Introduction
Leishmaniasis is a neglected infectious disease considered a public health problem, especially in developing countries such as Argentina, Bolivia, Brazil and other Latin American countries. The disease is caused by protozoa of the genus Leishmania and can develop as an infection with cutaneous, mucosal, and visceral lesions. These clinical forms have their development according to the pathogenicity of infecting species and host's immunity. The World Health Organization estimates that, in the last 5 years, about 1.3 million cases of cutaneous leishmaniasis and 300 000 cases of visceral leishmaniasis (WHO, 2010) have been reported, with around 20 000 deaths per year (Brazil, Ministry of Health, 2017). The importance of American tegumentary leishmaniasis (ATL) lies in its high incidence and wide geographic distribution, as well as in the capacity of assuming forms that can determine destructive, disfiguring and disabling lesions, with great repercussion in the individual's psychosocial approach field as self-stigma and social stigma, until suicidal ideations (Gontijo et al. Reference Gontijo, De and De Carvalho2003; Bennis et al. Reference Bennis, Thys, Filali, De Brouwere, Sahibi and Boelaert2017).
In the vector, the parasite survives in its salivary glands and the metacyclic promastigotes forms (flagellates) are inoculated into the individual through the bite on host's skin during the blood meal (WHO, 2010). Once in the skin, the parasites are phagocytosed by cells of the phagocytic mononuclear system and polymorphonuclear cells, transforming into amastigote form (not flagellated), which uses escape mechanisms to resist death, multiplying until cell lysis to infect new cells (WHO, 2010; Oliveira et al. Reference Oliveira, Ribeiro, Schrieffer, Machado, Carvalho and Bacellar2014). Also, there is evidence that mononuclear cells play an essential role in the spread of infection to other tissues, and that enzymes from metalloprotease complexes in the host and parasite are involved in this process (Chang and Werb, Reference Chang and Werb2001).
In general, metalloproteases are a group of enzymes necessary for cell proliferation, differentiation, extracellular remodeling, vascularization and cell migration in physiological and pathological processes (Chang and Werb, Reference Chang and Werb2001). These are divided into two groups: matrix metalloproteases (MMPs), such as MMP-9 and MMP-2 produced mainly by host activated macrophages (Birkedal-Hansen et al. Reference Birkedal-Hansen, Moore, Bodden, Windsor, Birkedal-Hansen, DeCarlo and Engler1993); and disintegrin metalloproteases (ADAMS), such as glycoprotein 63 (GP63), parasite metalloprotease present in the membrane of the Leishmania genus. This GP has been increasingly studied for the understanding of progression and clinical forms of leishmaniasis (Chang and Werb, Reference Chang and Werb2001; Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a; Shio and Olivier, Reference Shio and Olivier2010; Isnard et al. Reference Isnard, Shio and Olivier2012; de Oliveira et al. Reference de Oliveira, Vanessa Oliveira Silva, Damascena, Passos, Duthie, Guderian, Bhatia, de Moura, Reed, de Almeida and de Jesus2013). Infection with Leishmania sp. stimulates host MMPs production by infected macrophages, leading to the breakdown of cell matrix, which promotes the dissemination of parasite to another site (Liarte et al. Reference Liarte, Mendonca, Luz, Abreu, Mello, Farias, Ferreira, Millington and Costa2001; Carvalhal et al. Reference Carvalhal, Barbosa, D'El-Rei Hermida, Clarencio, Pinheiro, Veras and dos-Santos2004). The role of host MMP has been recently studied and it is related to the clinical development of visceral leishmaniasis in humans and dogs (Machado et al. Reference Machado, Melo, Moraes, Souza, Marcondes, Perri and Vasconcelos2010; Marangoni et al. Reference Marangoni, Melo, Moraes, Souza, Perri and Machado2011; Melo et al. Reference Melo, Marangoni, Marcondes, Lima and Machado2011; de Oliveira et al. Reference de Oliveira, Vanessa Oliveira Silva, Damascena, Passos, Duthie, Guderian, Bhatia, de Moura, Reed, de Almeida and de Jesus2013; Gadisa et al. Reference Gadisa, Tasew, Abera, Gelaye, Chanyalew, Abebe, Laskay and Aseffa2017). Host MMPs serological or tissue levels have been considered as new and simple biomarkers for monitoring the success of therapy and predicting favourable clinical outcome of human visceral leishmaniasis (de Oliveira et al. Reference de Oliveira, Vanessa Oliveira Silva, Damascena, Passos, Duthie, Guderian, Bhatia, de Moura, Reed, de Almeida and de Jesus2013; Gadisa et al. Reference Gadisa, Tasew, Abera, Gelaye, Chanyalew, Abebe, Laskay and Aseffa2017).
The host and parasite metalloproteases represent an important role in the understanding of host immune response to infection, parasite pathogenicity, progression and cure of the disease. There are no recent systematic reviews on the role of parasite and host metalloproteases in tegumentary leishmaniasis, specifically those caused by the New World Leishmania. Therefore, this paper is a systematic literature review about the function of the enzymatic complex of metalloproteases in infection by New World Leishmania species causing ATL.
