Introduction
The Nematoda is a diverse phylum of worm-like, free-living or parasitic animals that plays an important role in the decomposition process and recycling of nutrients in the aquatic environment (free-living forms) to the extent that it may influence host population dynamics (parasitic forms). This is considered the fifth most diverse phylum of metazoans, behind the Arthropoda, Mollusca, Craniata and Platyhelminthes (Hodda, Reference Hodda2022a, Reference Hodda2022b). Currently, almost 30 000 species of nematodes have been recognized as valid, but, according to the minimum estimate, the real number of species is around 500 000, half of which are represented by parasites (Hodda, Reference Hodda2022a, Reference Hodda2022b). This overwhelming diversity has represented a real challenge for better understanding these parasites, especially regarding their taxonomy and systematics that represent the basis for investigation of more complex questions related, for example, to life cycles, pathology and other host–parasite interactions (Moravec, Reference Moravec1998; Padial et al., Reference Padial, Miralles, de la Riva and Vences2010).
In aquatic environments, parasitic nematodes can be found within several different trophic levels, representing foodweb links (Anderson, Reference Anderson2000; Lafferty et al., Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marrcogliese, Martínez, Memmott, Marquet, McLaughlin, Mordecal, Pascual, Poulin and Thieltges2008; Lafferty, Reference Lafferty2013). In this sense, fish can act as paratenic, intermediate or definitive hosts for nematodes (Anderson, Reference Anderson2000), in which certain taxa of parasites, especially from marine environment, are important as zoonotic agents or causative of serious fish diseases resulting in considerable losses and problems for the seafood, fishing and fishery industries (Moravec and de Buron, Reference Moravec and de Buron2013; Shamsi and Suthar, Reference Shamsi and Suthar2016; Mattiucci et al., Reference Mattiucci, Cipriani, Levsen, Paoletti and Nascetti2018). This reinforces the importance of these organisms by their ecological, economic and health implications in addition to their high biodiversity potential.
Since the first mention of nematode parasites of fish from American waters by Rudolphi (Reference Rudolphi1819), it is possible to visualize 2 clear scenarios: firstly, these are still neglected organisms (Scholz and Choudhury, Reference Scholz and Choudhury2014; Luque et al., Reference Luque, Pereira, Alves, Oliva and Timi2017), and secondly, there are imbalances on the study efforts carried out on freshwater compared with marine environments, depending on the geographic area. For example, in South America, the number of studies including nematode parasites of freshwater fish is much higher than those marine (Luque et al., Reference Luque, Pereira, Alves, Oliva and Timi2017). An opposite situation is observed in Canada, where studies on nematodes from marine fish are more numerous (Arai and Smith, Reference Arai and Smith2016).
One of the best sources of assistance for estimating biodiversity patterns of a group are checklists with accurate taxonomic data and, in case of parasites, information on host and geographic occurrence. Currently, such checklists dealing with the parasitic nematodes of fish in the American continent are scattered, geographically limited to a local scale and mixed with other metazoan groups (see McDonald and Margolis, Reference McDonald and Margolis1995; Garrido-Olvera et al., Reference Garrido-Olvera, Garcia-Prieto and Pérez-Ponce de Léon2006; Luque et al., Reference Luque, Aguiar, Vieira, Gibson and Santos2011, Reference Luque, Cruces, Chero, Paschoal, Alves, Da Silva, Sanchez and Iannacone2016; Arai and Smith, Reference Arai and Smith2016; Santos et al., Reference Santos, Lopes, Gibson, Eiras, Velloso and Pereira2016; Lehun et al., Reference Lehun, Hasuike, Silva, Ciccheto, Michelan, Rodrigues, Nicola, de Lima, Correia and Takemoto2020; Santos-Reis et al., Reference Santos-Reis, Santos, Nunes and Mugnai2021; Ramallo and Ailán-Choke, Reference Ramallo and Ailán-Choke2022). This scenario along with taxonomic problems (e.g. inadequate description and dubious diagnosis of species) hampers the understanding of the real and general biodiversity patterns associated with nematode parasites of marine fish from the Americas, and its configurations within host and geographic gradients.
