (GutA) on cellular oxygen consumption of L. tarentolae promastigotes. Respiration rates (15·9 ± 3·2 nmol O/min/108 cells) in the absence of xenobiotics were set to 100% and used as a reference for other measurements. Data represent mean ± s.d. of five independent experiments. For curve fitting and calculation of IC50 values a four parameter logistic model was used.
Influence of APD on activities of mitochondrial complexes
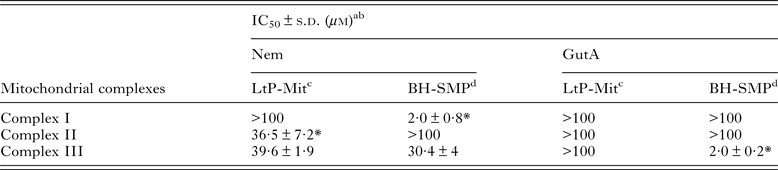
Since inhibition of oxygen consumption was observed in LtP cells, it was obvious to study the influence of Nem and GutA on the possible targets in the respiratory chain. Both APD are lipophilic and, therefore, competition with the lipophilic electron carrier ubiquinone at its binding sites (complex I – III) is expected. Table 2 shows the effect of both APD on complex I–III activities in LtP-Mit and BH-SMP. Nemorosone caused species-specific inhibition (P < 0·05) of succinate:ubiquinone oxidoreductase (complex II) from LtP-Mit and was less active in BH-SMP. However, for NADH:ubiquinone oxidoreductase (complex I) Nem strongly inhibited the activities in BH-SMP but not in LtP-Mit under our conditions (P < 0·05). For the ubiquinol:cytochrome