Methodology
Research strategy
The PRISMA (Beller et al. Reference Beller, Glasziou, Altman, Hopewell, Bastian, Chalmers, Gøtzsche, Lasserson and Tovey2013) was used for making this systematic literature review. The strategy for research in the PubMed (US National Library of Medicine) consisted of the use of MeSH database tool, by employing the descriptors ‘leishmaniasis’, ‘cutaneous leishmaniasis’, ‘mucocutaneous leishmaniasis’, ‘diffuse cutaneous leishmaniasis’, ‘Leishmania’ and ‘metalloproteases’. The same terms were also used for the research in EMBASE databases, LILACS (Latin American and Caribbean Center on Information in Health Sciences) and ISI Web of Science.
Inclusion and exclusion criteria
Articles published from 01/01/2006 to 12/31/2016 have been included in this review. As inclusion criteria, only the articles that studied the role of metalloproteases in New World Leishmania species causative of ATL, following the WHO clasification (2011), were included. Thus, papers which studied metalloproteases in Leishmania braziliensis, Leishmania amazonensis, Leishmania mexicana, Leishmania guyanensis, Leishmania panamensis, Leishmania lainsoni, Leishmania naiffi and Leishmania peruviana were selected. Also, we included Leishmania infantum, responsible for ATL or atyphical CL. Therefore, studies that investigated other Leishmania species or species that do not cause human infection and visceral leishmaniasis were excluded from the review. Only articles that were published in Spanish, Portuguese or English and had available abstract were selected. Case reports, commentary, editorials, errata publications, interviews and guidelines were also excluded from this review.
Selection of articles
Independently, six researchers belonging to Group I (IGD, LSM, TFPM, BMC, QALN and JVPS) searched the databases for relevant abstracts to be included in the study. The selected articles were randomly distributed into Group I, which judged the articles based on the inclusion and exclusion criteria. The first step of article selection was the research for potential publications through the reading of the titles and abstracts, excluding this way the articles that did not attend to selection criteria. After the initial selection, the articles were chosen by pairs of judges to establish a consensus on articles to be selected. Publications in which four or more researchers agreed to include the article for the systematic review were included as definitive articles. If the consensus was found between three or two researchers, the articles were considered as indeterminate. Following the same consensus, those articles selected by one researcher were excluded from the review.
In a second step, three reviewers from Group II (JVPS, LSM and IGD) independently evaluated the full articles selected in the first stage. After consensus eligibility assessment of Group II researchers, the articles that were finally included in this systematic review were chosen.
Methodological quality and risk of bias evaluation
At the second moment of selection, the three researchers from Group II independently assessed the quality and risk of biases in each included study. The researchers also evaluated the selected articles according to data analysis, sample selection, animal species or cell type and methods for detection of metalloproteases. Regarding the biases, the articles were evaluated for their limitations pointed out by the researchers themselves and for those detected by the reviewers.
Extraction of data and analysis of results
The data extraction was carried out by structuring the relevant information of the articles in the form of tables. From the selected articles, the researchers in Group I extracted and tabulated the data. After the synthesis of the data in the form of tables, the judges of Group II made their corrections, checking the data extracted in the tables in two stages. The first stage consisted of the random distribution of publications among the judges of Group I, who promoted the first corrections. In the second stage, the judges of Group II did the correction in pairs and randomly.
Results and discussion
In this systematic review, 17 original articles were included (Fig. 1) after applying the inclusion and exclusion criteria and consensus among the researchers. Thirteen articles were related to the GP63 of New World Leishmania sp. species (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006, Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006; Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007; Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008, Reference Kulkarni, Olson, Engman and Mcgwire2009; Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a, Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblayb; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014) and four articles were related with host's MMP-9 (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb; Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014).

Fig. 1. PRISMA flowchart with selection criteria of the articles included in the systematic review.
Leishmania sp. Glycoprotein 63
GP63 is a multifunctional enzyme related to complement components inactivation (Isnard et al. Reference Isnard, Shio and Olivier2012), matrix extracellular degradation, contributing to cellular migration (Yao, Reference Yao2010) and inhibition of natural killer cells activation, which contributes to infection persistence and disease progression (Shio and Olivier, Reference Shio and Olivier2010; Shio et al. Reference Shio, Hassani, Isnard, Ralph, Contreras, Gomez, Abu-Dayyeh and Olivier2012). In addition, the invasive and immunomodulatory role of GP63 and other proteases differ between the clinical form of the disease and the virulence of strains in patients from the same region (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006).
GP63 studies characteristics
From the articles on GP63 (n = 13), four were conducted in Canada (Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a, Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblayb; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010), four in Brazil (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006, Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014), two in the USA (Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008, Reference Kulkarni, Olson, Engman and Mcgwire2009), one in the UK (Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007), one in India (Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008), and one in Greece (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006). In 13 studies, 11 performed in vitro studies (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006, Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007; Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008; Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008, Reference Kulkarni, Olson, Engman and Mcgwire2009; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a, Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblayb; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014;), one was retrospective (Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008) and one in vivo (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006). Six studies performed statistical analysis of their results (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006; Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008; Gomez et al. Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblay2009b; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014), while five did not report the statistics (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a; Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010) and one did not perform the analysis (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006; Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008).
Different leishmaniasis clinical forms were investigated in the studies, with three studies addressing cutaneous form (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014), one studied cutaneous and mucosal forms (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008), two studied cutaneous and visceral forms (Gomez et al. Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblay2009b; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010), five studied different forms of disease manifestation (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006; Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007; Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008; Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a) and one investigated the diffuse cutaneous and cutaneous form (Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009). The New World Leishmania studied were L. amazonensis, L. mexicana, L. guyanensis, L. panamensis, L. braziliensis and Leishmania venezuelensis (Table 1).