With the technological advances of genetic studies in the last 20 years, the systematics of Nematoda has changed significantly (see Blaxter et al., Reference Blaxter, De Ley, Garey, Liu, Scheldeman, Vierstraete, Vanfleteren, Mackey, Dorris, Frisse, Vida and Thomas1998; De Ley and Blaxter, Reference De Ley, Blaxter and Lee2002, Reference De Ley and Blaxter2004; Meldal et al., Reference Meldal, Debenham, De Ley, De Ley, Vanfleteren, Vierstraete, Bert, Borgonie, Moens, Tyler, Austen, Blaxter, Rogers and Lambshead2007; Anderson et al., Reference Anderson, Chabaud and Willmott2009). In fact, genetic approaches have been crucial for the advancement of knowledge pertaining to nematodes reported parasitizing marine fish in the American continent, such as supporting species validity, improving identification of larval forms and clarifying phylogenetic relationships (see e.g. Mejía-Madrid and Aguirre-Macedo, Reference Mejia-Madrid and Aguirre-Macedo2011a, Reference Mejía-Madrid and Aguirre-Macedo2011b; Sardella et al., Reference Sardella, Pereira and Luque2017; Roca-Geronès et al., Reference Roca-Geronès, Montoliu, Godínez-González, Fisa and Shamsi2018; Aguilar-Aguilar et al., Reference Aguilar-Aguilar, Ruiz-Campos, Martorelli, Montes and Martínez-Aquino2019; Barton et al., Reference Barton, Moravec, Zhu and Shamsi2022). However, the related genetic databases still remain very scarce proportionally to the biodiversity of the group.
Based on the importance of the nematode parasites of marine fish from off the American continent previously highlighted, a review on the aspects of the diversity of these organisms is herein shown, associating it with host taxa and geographic distribution, as well as aspects of their current taxonomy, systematics and knowledge on life cycles, genetics and phylogeny.
Survey of species recorded and related data
A review of the parasitic nematodes in marine fish from off the Americas (including the Caribbean Islands and Hawaii) is presented herein. Only reports of adult specimens identified to species level and occurring in hosts from marine or brackish environments were included. There are some reports of nematodes in anadromous and catadromous fishes which were excluded from the present survey, since they are nematode genera or species of freshwater origin, such as Camallanus, Salmonema, Salvelinema and Truttaedacnitis. The taxonomic classification of Hodda (Reference Hodda2022a) was followed, although some taxonomic authorities were changed. The review was based on an extensive literary search gathered from different databases, i.e. Google Scholar, Web of Science and Biological Abstracts, and also supplemented by data from the Host-Parasite Database of the Natural History Museum, London, UK (Gibson et al., Reference Gibson, Bray and Harris2005), the World Register of Marine Species (WoRMS Editorial Board, 2022) and personal libraries. Data from the so-called ‘grey literature’ (theses) were excluded, since they represent unverified and/or unpublished information. GenBank was used as a source for searching of genetic data of the nematode species.
Patterns of nematode diversity associated with host taxa
Species richness was used as the indicator for evaluating diversity of the nematodes parasitizing marine fish from off the American continent. As previously stated, only those reports of nematodes identified to species were considered; consequently, most reports of larval forms were not accounted, except when molecular identification was used. For the association of nematode diversity with host taxa orders, including those recently proposed incertae sedis, and families of bony and cartilaginous fish were considered, according to Froese and Pauly (Reference Froese and Pauly2022).
A total of 209 nematode species were accounted in 504 marine fish from 46 orders and 114 families. Chondrichthyan hosts were much less numerous than Osteichthyes, represented by 62 (12% of the total) species from 10 (22%) orders and 17 (15%) families. The highly diverse order Perciformes was by far the most representative with 27 (24%) families and the second was the incertae sedis taxon Eupercaria that included 11 families (10%), most of which were previously allocated in Perciformes (see Betancur-R et al., Reference Betancur-R, Wiley, Arratia, Acero, Bailly, Miya, Lecointre and Orti2017). In addition to Carcharhiniformes and Centrarchiformes that were represented by 5 (4%) and 4 (3%) families, respectively, the remaining orders of bony and cartilaginous fish were represented by less than 3 families, most of which included only 2 or 1.