c oxidoreductase activity (complex III) similar IC50 values (P > 0·05) were obtained for both mitochondrial models treated with Nem. In the case of GutA, the data show that it exclusively interfered in the complex III activity of BH-SMP.
Table 2. Effect of nemorosone (Nem) and guttiferone A (GutA) on electron transport chain complexes from LtP-Mit and BH-SMP
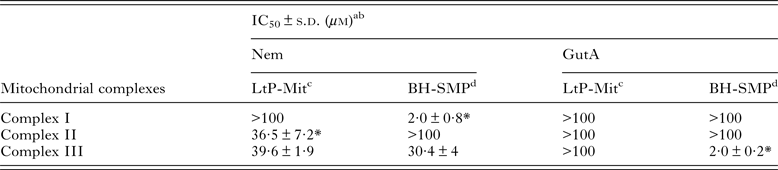
a IC50: Concentration that caused 50% of inhibition.
b s.d.: Standard deviation.
c LtP-Mit: Mitochondrial fraction from L. tarentolae promastigotes.
d BH-SMP: Submitochondrial particles from bovine heart.
* Depicts statistically significant differences (P < 0·05, Student's t-test) between LtP-Mit and BH-SMP.
Influence of APD on O2 •– production
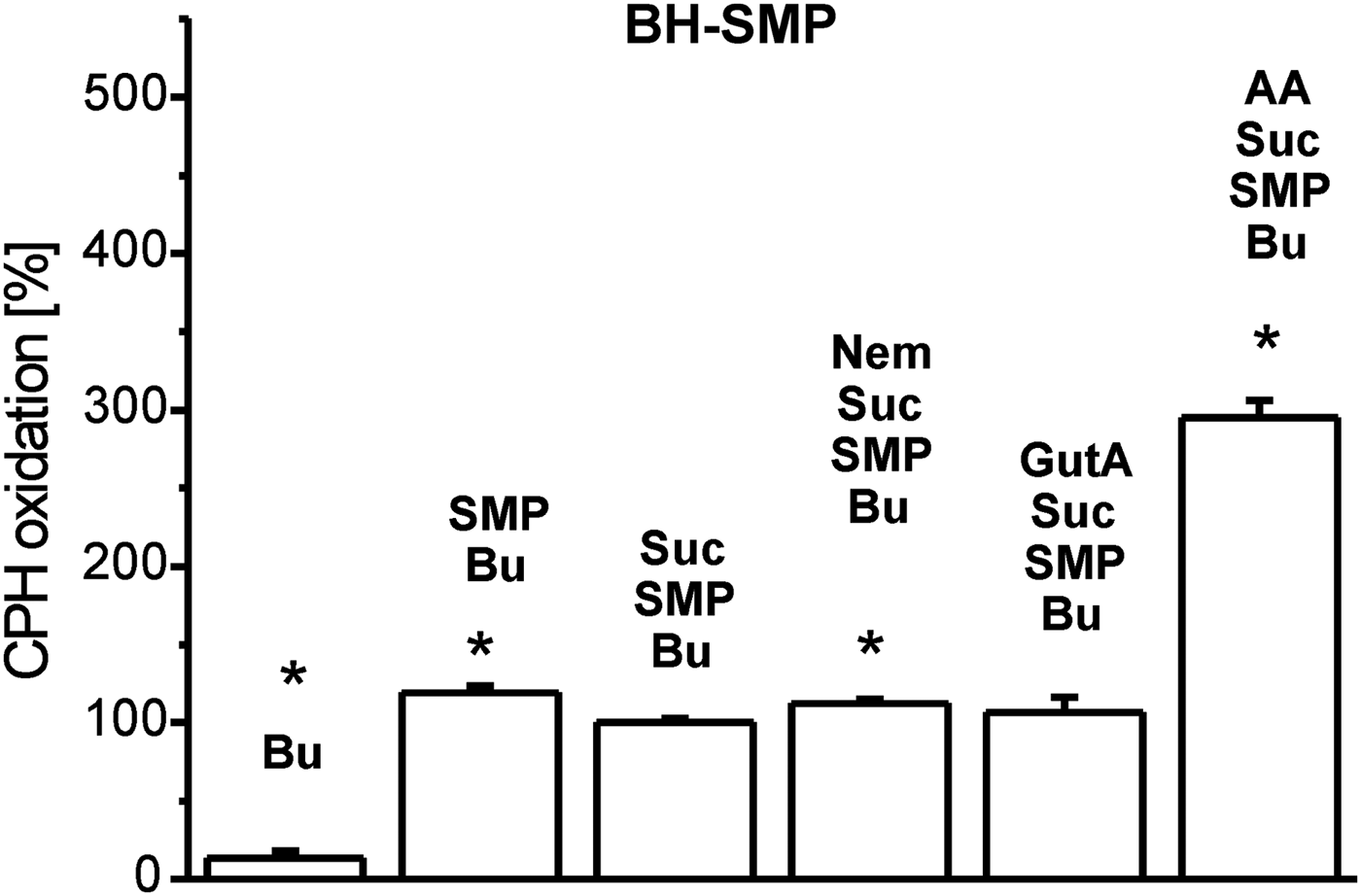
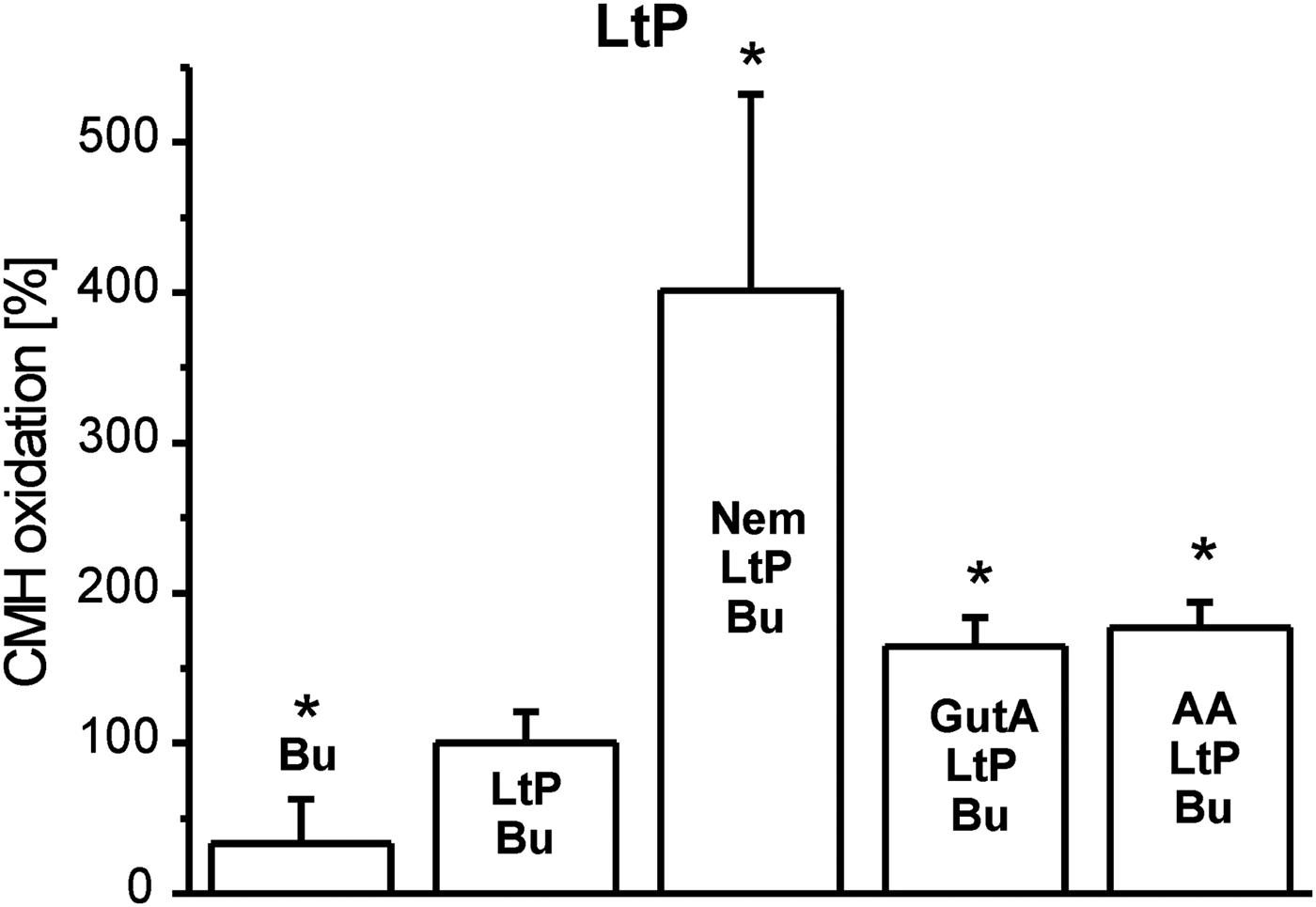
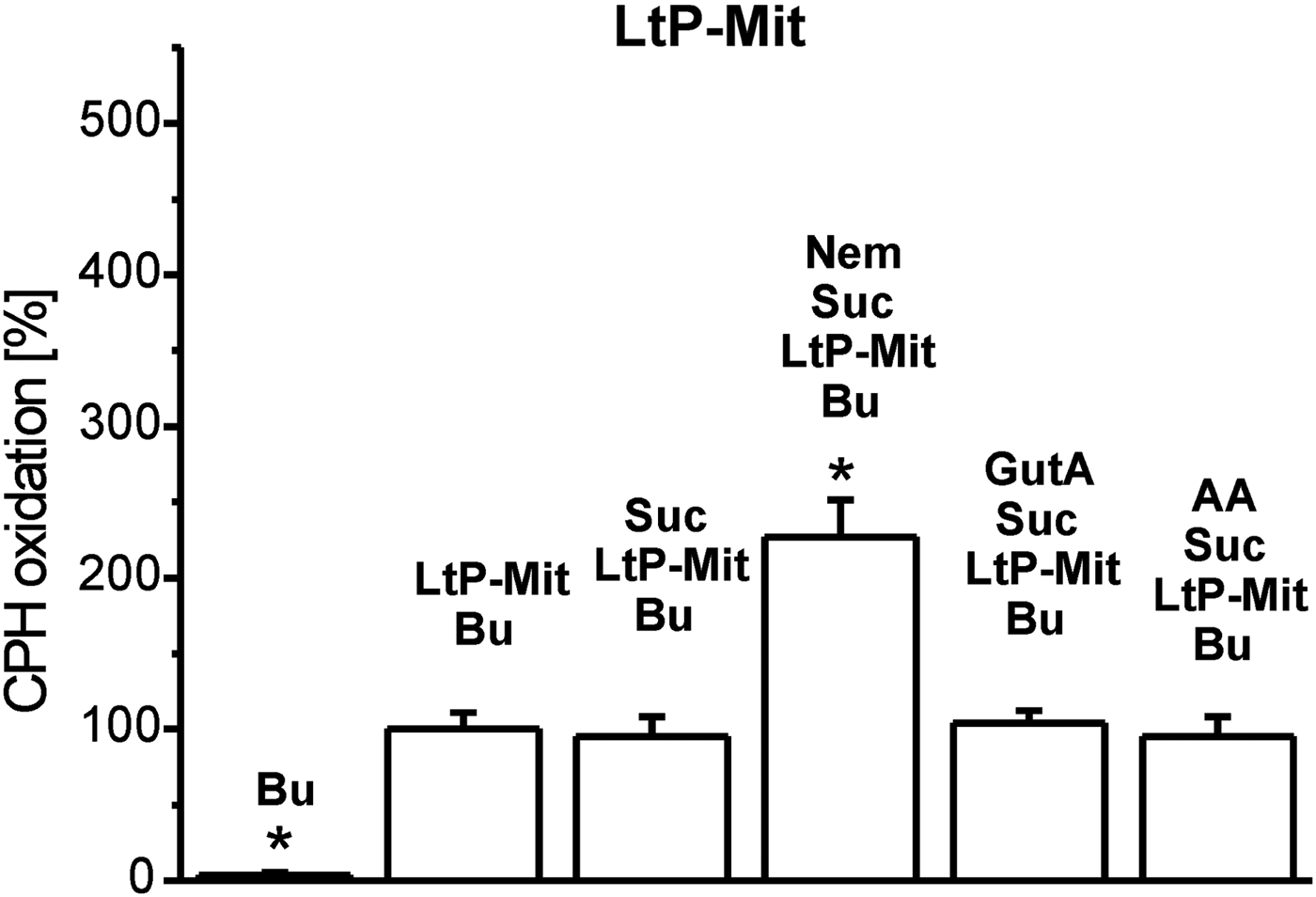
Inhibition of mitochondrial respiratory complexes can trigger mitochondrial superoxide radical production. The effectivity of xenobiotics in this respect strongly depends on their structure and corresponding binding sites. Therefore, we studied this parameter in BH-SMP, LtP-Mit and LtP cells using the oxidation of cyclic hydroxylamines (CMH and CPH) in combination with ESR spectroscopy as detection method. In all experiments, oxidation rates of CPH or CMH induced by mitochondrial fractions and cells in the presence of substrates (succinate or glucose) were considered as appropriate control (100%). In mitochondrial fractions without succinate, CPH oxidation rates were slightly higher in comparison with the respective controls possibly due to direct interaction of oxidized cyt c with CPH involving no O2 •– . In BH-SMP (Fig. 3), the well-known inhibitor of mammalian mitochondria antimycin A (AA) triggered an enormous increase of CPH oxidation. Likewise, Nem caused a significant increase of CPH oxidation rates; while GutA caused only a non-significant elevation. Using the same concentrations of xenobiotics in LtP (Fig. 4) Nem, GutA and AA resulted in a significant increase in CMH oxidation; however, only Nem caused a more then triplication of CMH oxidation rates. This effect is qualitatively confirmed in LtP-Mit respiring succinate (Fig. 5). In this system, again CPH oxidation rates were more than duplicated in the presence of Nem. In contrast, both GutA and AA caused only non-significant changes in succinate-respiring LtP-Mit.