Table 1. Characteristics of the selected articles on the role of GP63 in New World Leishmaniasis

CL, cutaneous Leishmaniasis; ML, mucosal Leishmaniasis; VL, visceral Leishmaniasis; MMPs, metalloproteases; FN, fibronectin; NR, not related; gp63EXT, class of GP63 genes; OW, Old World; NF-kB, nuclear factor kappa beta; PCR, polymerase chain reaction; TcGP63, recombinant GP63 of T. cruzi; AP-1, activated protein-1; SNAREs, soluble N-ethylmaleimide-sensitive factor attachment receptor; 3D COL I, 3D Collagen I.
About the objectives of the selected articles, the role/mechanism and structure of GP63 in promastigotes (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006, Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009), amastigotes (Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008), animal model (BALB/c mice) (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006) and in 3D type I collagen matrices (Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014) were studied. Another study (Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008) evaluated a new method to detect Leishmania sp. Metalloproteases, and two other studies investigated cellular (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008) and gene localization (Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009) of metalloproteases (Table 1).
Most of the studies used cell culture as the sample, while others used cell (Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009) and nuclear (Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008) extracts from promastigotes. Besides these, three articles used murine macrophages (Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008), one used embryonic fibroblasts (Gomez et al. Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblay2009b) and others used recombinants proteins (Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a) and genes (Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009). Many methods have been used to detect GP63: most of the studies used Western Blotting (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006; Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008, Reference Kulkarni, Olson, Engman and Mcgwire2009; Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a, Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblayb; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010) and the other methodologies employed were gelatin SDS-PAGE (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014), complement lysis assays (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006), DNA sequencing (Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007), zymography (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008, Reference Kulkarni, Olson, Engman and Mcgwire2009; Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014), indirect fluorescence microscopy (Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a; Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009), immunoprecipitation (Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010), evaluation of protein complex by Bradford method (Gomez et al. Reference Gomez, Contreras, Halle, Tremblay, McMaster and Olivier2009a), electrophoretic mobility shift assay (EMSA) (Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008), confocal microscopy (Gomez et al. Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblay2009b; Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010), confocal fluorescence microscopy (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008), flow cytometry (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009) and polymerase chain reaction (PCR) (Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008) (Table 2).
Table 2. Samples, methods of detection and conclusion of articles included on GP63 of New World Leishmania sp.

WB, Western Blotting; DLN: IFM, immunofluorescence microscopy; IP, immunoprecipitation; PCA, protein complex analysis; EMSA, electrophoresis mobility shift assay; CM, confocal microscopy; CFM, confocal fluorescence microscopy; FC, flow cytometry; MMP, metalloproteases; PTP, protein tyrosine phosphatase; PCR, polymerase chain reaction; FN, fibronectin; BMMΦ, bone marrow–derived MØ; SDS-PAGE; DLNs, draining lymph nodes; GP63EXT, class of GP63 genes; OW, Old World; MMP, metalloprotease; NF-kB, nuclear factor kappa-beta; FN, fibronectin; SHP-1, type of tyrosine phosphatase; TCPTP, T cell phosphatase; PTP1B, type of tyrosine phosphatase; TcGP63, recombinant GP63 of T. cruzi; AP-1, activated protein-1; VAMP8, SNAREs present on phagosomes; SNAREs, soluble N-ethylmaleimide-sensitive factor attachment receptor.
Role of new world Leishmania sp. (GP63)
Lima et al. showed that there are alterations in the GP63 expression according to the Leishmania strain and its ability to interact with macrophages, which confirms that the presence of peptidases is essential to genus virulence in nutrition, cell invasion, intracellular survival, differentiation and proliferation. The authors observed that the virulent strain of L. braziliensis presented a distinct and complex proteolytic profile, composed of metal and cysteine peptidases, whose hydrolytic activities were modulated by pH conditions. During analysis of the SDS-PAGE gel, the virulent strain of L. braziliensis showed a more complex peptidase pattern than the non-virulent, presenting four proteolytic bands at acid and alkaline pH, while the non-virulent strain showed only one band, representing GP63 metalloprotease. All differences found in the production of proteases are possibly associated with the mechanism of infection and virulence of L. braziliensis. The comparison of proteolytic activity in strains of different virulence patterns may contribute to the study of the participation of these enzymes during infection in the host (Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009).
Cuervo et al. (Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006) has studied the proteases activity of several strains of L. braziliensis using promastigotes and SDS-page. The authors tested different pHs and conditions and obtained distinct profiles of metalloproteases. They observed distinct zymographic patterns in L. (V). braziliensis strains isolated from cutaneous, mucosal and disseminated leishmaniasis from subjects coming from Brazil and Colombia. Brazilian isolates exhibited the same protease profile as the reference isolate, while isolates from Colombian patients showed a different pattern. While Brazilian isolates remained infective, the other did not show infectivity after the cryopreservation process. Differences among the microorganisms can be related to the geographical origin of the strains and/or to the clinical manifestations (Cuervo et al. Reference Cuervo, Saboia-Vahia, Costa Silva-Filho, Fernandes, Cupolillo and De Jesus2006).