The nematode diversity followed that observed for host taxa, in which a total of 96 (45%) and 66 (31%) species were reported parasitizing the most diverse host groups, Perciformes and Eupercaria, respectively (Fig. 1A). The highest numbers of nematode species were also observed in families belonging to Eupercaria (Scieanidae and Lutjanidae) and Perciformes (Serranidae) (Fig. 1B). Parasite diversity usually is proportional to that of their hosts (Hechinger and Lafferty, Reference Hechinger and Lafferty2005; Poulin, Reference Poulin2014), which may be a result of numerous pathways for speciation processes. Moreover, combined with the high diversity, many species of sciaenid, lutjanid and serranid fish have commercial importance (Polovina and Ralston, Reference Polovina and Ralston1987; Chiappone et al., Reference Chiappone, Sluka and Saeley2000; Claro et al., Reference Claro, Lindeman and Parenti2001; Ramcharitar et al., Reference Ramcharitar, Gannon and Arthur2006), attracting interest for parasitological studies on such hosts. In this sense, other fish families including species with remarkable economic value, i.e. Carangidae, Paralichthyidae and Scombridae (Honebrink, Reference Honebrink2000; Astarloa, Reference Astarloa2002; Juan-Jordá et al., Reference Juan-Jordá, Mosqueira, Freire, Ferrer-Jordá and Dulvy2016), showed nematode richness that stands out in relation to most of the others (Fig. 1B). Only 2 or 1 nematode species have been reported in the orders Chimeariformes, Hexanchiformes, Squatiniformes and Torpediniformes (all Chondrichthyes) and Acanthuriformes, Albuliformes, Cichliformes, Lampriformes, Mugiliformes, Myliobatiformes, Osmeriformes, Ovalentaria (incertae sedis), Syngnathiformes and Zeriformes (all Osteichthyes), as well as in several families of Perciformes (see Supplementary Material 1). These numbers do not necessarily mean that the nematode richness is indeed low in these host taxa, but most likely indicates that some of these fish can harbour larval stages and not adults considered in the present review or that parasitological studies are incipient for most of them, especially those focused on nematode taxonomy.
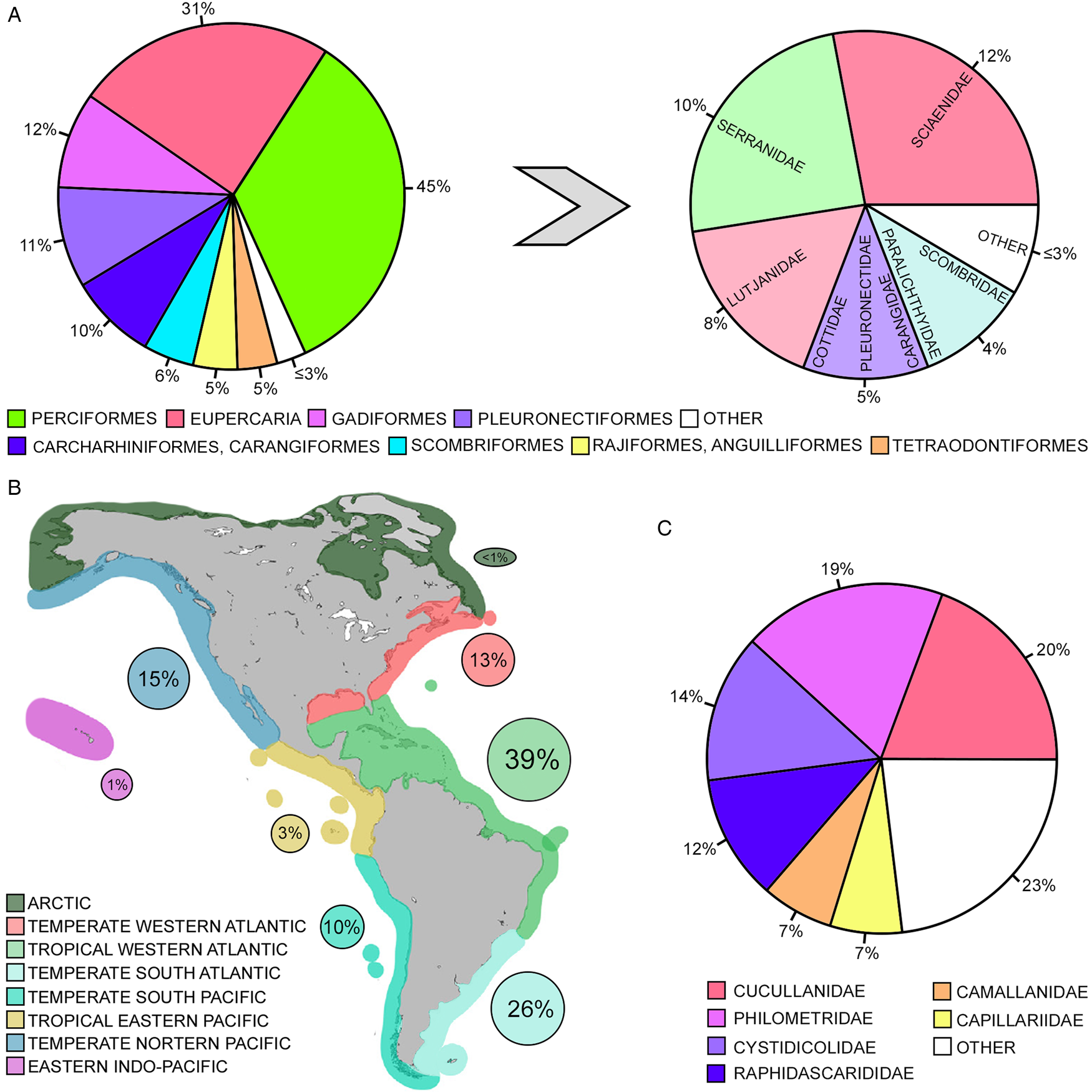