Fig. 3. Superoxide radical production in submitochondrial particles from bovine heart (BH-SMP) measured by oxidation of CPH (400 μ m) and detection by ESR spectroscopy. The CPH oxidation rate in the presence of buffer (Bu), succinate (Suc, 12 mm) and SMP (1·08 mg mL−1) was set to 100% and other rates were expressed in relation to this reference. Xenobiotics present were nemorosone (Nem, 100 μ m), guttiferone A (GutA, 100 μ m) and antimycin A (AA, 5 μ m). Data represent mean ± s.d. of four independent experiments. *Statistical significant differences in comparison with the Suc/SMP/Bu sample were identified on the level P < 0·05 by Student's t-test.

Fig. 4. Superoxide radical production in L. tarentolae promastigotes (LtP) measured by oxidation of CMH (400 μ m) and detection by ESR spectroscopy. The CMH oxidation rate in the presence of PBS containing 15 mm glucose (Bu), and LtP (5 × 108 cells mL−1) was set to 100% and other rates were expressed in relation to this reference. Xenobiotics present were nemorosone (Nem, 100 μ m), guttiferone A (GutA, 100 μ m) and antimycin A (AA, 5 μ m). Data represent mean ± s.d. of four independent experiments. *Statistical significant differences in comparison with the LtP/Bu sample were identified on the level P < 0·05 by Student's t-test.
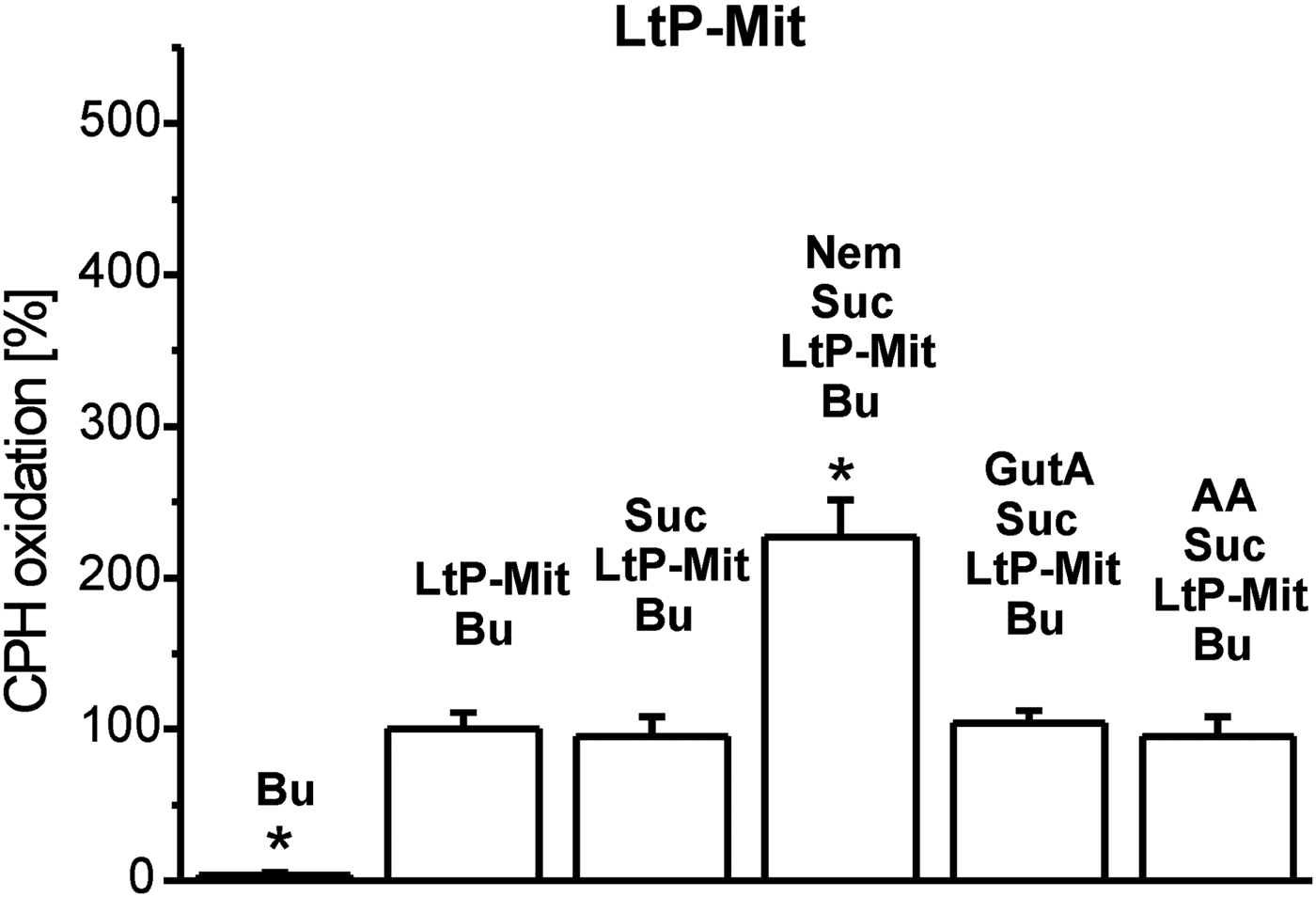
Fig. 5. Superoxide radical production in mitochondria from L. tarentolae promastigotes (LtP-Mit) measured by oxidation of CPH (400 μ m) and detection by ESR spectroscopy. The CPH oxidation rate in the presence of buffer (Bu), succinate (Suc, 12 mm) and LtP-Mit (4·04 mg mL−1) was set to 100% and other rates were expressed in relation to this reference. Xenobiotics present were nemorosone (Nem, 100 μ m), guttiferone A (GutA, 100 μ m) and antimycin A (AA, 5 μ m). Data represent mean ± s.d. of four independent experiments. *Statistical significant differences in comparison with the Suc/LtP–Mit/Bu sample were identified on the level P < 0·05 by Student's t-test.