Real Time PCR technique has been shown as a sensitive assay for GP63 detection and quantification of the parasite load in the absence of lesions caused by Leishmania infection, and still allows verification of the systemic parasite distribution (Tupperwar et al. Reference Tupperwar, Vineeth, Rath and Vaidya2008). Also, in the methods used for GP63 identification, the GP63EXT gene (GP63 gene) may be an excellent marker for Leishmania genotyping using polymorphism in the recombinant domain of this gene. The authors also pointed out that from this genotyping it is possible to distinguish two species of the Old World, L. infantum and Leishmania archibaldi. The gene conversion between various alleles of the same GP63 gene class and between different members of this gene family is not only a determining factor in the generation of genetic diversity but also in the maintenance of homozygosis and heterozygosis among genes of the GP63 family (Mauricio et al. Reference Mauricio, Gaunt, Stothard and Miles2007).
GP63 may be related to host complement proteins resistance, protecting the parasite from complete lysis mediated by this system (Kulkarni et al. Reference Kulkarni, Olson, Engman and Mcgwire2009). This pattern of proteolytic activity of GP63 is a stable phenotypic characteristic of Leishmania sp. (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008). Gregory et al. demonstrated in vitro that promastigote forms of pathogenic species, including L. mexicana and L. braziliensis, promote the cleavage of the D-subunit of nuclear factor kappa-B (NF-κBp65RelA) in the cytoplasm of infected murine macrophages, in a process depending on GP63 protease activity (Gregory et al. Reference Gregory, Godbout, Contreras, Forget and Olivier2008). NF-κB plays an important role in the onset of the innate response after the pathogen invasion in the host, regulating the expression of chemokines, cytokines, adhesion molecules and enzymes that produce secondary inflammatory mediators, which reduces the inflammatory response capacity (Calegari-Silva et al. Reference Calegari-Silva, Pereira, Dione, De-melo, Saraiva, Soares, Bellio and Lopes2009).
In both parasitic stages (promastigote and amastigote), GP63 from L. amazonensis binds and degrades host fibronectin, influencing the activation of macrophages infected by the parasite. Fibronectin is a multifunctional protein that has domains for interaction with matrix extracellular (ECM) components such as heparin, collagen and fibrin, exerting structural function in ECM and decreasing the activation capacity of macrophages (Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008). Petropolis et al. studied the interaction between promastigotes and the extracellular matrices of the host, using a three-dimensional collagen matrix composed of type I collagen. It was observed that L. amazonensis promastigotes adhered strongly to the fibres of the matrix of collagen, altering the organization of these fibers, remodeling the entire matrix. Also, the parasite degrades collagen, and this degradation is reduced only in the presence of protease inhibitors. Incubation with anti-GP63 showed a small but significant reduction in the degradation of collagen fibres, suggesting that this metalloprotease is involved in this process. The group showed that the metalloproteases played an important role in the migration of 3D matrices so that fibre remodeling contributes to the invasion of the parasite (Petropolis et al. Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014).
GP63 is also responsible for cleaving tyrosine phosphatases (PTPs) in murine macrophages infected with Leishmania, such as SHP-1 (protein tyrosine phosphatase), TCPTP (T cell phosphatase) and PTP1B (protein tyrosine phosphatase). These enzymes are activated in the infection by a GP63-dependent cleavage process, and PTP1B is required for the development of Leishmaniasis in its early stages. Therefore, in addition to the GP63 cleavage of kinases and transcriptional factors, it further modulates tyrosine phosphatases that regulate cell signaling pathways and may subvert macrophage signaling and its functions (Isnard et al. Reference Isnard, Shio and Olivier2012). Thus, GP63 acts as an important virulence factor in the development of the intracellular parasite and establishment of the infection (Gomez et al. Reference Gomez, Stuible, Shimizu, Mcmaster, Olivier and Tremblay2009b).
The in vivo reduction in the amount of GP63 from Leishmania sp. seems to be related to the increase of extracellular lysis by complement, decreasing infection in macrophages. Thiakaki et al. observed that single GP63 mutants exposed to cellular and humoral attacks in vivo were more susceptible and had greater difficulty in establishing intracellular parasitism. The reduction of GP63 also induced a Th1-like cellular immune response (T helper 1), and it is crucial in host defense against Leishmania infection (Thiakaki et al. Reference Thiakaki, Kolli, Chang and Soteriadou2006). The Th1 immune response is essential due to its inducting of the production of interferon-gamma (IFN-γ) and interleukin 12 (IL-12), which are responsible for the activation of macrophages to produce leishmanicidal substances, leading to the death of the parasite. The induction of Th1 cell subpopulations is known to inhibit the proliferation and differentiation of Th2 cells, which are involved in the production of cytokines that inhibit the activation of macrophages and promote inflammation. Thus, these cells promote parasite persistence and evolution of the infection (Espir et al. Reference Espir, Figueira, Naiff, Guimarães, Ramalho-ortigão, Malheiro, Maria and Franco2014). Thus, decreasing of GP63 would be necessary for the development of Th1 type immune response and to favor parasite death (Fig. 2).