Fig. 1. Graphic representations (percentage, %) of the fish orders and families (A), marine regions (B) and nematode families (C) related to the 209 nematode parasites in marine fish of the Americas. More details regarding number of nematode species by host taxa, geographic distribution and parasite taxa are shown in Supplementary Materials 1 and 2.
Currently, specific studies estimating only nematode diversity in marine fish from the American continent are inexistent. Studies that include diversity estimations normally analyse parasite community structures rather than be focused on specific parasite taxa. Moreover, there is no approach specifically relating nematode diversity with that of fish taxa in the referred region, which complicates a general and conclusive discussion regarding the subject. Almost all studies that deal with parasite diversity, including nematodes, and its relations with marine fish populations in off the Americas are restricted to 1 or few host species within a limited geographic area. The study by Luque and Poulin (Reference Luque and Poulin2008) represents one of the few exceptions, in which authors performed a large-scale investigation on factors influencing parasite diversity in Neotropical marine fish. These authors concluded that host traits were weakly correlated with parasite species richness; however, habitat exploitation, diet and behaviour intrinsic to fish species were important for taxonomic diversity of parasites. Recently, Silva et al. (Reference Silva, Benicio, Moreira, Paschoal and Pereira2022) observed similar results on parasites of clupeiform fish in South Atlantic, indicating that fish species is a strong determinant for parasite diversity, especially their diet composition and infection by endoparasites as nematodes, which are mostly transmitted by food chain links in marine environments (Lafferty, Reference Lafferty2013). It should be highlighted that several approaches including parasite diversity estimations use parasites as biological tags for discriminating host populations from the South Atlantic and Pacific Oceans, where nematodes always show representative richness in the parasite communities (Timi, Reference Timi2003; Timi et al., Reference Timi, Luque and Sardella2005, Reference Timi, Lanfranchi and Luque2010; Braicovich and Timi, Reference Braicovich and Timi2010; Canel et al., Reference Canel, Levy, Soares, Braicovich, Haimovici, Luque and Timi2019; Espínola-Novelo and Oliva, Reference Espínola-Novelo and Oliva2021).