Influence of APD on mitochondrial membrane potential in LtP
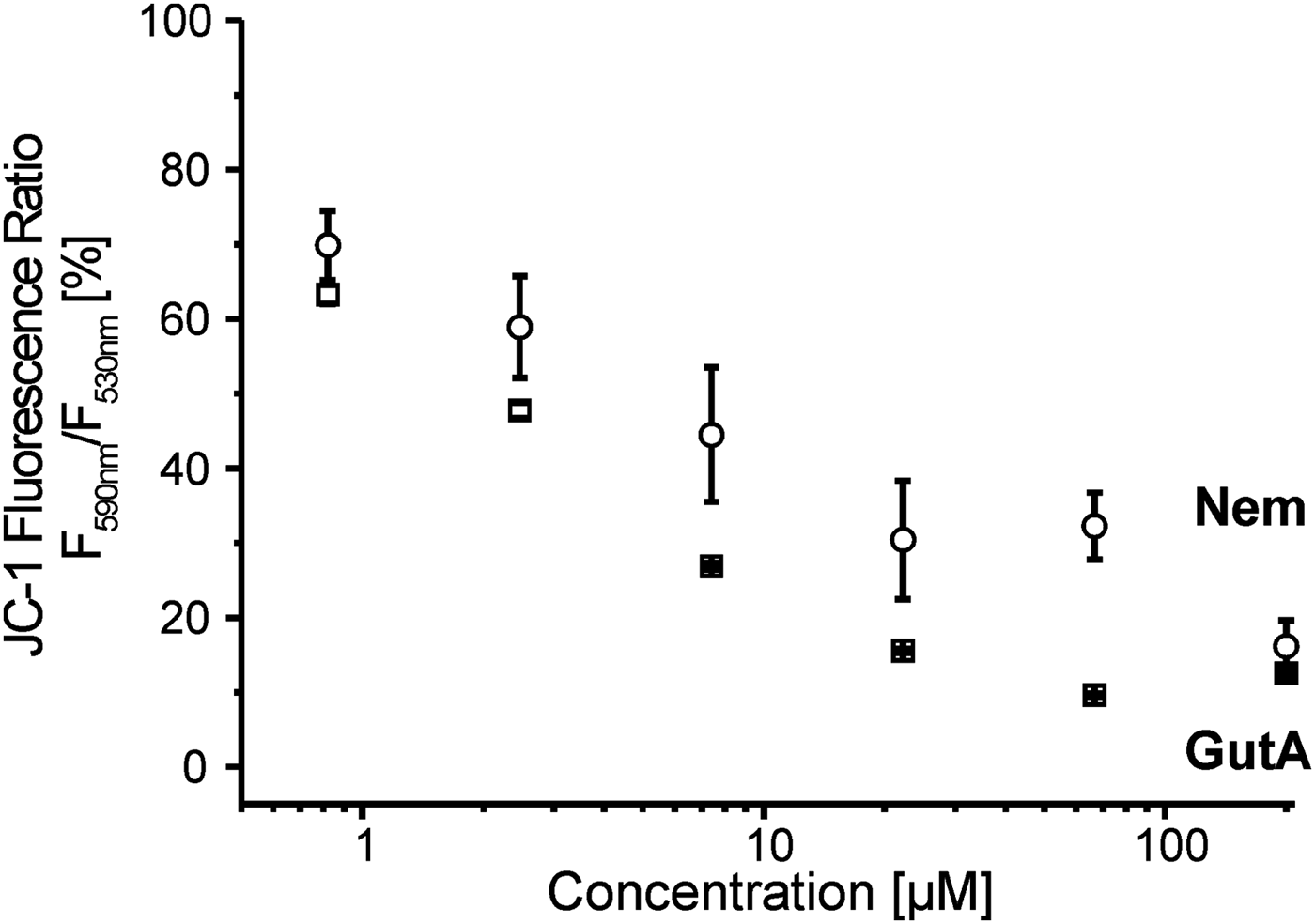
Both Nem and GutA decreased the mitochondrial membrane potential in LtP (Fig. 6), while the effect of GutA was stronger than that of Nem at equal concentrations. In comparison with the positive control with the ionophore valinomycin (10 μ m) which reduced the membrane potential to 42 ± 0·2%, GutA showed an even stronger effect at similar concentrations.