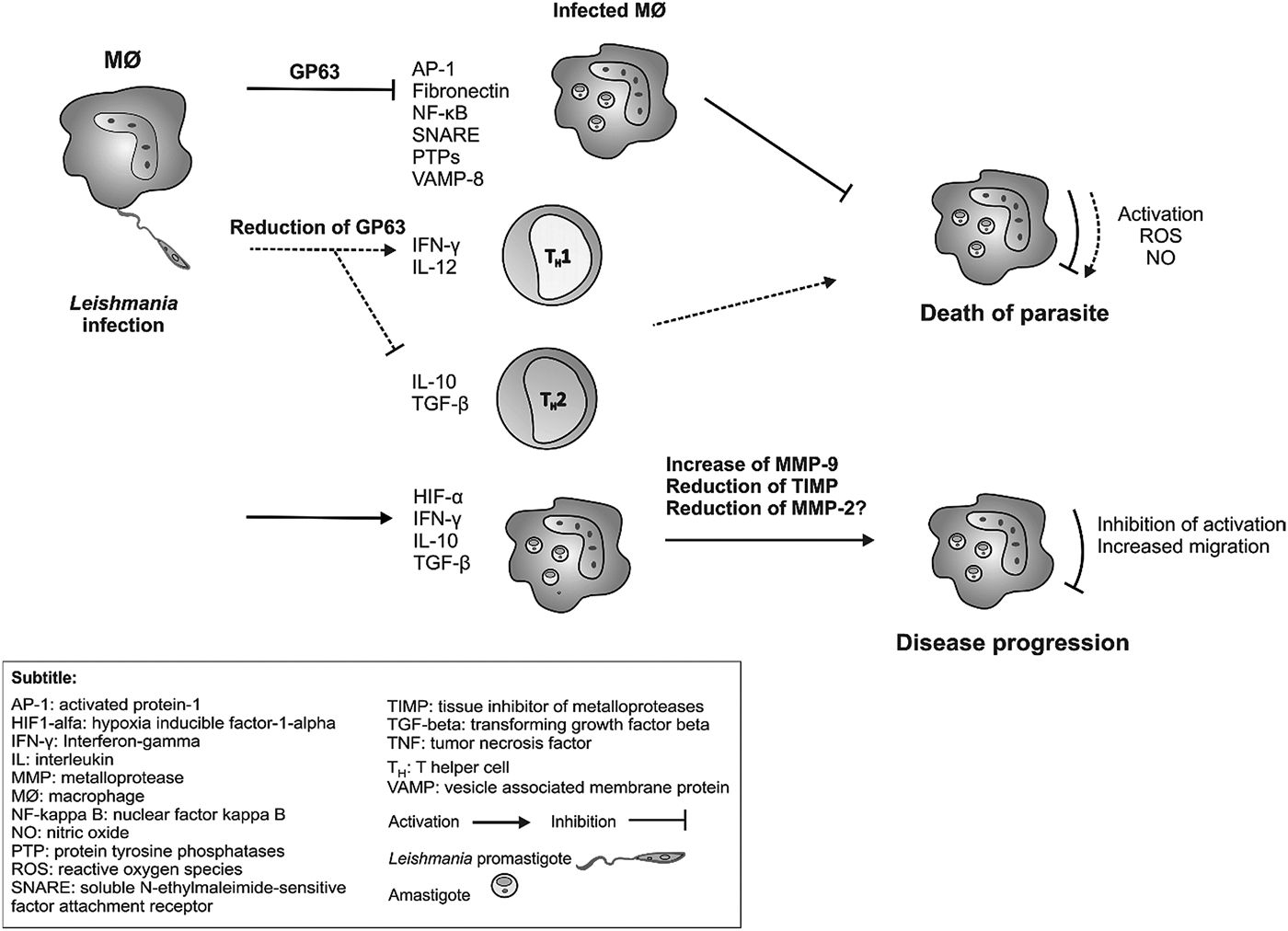
Fig. 2. Role of GP63 and MMP-9 metalloproteases in the induction of immune response during New World Leishmania sp. infection (hypothetical scheme). GP63 of Leishmania sp. promotes the cleavage of several molecules involved in the activation of macrophages, such as activated protein-1 (AP-1), fibronectin, nuclear factor kappa B (NF-kappa B), soluble N-ethylmaleimide-sensitive factor attachment receptor (SNAREs), protein tyrosine phosphatases (PTPs) and vesicle associated membrane protein 8 (VAMP-8). This inhibition of the macrophage does not lead to the production of microbicidal substances, such as oxygen reactive species (ROS) and nitric oxide (NO) and, consequently, does not lead to the death of the parasite. Some studies show that the reduction of GP63 is able to induce the production of cytokines interferon-gamma (IFN-γ) and interleukin (IL) 12 by Th1 cells and macrophages, which in turn activate the macrophages to produce the microbicidal substances with consequent death of the parasite. Furthermore, this reduction of GP63 is able to inhibit the production of IL-10 and to transform growth factor beta (TGF-β) by reducing the Th2-type response. During the infection, cells from the phagocytic mononuclear system produce IFN- γ, IL-10, TGF-β and hypoxia-induced factor 1-alpha (HIF1-α), which stimulate increased production of metalloprotease 9 (MMP-9) and (TIMP) inhibitor, all of which promote macrophage inhibition and cell migration to other tissues, persistence of infection and disease progression.
In Leishmania tarentolae infection (not included for this review), GP63 was mentioned as responsible for the cleavage of vesicle-associated membrane proteins (VAMP8) and vesicular surface proteins (SNARE, Soluble NSF Attachment Receptor) present in phagocytic phagosomes. GP63 cleaves a subgroup of SNAREs, including VAMP8, and this phenomenon prevents the binding of the NADPH oxidase complex to phagosomes by altering the pH and degradation properties of phagosomes. Therefore, the presentation of the exogenous antigens of Leishmania by MHC molecules (Major Histocompatibility Complex) class I does not occur, so that there is reduced activation of T lymphocytes, persisting infection and significantly reducing parasitic death (Matheoud et al. Reference Matheoud, Moradin, Bellemare-Pelletier, Shio, Hong, Olivier, Gagnon, Desjardins and Descoteaux2013).