Based on the present survey, patterns of nematode diversity in marine waters of the American continent are proportional to that of their host taxa, in which Perciformes and Eupercaria that allocate highly diverse fish families support the richest nematofauna. It cannot be discarded that several parasitological studies including nematode parasites of marine fish in the Americas are driven by economic importance of host species (Luque et al., Reference Luque, Pereira, Alves, Oliva and Timi2017; see also the following examples Cantatore and Timi, Reference Cantatore and Timi2015; George-Nascimento and Oliva, Reference George-Nascimento and Oliva2015). In this sense, a significant part of these studies is focused on larval forms of Anisakidae and Raphidascarididae due to their zoonotic potential (Siendermann, Reference Siendermann1961; Kuhn et al., Reference Kuhn, Cunze, Kochmann and Klimpel2016; Alves et al., Reference Alves, Souza, Takemoto, Melo, Madi and Jeraldo2020; Ferreira et al., Reference Ferreira, Rojas, Queiroz, Vidal, Fonseca, Júnior, Luque and Oliveira2020; Diniz et al., Reference Diniz, Knoff, Fonseca, Gomes and São Clemente2022). The nematode larval forms were excluded for diversity estimations, since their identification is mostly not to species level reducing the precision of the results or making them biased. Despite the current scenario of nematode diversity among the taxa of marine fish herein observed indicates interesting trends, it is still superficial since several fish species remain unstudied for parasites and the parasite fauna of hosts from several orders and families are poorly known.
Regional patterns of nematode diversity
For analysing regional patterns of nematode diversity along the coast of the American continent, species richness was accounted by marine ecoregions according to the classification of Poore and Bruce (Reference Poore and Bruce2012). About 78% of the nematode species in the present survey was reported in the coast of the Atlantic Ocean vs only 29% in the Pacific. The highest percentage of species (39%) was observed in the tropical part of the Atlantic, followed by that observed in the temperate southern region of the same ocean (26%) (Fig. 1B). These Atlantic ecoregions were by far the most representative in terms of nematode species richness. The tropical and southern temperate regions of the Pacific Ocean accounted only by 3 and 10% of the nematode species, respectively (Fig. 1B). The percentages of nematode species reported in northern temperate zones of the Atlantic and Pacific were 13 and 15%, respectively (Fig. 1B). The less representative regions regarding nematode species were the Arctic and Indo-Pacific, in which 1% or less of the total richness was observed (Fig. 1B).
It has been documented that local characteristics of marine environments may be strongly related to the parasite community structure of fish, including patterns of species richness (Luque and Poulin, Reference Luque and Poulin2008; Merckx et al., Reference Merckx, Goethals, Steyaert, Vanreusel, Vincx and Vanaverbeke2009; Timi et al., Reference Timi, Lanfranchi and Luque2010). In large scale as within latitudinal diversity gradient (LGD), the relationships between endoparasites of fish and diversity are unclear (Poulin, Reference Poulin1995; Rohde and Heap, Reference Rohde and Heap1998; Rohde, Reference Rohde1999; Preisser, Reference Preisser2019). However, for both bony and cartilaginous fish, LGD is clear in that species richness reaches its maximum in low latitudes (i.e. intertropical zone) and decreases in high latitudes (i.e. temperate and polar zones) (Miloslavich et al., Reference Miloslavich, Klein, Díaz, Hernández, Bigatti, Campos, Artigas, Castillo, Penchaszadeh, Neill, Carranza, Retana, de Astarloa, Lewis, Yorio, Piriz, Rodríguez, Yoneshigue-Valentin, Gamboa