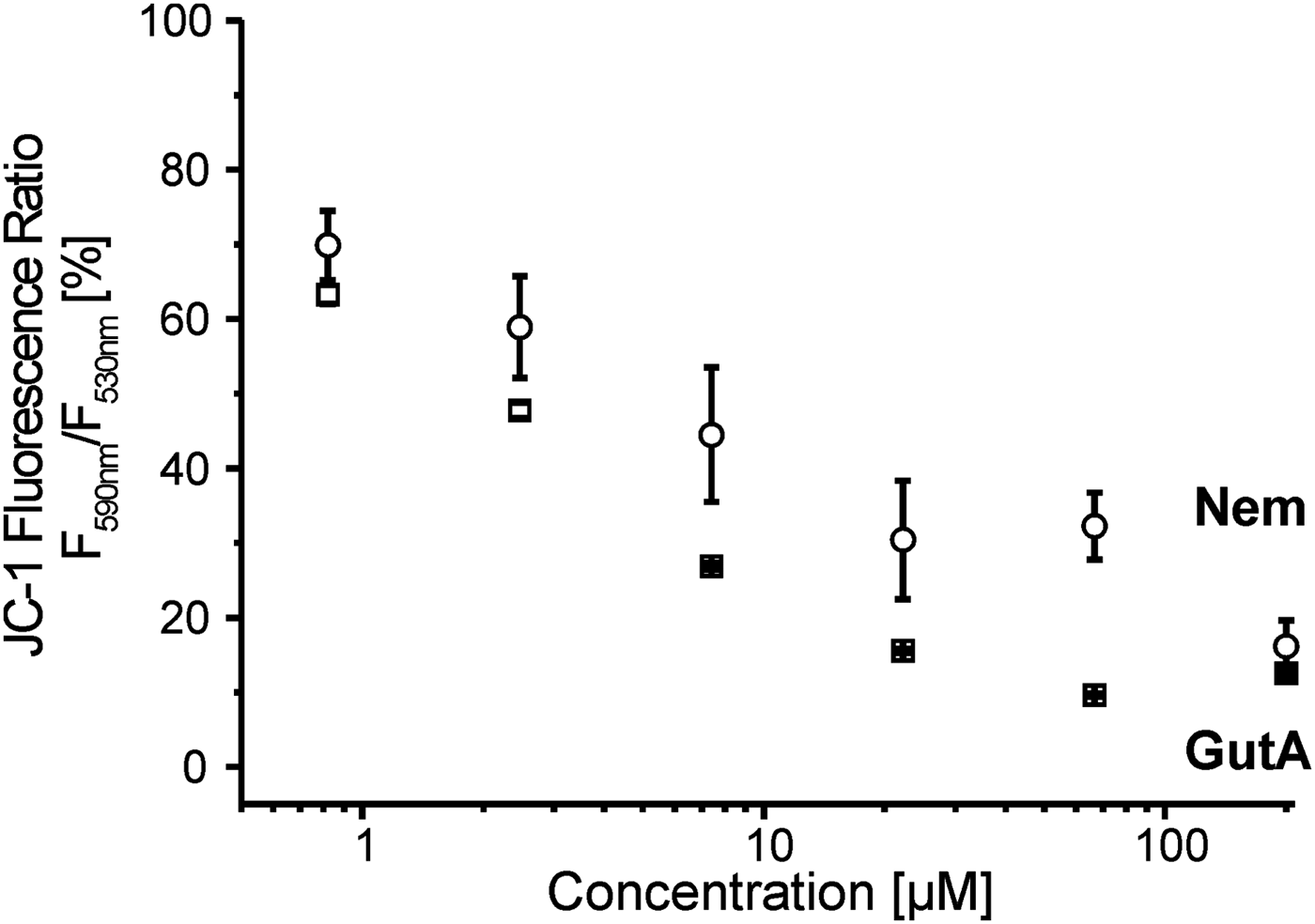
Fig. 6. Mitochondrial membrane potential in L. tarentolae promastigotes (LtP) measured by JC-1 in the presence of increasing concentrations of nemorosone (Nem) and guttiferone A (GutA). LtP (5 × 107 cells mL−1) were incubated in PBS containing 15 mm glucose and 15 μ m JC-1 for 4 h at 26 °C. Control LtP and LtP with the highest amount of vehicle (DMSO 0·75%) showed 100·0 ± 0·9% and 102·0 ± 0·5% relative membrane potential, respectively. The positive control in the presence of 10 μ m valinomycin was 42·0 ± 0·2%. Data represent mean ± s.d. of three independent experiments.
DISCUSSION
A substantial number of leishmanicidal drugs have the mitochondrion as at least one of their targets. These include drugs clinically used or in clinical trials such as: pentamidine, sitamaquine and paromomycin, and anti-leishmanial agents in pre-clinical studies, including licochalcones, phenylphenalenones, aurones and others (Monzote et al. Reference Monzote, Cuesta-Rubio, Matheeussen, Van Assche, Maes and Cos2011). The study of the mitochondrion as a likely target for APD is obvious since previous evidences exist that these compounds target mitochondria in cancer cells.