GP63 also contributes to the reduction of immune functions in the early stages of infection through changes in the activity of protein 1 (AP-1), a transcription factor that controls gene regulation in response to physiological and pathological stimuli, such as cytokines, growth factors, stress signals, apoptosis, bacterial and viral infections and oncogenic responses. Changes in AP-1 caused by GP63 during infection by Leishmania sp. allow an adaptation of the parasite to the harmful intracellular environment, leading to its survival and spreading of the infection through the organism (Contreras et al. Reference Contreras, Gómez, Nguyen, Shio, McMaster and Olivier2010).
From these GP63 articles, three studies reported biases, such as lack of information on clinical data and injury time (Cuervo et al. Reference Cuervo, Santos, Alves, Menezes, Silva, Britto, Fernandes, Cupolillo and de Jesus2008; Kulkarni et al. Reference Kulkarni, Jones, Mcmaster, Mcgwire, Kulkarni, Jones, Mcmaster and Mcgwire2008; Lima et al. Reference Lima, Elias, Souza, Santos and Dutra2009). Petropolis et al. (Reference Petropolis, Rodrigues, Viana, Pontes, Pereira and Silva-Filho2014) used the stationary phase promastigotes and not enriched or metacyclic forms. The research group has not accessed the metacyclic/procyclic ratio of the promastigote culture so that it is possible that these forms behavior in 3D cultures. Also, other classes of proteases could not be detected by zymography under the conditions tested, which may limit the use of this method for Leishmania proteases. Tupperwar et al. (Reference Tupperwar, Vineeth, Rath and Vaidya2008) suggest that the qRT-PCR performed using varied labeled fluorescent probes become limited for the detection of unique DNA sequences that could be involved in the detection of GP63.
Host metalloproteases
Cells of the immune response produce MMP and other proteases involved in the pathogenesis of the disease. In this review, the selected articles reported that immune cells, mainly activated macrophages, produce high levels of MMP-9 during infection by New World Leishmania species. This enzyme has been considered one of the factors responsible for digesting extracellular matrix components and regulating microvascular permeability in the primary inflammatory response, in addition to being involved in leukocyte recruitment (Reichel et al. Reference Reichel, Rehberg, Bihari, Moser, Linder, Khandoga and Krombach2008). Although the macrophages are the main route of infection elimination, the parasite possesses escape mechanisms of immune response allowing its survival and dissemination, which alters the production of cytokines and other mediators of immune response (Oliveira et al. Reference Oliveira, Ribeiro, Schrieffer, Machado, Carvalho and Bacellar2014).
MMP-related studies were conducted in Brazil (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb; Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014). From the five articles included in this review, two performed in vitro studies (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014), one conducted a retrospective study (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012), one a prospective study (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a) and another one clinical essays (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a). Four studies have reported a statistical analysis of their results (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb; Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014). The cutaneous (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014), and mucosal (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012) clinical forms were studied. The species of Leishmania related were L. braziliensis (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb) and one did not report the specie (Table 3). Regarding the objectives of the selected articles, three studies have investigated the role of MMP-9 in the pathogenesis and development of infection in human macrophages (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a), cells from human skin fragments (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b), peripheral blood cells and biopsy of lesions (Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014). Also, Maretti-Mira et al. (Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b) also investigated the role of MMP-2 in chronic cutaneous form and skin re-epithelialization.
Table 3. Characteristics of selected articles on the role of host metalloproteases in New World Leishmaniasis

NR, not related; CL, cutaneous Leishmaniasis; ML, mucosal Leishmaniasis; ATL, American tegumentary Leishmaniasis; ACL, American cutaneous Leishmaniasis; Y, yes; HIF-1a, hypoxia inducible factor-1-alpha; VEGF-A, vascular endothelial growth factor A; VEGFR2, vascular endothelial growth factor; MMP, metalloprotease.
The studies used blood, (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a; Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014), human macrophages (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a), skin fragments (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b), skin lesions (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012) and culture of promastigotes and amastigotes of L. braziliensis (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a). The methods for detection of MMP, which were used in the selected studies, were zymography (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb), immunoprecipitation (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a), Western blotting (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a), RNA Real Time PCR (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a, Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmezb), immunohistochemistry (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b; Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012), flow cytometry (Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014) and ELISA (enzyme-linked immunosorbent assay) (Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014) (Table 4).
Table 4. Samples, methods of detection and completion of articles included on host metalloproteases in New World Leishmania sp.

kDa, kilodalton; WB, Western Blotting; FC, flow cytometry; MMPs, metalloproteases; IHC, Immunohistochemistry; IP, immunoprecipitation; qPCR, quantitative polymerase chain reaction; ELISA, enzyme-linked immunesorbent assay; ML, mucosal Leishmaniasis; LCL, localized cutaneous Leishmaniasis; IFN, interferon gamma; IL, interleukin; TGF, transforming growth factor; VEGFR2, vascular endothelial growth factor; HIF-1, hypoxia inducible factor-1-alpha; VEGF-A, vascular endothelial growth factor A; MIP1a, macrophage inhibitory protein 1a.