As a Leishmania model the LtP, a lizard-infecting species (Sauroleishmania) was used which is not pathogenic to humans.
In a previous study, both compounds caused growth inhibition against intracellular amastigotes of Leishmania amazonensis (Nem: IC50 = 11·2 ± 0·6 μ m, GutA IC50 = 15·6 ± 0·1 μ m) and Leishmania infantum (Nem: IC50 = 32·9 ± 5·4 μ m, GutA IC50 = 13·5 ± 0 μ m), causal pathogens of cutaneous and visceral leishmaniasis, respectively (Monzote et al. Reference Monzote, Cuesta-Rubio, Matheeussen, Van Assche, Maes and Cos2011). Although in the present study different parasite species and models have been used, in most cases Nem demonstrated a stronger inhibitory activity and higher selectivity for Leishmania (Table 1) compared with GutA. As LtP showed also sensitivity to both studied APD, experiments were carry out to explore the role of mitochondria in the leishmanicidal and toxicity effects caused by Nem and GutA. In addition, LtP have been employed in several pharmacological investigations, such as drug screening (George et al. Reference George, Bishop, Titus and Selitrennikoff2006), amplification of genes involved in the resistance to certain anti-leishmanial drugs such as amphotericin B (Singh et al. Reference Singh, Papadopoulou and Ouellette2001) and in the purification and characterization of proteins used as potential anti-leishmanial targets (Fritsche et al. Reference Fritsche, Sitz, Weiland, Breitling and Pohl2007).
Since mitochondria are the principal site for generation of cellular ATP, which is vital for cell function, mitochondrial dysfunction could trigger death. As in most eukaryotes, the Leishmania mitochondrion is also responsible for cellular respiration, which occurs via the electron transport chain (ETC) containing four complexes located in the inner mitochondrial membrane (Roy et al. Reference Roy, Das, Ganguly, Bose, Khalkho, Pal, Dey, Giri, Jaisankar, Dey and Majumder2008). Mitochondrial functions can be affected by the compounds directly or indirectly. To assess mitochondrial involvement in the anti-leishmanial effects of APD we compared the influence of APD on mitochondrial functions in mammalian mitochondria (BH-SMP) and leishmanial mitochondria (LtP-Mit). Inhibition of oxygen consumption was observed in LtP with both compounds. However, only Nem produced significant inhibition of oxygen consumption at concentrations (IC50 = 40·3 ± 9·5 μ m, Fig. 2) which could be relevant for anti-leishmanial effects. GutA with an IC50 value of 163·8 ± 20·0 μ m was much less effective in respiration inhibition.
For Nem higher concentrations were required to inhibit oxygen consumption than to inhibit viability, which suggests a cumulative effect. Due to the different time scales of viability (72 h) and oxygen consumption assays (0·5 h) both assays reflect different phases of Nem and GutA action on Leishmania. While the viability assay gives information about the final outcome of the experiments, oxygen consumption inhibition is an indicator of direct targets in the mitochondrial ETC. Typical consequences of such processes can be elevated formation of reactive oxygen species (ROS) and/or shortage of cellular ATP (by ETC inhibition or uncoupling) which could gradually contribute to cell death. Depending on the cellular ATP and ROS status already minor inhibition of ETC in the long run could be sufficient to compromise viability. In addition, the possibility that Nem targets other cellular functions in Leishmania parasites cannot be excluded, since many known anti-leishmanial compounds act via multiple mechanisms.
Likewise the assays on individual electron transfer complexes (Table 2) gave information about a direct effect of APD on mitochondria. In our assays Nem displayed a versatile activity on mitochondrial complexes. A specific inhibition of complex II from LtP-Mit was observed, with IC50 values in same range (P > 0·05) as IC50 values for oxygen consumption. Although complexes I and III are the main producers of ROS in mammalian cells (Murphy, Reference Murphy2009), complex II may also act as a source of ROS (Ishii et al. Reference Ishii, Ishii and Hartman2007). Since Leishmania parasites have a limited electron transport between complexes I–III, succinate might be a primary electron donor for energy production (Santhamma and Bhaduri, Reference Santhamma and Bhaduri1995). Notably, recent reports described specific inhibition of complex II as good target in Leishmania, including benzophenone-derived bisphosphonium salts (Luque-Ortega et al. Reference Luque-Ortega, Reuther, Rivas and Dardonville2010), phenyl-phenalenones (Luque-Ortega et al. Reference Luque-Ortega, Martinez, Saugar, Izquierdo, Abad, Luis, Pinero, Valladares and Rivas2004) and thenoyltrifluoroacetone (Mehta and Shaha, Reference Mehta and Shaha2004). In parallel, inhibition of complex III was observed, although a similar activity was also observed in BH-SMP. Based on these data, complex II inhibition by Nem in LtP could be responsible for the observed inhibition of oxygen consumption in LtP.