Whereas some studies associate MMP-9 as prognostic biomarker for visceral leishmaniasis (de Oliveira et al. Reference de Oliveira, Vanessa Oliveira Silva, Damascena, Passos, Duthie, Guderian, Bhatia, de Moura, Reed, de Almeida and de Jesus2013; Gadisa et al. Reference Gadisa, Tasew, Abera, Gelaye, Chanyalew, Abebe, Laskay and Aseffa2017), one study related that MMP-9 may represent a biomarker to prognosticate an increased risk for parasite dissemination and development of mucosal leishmaniasis (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a). The high levels of this enzyme indicate an increased risk of parasitic spread and development of the mucosal form of leishmaniasis. Macrophage cultures infected by L. braziliensis increased MMP-9 activity levels, so the authors suggest that the infection stimulates macrophages to increase MMP-9 production, which is related to increased cell migratory activity. This shows that the metalloprotease plays an important role in host defense against the progression of leishmaniasis. Furthermore, macrophages from patients with different forms of ATL showed several patterns in the production and activity of MMP-9. Some studies obtained human monocyte-derived macrophages from peripheral blood mononuclear cells (PBMC) and isolated from patients with the mucosal leishmaniasis. In these cells increased levels of MMP-9 were observed before and after infection when compared with samples from patients with localized cutaneous form. The superior gelatinolytic activity of macrophages from individuals that have evolved into the mucosal form may be related to the migration of these cells to other tissues. The matrix degradation acts facilitating this migration process to tissues such as the mucosa, leading the individual to develop the mucosal form of the disease. Conversely, macrophages from individuals with reduced MMP-9 activity may have a L. braziliensis infection controlled at the entry site, so that these individuals develop only the localized cutaneous form of the disease (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a).
Thus, the therapeutic modulation of MMP-9 could be a useful approach to improve the prognosis of patients infected with L. braziliensis (Maretti-Mira et al. Reference Maretti-Mira, de Pinho Rodrigues, de Oliveira-Neto, Pirmez and Craft2011a). The patients in the pre-ulcerative stage of the disease produce lower levels of MMP-9 than patients in the ulcerative phase, suggesting that progression of the disease to the ulcerative phase may be associated with increased MMP-9 levels, as well as by an imbalance between MMP-9 and its inhibitor, TIMP-1. These results corroborate with the studies cited above, which suggest that the monitoring of MMP-9 early in the disease to establish the likelihood of clinical progression and therapeutic cure. The inflammatory cytokine TNF (tumor necrosis factor), besides playing a role in cellular apoptosis and other biological processes, also participates in the induction of metalloproteases. In cutaneous leishmaniasis, TNF works in the regulation of MMP-9, since the addition of exogenous TNF increases the production of MMP-9 in healthy patients, whereas the neutralization of this cytokine negatively regulates the synthesis of MMP-9 in patients with the cutaneous form of the disease. The excessive production of TNF by monocytes from patients with the cutaneous form increases the production of MMP-9 leading to the imbalance of the enzyme and its inhibitor, degradation of the basal membrane, migration of inflammatory cells and development of the lesion. These results propose that TNF cytokine is the primary regulator of MMP-9 production during L. braziliensis infection (Campos et al. Reference Campos, Passos, Novais, Beiting, Costa, Queiroz, Mosser, Scott, Carvalho and Carvalho2014).
In addition to TNF and TIMP-1, other factors are involved in the increased production of MMP-9 by immune cells, such as hypoxia-inducible factor-1 (HIF1-α). This factor, when associated with its beta subunit, acts as a transcription factor of VEGF-A (vascular endothelial growth factor A) in the lesions of patients infected with Leishmania sp. These factors are involved in essential body processes, such as embryogenesis, angiogenesis, cell proliferation, metastasis and also play an important role in response to hypoxia. The HIF1-α protein is maintained in response to hypoxia. HIF1-α is expressed mainly in parasitophorous vacuoles and cytoplasm of infected macrophages. In leishmaniasis lesions, a microcirculation deficiency, leukocyte metabolic demand, parasite proliferation and secondary bacterial infection are observed, with HIF1- α protein representing a hypoxic event in the lesion (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012). There is evidence that HIF1-α accumulates in the inflamed tissue of Leishmaniasis lesion due to the presence of macrophages (Arrais-Silva et al. Reference Arrais-Silva, Paffaro, Yamada and Giorgio2005). In the study by Fraga et al. (Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012), the researchers observed an increase in MMP-9 in the group of individuals who had high levels of HIF1-α. Also, levels of VEGF-A and VEGFR2 (vascular endothelial growth factor receptor 2) are also positively correlated with those of MMP-9. Since HIF1-α factor increases the expression of VEGFR2 and MMP-9, inflammatory cellular response and angiogenesis in leishmanial lesion sites are promoted (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012). The increase of MMP-9 occurs along with HIF1-α and VEGF (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012), the expression of HIF1-α may induce the elements necessary to provide the establishment of angiogenesis in the inflammatory response, aiding these specific sites of lesions caused by Leishmania (Zarember and Malech, Reference Zarember and Malech2005).