A complex I assay was also performed for LtP-Mit and BH-SMP, which revealed a strong inhibition by Nem in mammalian mitochondria. However, since complex I in LtP is considerably different from mammalian complex I (not rotenone sensitive, not significantly linked to oxygen consumption) this difference in inhibition could also have principal reasons. In contrast, GutA did not interfere with mitochondrial complexes, except a strong inhibition of complex III of BH-SMP. In this context, it is noteworthy that effects of GutA on rat liver mitochondria have been reported, which demonstrated a dissipation of mitochondrial membrane potential, depleted ATP and increased ROS levels (Pardo-Andreu et al. Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti2011a ).
Mitochondria have been recognized to produce most ROS via electron transfer in the respiratory chain. Mitochondrial ROS production could be an indirect evidence of impaired mitochondria (Halliwell and Gutteridge, Reference Halliwell and Gutteridge1990). In our study, in BH-SMP (Fig. 3), LtP (Fig. 4) and LtP-Mit (Fig. 5) Nem significantly increased the mitochondrial superoxide production above control levels at 100 μ m, while strongest effects of Nem were observed in LtP and LtP-Mit. Although GutA also triggered superoxide radical production in LtP, it was much less effective than Nem. In liver mitochondria a decrease of H2O2 release at low micromolar concentrations of Nem was observed, which, however, was attributed to its uncoupling effect al lower concentrations (Reis et al. Reference Reis, Pardo-Andreu, Nunez-Figueredo, Cuesta-Rubio, Marin-Prida, Uyemura, Curti and Alberici2014).
As a parameter susceptible to both direct and indirect effects (lipid peroxidation, permeability transition pore formation) on mitochondria the mitochondrial membrane potential in LtP was studied by the JC-1 method (Fig. 6). Our data demonstrated that at concentrations relevant for inhibiting leishmanial viability, both compounds decreased the membrane potential; however, GutA is stronger than Nem over a wide concentration range. This is also supported by the structural differences: GutA structure provides three dissociable OH groups for H+ shuttling over the inner mitochondrial membrane while Nem has only one OH group. In addition, non-protonophoric mechanisms for uncoupling by GutA in mammalian mitochondria were proposed (Pardo-Andreu et al. Reference Pardo-Andreu, Nunez-Figueredo, Tudella, Cuesta-Rubio, Rodrigues, Pestana, Uyemura, Leopoldino, Alberici and Curti2011a ).
Thus, the mammalian toxicity of GutA could be related to rather specific inhibition of complex III, while the observed anti-leishmanial effects of GutA could have extra-mitochondrial reasons or are related to uncoupling of leishmanial mitochondria but rather not to inhibition of the ETC or to excessive O2 •– formation. In this case, the mitochondrial dysfunction observed could be only an indirect consequence. Anti-leishmanial effects of Nem are supported by inhibition of mitochondrial complexes II and III, strongly increased O2 •– formation (at higher concentrations) and disruption of the membrane potential (already at lower concentrations). In mammalian mitochondria obviously rather complex I is a major target in the ETC, which differs, however, structurally and functionally from leishmanial complex I equivalent.
Also toxicological effects of other APD explained by mitochondrial dysfunction have been reported. In this context, garcinol (Pan et al. Reference Pan, Chang, Lin-Shiau, Ho and Lin2001), diphenyl-acetaldehyde (Almeida et al. Reference Almeida, Bechara, Vercesi and Nantes1999), xanthochymol, guttiferone E and guttiferone H (Protiva et al. Reference Protiva, Hopkins, Baggett, Yang, Lipkin, Holt, Kennelly and Bernard2008) were mentioned. Despite Nem and GutA sharing some structural core elements, the mechanism of action involved in their anti-leishmanial activity seems to be different. This was also observed with other APD, the 7-epi-nemorosone and plukenetione A, which caused anti-retroviral activities by different mechanism of action (Diaz-Carballo et al. Reference Diaz-Carballo, Ueberla, Kleff, Ergun, Malak, Freistuehler, Somogyi, Kucherer, Bardenheuer and Strumberg2010). In scientific literature, other cellular targets have been suggested for APD, including kinases (Diaz-Carballo et al. Reference Diaz-Carballo, Gustmann, Acikelli, Bardenheuer, Buehler, Jastrow, Ergun and Strumberg2012), G₀/G₁ phase of cell cycle (Popolo et al. Reference Popolo, Piccinelli, Morello, Sorrentino, Osmany, Rastrelli and Pinto2011) and microtubule inhibition (Roux et al. Reference Roux, Hadi, Thoret, Guenard, Thoison, Pais and Sevenet2000).
In summary, the present work represents the first investigation into the mode of anti-leishmanial activity of Nem and GutA. We suggest that Nem caused its anti-leishmanial action due to inhibition of complexes II and III of mitochondrial ETC of Leishmania parasites that could cause an increase in ROS production and triggers parasites death. In this sense, inhibition of mammalian mitochondrial complex I by Nem could also explain the cytotoxicity seen in mammalian cells. However, in case of GutA, mitochondrial complex III is involved in mammalian toxicity caused by this compound, but obviously mitochondria are not the primary anti-leishmanial site of action.
Acknowledgements
The award of an Ernst Mach scholarship to Dr. Lianet Monzote by the Austrian Exchange Office and the support by the Austrian Science Fund (FWF) Grant P27814 are gratefully acknowledged. In addition, we are grateful for the support of Prof. Veronika Sexl.