In the present review, just one study related MMP-2 to ATL (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b), while concerning VL, there are some studies describing its role in the pathogenesis of disease in human and dogs (Machado et al. Reference Machado, Melo, Moraes, Souza, Marcondes, Perri and Vasconcelos2010; Marangoni et al. Reference Marangoni, Melo, Moraes, Souza, Perri and Machado2011; Melo et al. Reference Melo, Marangoni, Marcondes, Lima and Machado2011; Melo et al. Reference Melo, Marcondes and Machado2012). In ATL, Maretti-Mira et al. (Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b) reported low levels of MMP-2 and high levels of MMP-9 gelatinolytic activity in lesions of patients with low response to antimony treatment. This enzymatic profile may be related to the increased number of cells producing IFN-γ, TGF-β (growth-transforming factor) and IL-10. The increase of MMP-2 is also associated with the process of re-epithelialization of the lesions and therapeutic success. In cutaneous leishmaniasis caused by L. braziliensis, various cytokine profiles can be found in the lesions. The TGF-β and IL-10 cytokines characterize a Th2-type cellular immune response profile, being related to persistent infections and chronic lesions, whereas IFN-γ is important in the resolution of cutaneous form. Lesions from individuals with low response to treatment had cells producing IFN-γ and high levels of MMP-9 gelatinase activity; this shows that the production of these cytokines in recent lesions suggests that the first few months are the most important to establish an effective immune response that can result in the success or failure of treatment. The high levels of proinflammatory cytokines found in lesions of patients with low response also suggest that excessive IFN-γ may have the opposite effect and impair resolution of lesions, whereas, in individuals with a good immune response, the predominance of these cytokines such as IFN-γ may be responsible for the low level of gelatinolytic activity of MMP-9 in the lesions (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b).
IL-10 appeared to be the only cytokine capable of suppressing the production and activity of metalloproteases, thus playing a necessary role in protecting the matrix during infection. These results show the importance of gelatinases activity in skin lesions of leishmaniasis. The modulation of IL-10, IFN-γ and TGF-β may be responsible for the activity pattern of the metalloproteinases early in the infection, and may influence the persistence or cure of lesions (Maretti-Mira et al. Reference Maretti-Mira, De Oliveira-Neto, Da-Cruz, De Oliveira, Craft and Pirmez2011b; de Oliveira et al. Reference de Oliveira, Vanessa Oliveira Silva, Damascena, Passos, Duthie, Guderian, Bhatia, de Moura, Reed, de Almeida and de Jesus2013; Oliveira et al. Reference Oliveira, Ribeiro, Schrieffer, Machado, Carvalho and Bacellar2014) (Fig. 2).
Regarding biases, only one study reported them. The limitation of the study was the lack of information on the clinical data of lesions and their time of evolution. Therefore, to confirm its results, in vitro and in vivo studies could clarify the mechanism underlying the induction and activity of the factors involved in cutaneous leishmaniasis (Fraga et al. Reference Fraga, Oliveira, Alves, Viana, Sousa, Carvalho, De Paula, Botelho and Guimarães2012).
Conclusions
In this systematic review, we found sufficient literature about parasite metalloproteases related pathogenicity, virulence and specie detection, but some species have not been studied and their role remains unclear, such as Leishmania lainsoni, L. naiffi, L. peruviana and L. infantum (causing atypical CL and ATL). It was observed that the GP63 complex has different enzymatic profiles according to the Leishmania species that causes ATL in the New World. GP63 is significantly related to the development and establishment of the infection. It can inhibit complement system activity, stimulate the degradation of fibronectin and promote cleavages of transcriptional molecules, such as NF-κB, membrane proteins and tyrosine phosphatases. In addition, GP63 has been shown as an essential virulence factor in the establishment of infection, contributing to the reduction of immune functions in the early stages of the disease, which provides the adaptation of the parasite to the intracellular environment and allows the survival and propagation of the protozoan to other tissues of the body.
Based on the results of this review, there are few studies on host metalloprotease involved in ATL, but there is a consensus that these studies have high methodology quality and scientific rigor, and, due to it, we considered relevant to compile the results about host metalloprotease. Thus, it becomes evident that new clinical and experimental studies are needed to demonstrate the role of these enzymes and the mechanisms that are involved in the progression of cutaneous and mucocutaneous leishmaniasis caused by New World Leishmania.
Among the host matrix metalloproteases, MMP-9 can be considered a biomarker of bad prognostic that indicates a high risk of parasite dissemination and development to the mucosal form of leishmaniasis, especially if detected at the beginning of the cutaneous lesion. Also, its modulation may improve prognosis in L. braziliensis infections and may be considered a therapeutic and laboratory follow-up target. Since MMP-2 has also been considered an important marker to diagnosis and prognosis of VL in human and dogs, MMP-2 activity appears to be involved with therapeutic success in ATL when present at elevated levels and with the re-epithelialization process, but other studies must be conducted to affirm the potential of MMP-2 for the ATL development. From this systematic review of the literature on the role of metalloproteases in ATL, it is suggested that the serological and tissue levels of MMP-9 should be monitored early in the onset of the cutaneous lesion and used as a marker of disease progression to the mucosal form. The monitoring of MMP-9 levels could be performed using Western Blot, zymography and Real Time PCR in serological and tissue samples. The inclusion of one or more techniques in the laboratory routine can improve the diagnosis and monitoring of cases of cutaneous leishmaniasis, since, if it is detected in the first months of infection, it can predict the prognosis of the clinical form and resolution of leishmaniasis after treatment.
Acknowledgement
The authors gratefully acknowledge Dr Ricardo Moliterno of Universidade Estadual de Maringá for his valuable suggestions and careful analysis of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
Nothing to declare.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018000367