and Martín2011; Carrillo-Briceño et al., Reference Carrillo-Briceño, Carrillo, Aguilera and Sanchez-Villagra2018; Manel et al., Reference Manel, Guerin, Muillot, Blanchet, Velez, Albouy and Pellissier2020). Moreover, fish diversity is higher in the Atlantic than in the Pacific coast of America (Miloslavich et al., Reference Miloslavich, Klein, Díaz, Hernández, Bigatti, Campos, Artigas, Castillo, Penchaszadeh, Neill, Carranza, Retana, de Astarloa, Lewis, Yorio, Piriz, Rodríguez, Yoneshigue-Valentin, Gamboa and Martín2011; Carrillo-Briceño et al., Reference Carrillo-Briceño, Carrillo, Aguilera and Sanchez-Villagra2018; Manel et al., Reference Manel, Guerin, Muillot, Blanchet, Velez, Albouy and Pellissier2020). This uneven diversity of hosts, according to oceans and marine ecoregions, most likely is the main factor responsible for the regional patterns of nematode diversity observed here, since parasite diversity tends to be accompanied by that of hosts as previously discussed (Hechinger and Lafferty, Reference Hechinger and Lafferty2005; Poulin, Reference Poulin2014). An interesting observation is that the diversity of possible intermediate hosts for nematode parasites of marine fish, such as crustaceans and molluscs (Anderson, Reference Anderson2000; Lafferty, Reference Lafferty2013), is more or less similar between the Atlantic and Pacific Oceans (Miloslavich et al., Reference Miloslavich, Klein, Díaz, Hernández, Bigatti, Campos, Artigas, Castillo, Penchaszadeh, Neill, Carranza, Retana, de Astarloa, Lewis, Yorio, Piriz, Rodríguez, Yoneshigue-Valentin, Gamboa and Martín2011), reinforcing that differences on fish diversity may be important for the regional patterns of nematode diversity observed here.
Another important fact is that the most prolific research groups on parasites of marine fish in the Americas, including nematode taxonomists, are based near the Atlantic coast and, consequently, most of their studies are developed along this region, boosting the knowledge about nematode diversity. It should be highlighted that the number of nematode species described, infecting marine fish in the Atlantic coast, has been increasing considerably in the last 20 years as a result of the work of research groups mainly from Argentina (e.g. Timi and Sardella, Reference Timi and Sardella2002; Timi and Lanfranchi, Reference Timi and Lanfranchi2006; Timi et al., Reference Timi, Rossin and Lanfranchi2006, Reference Timi, Rossin, Lanfranchi and Etchegoin2007, Reference Timi, Lanfranchi, Tavares and Luque2009; Rossin and Timi, Reference Rossin and Timi2009; Rossin et al., Reference Rossin, Incorvaia and Timi2012), Brazil (e.g. Santos et al., Reference Santos, Lent and Gibson2004; Pereira et al., Reference Pereira, Timi, Vieira and Luque2012, Reference Pereira, Pereira, Timi and Luque2013, Reference Pereira, Pantoja, Luque and Timi2014a, Reference Pereira, de Nazaré Pereira and Luque2014b, Reference Pereira, Vieira and Luque2014c; Paschoal et al., Reference Paschoal, Vieira, Cezar and Luque2014; Vieira et al., Reference Vieira, Pereira, Pantoja, Soares, Pereira, Timi, Scholz and Luque2015) and Mexico (e.g. González-Solís et al., Reference González-Solís, Argáez-García and Guillén-Hernández2002a, Reference González-Solís, Moravec and Vidal-Martínez2002b, Reference González-Solís, Moravec and Tuz Paredes2007a, Reference González-Solís, Tuz-Paredes and Quintal-Loria2007b; Gopar-Merino et al., Reference Gopar-Merino, Osorio-Sarabia and García-Prieto2005; Mejía-Madrid and Pérez-Ponce de León, Reference Mejía-Madrid and Pérez-Ponce de León2007; López-Caballero et al., Reference López-Caballero, Osorio-Sarabia and García-Prieto2009; Mejía-Madrid and Guillén-Hernández, Reference Mejía-Madrid and Guillén-Hernández2011), as well as from USA in collaboration with the renowned Czech taxonomist of nematode parasites of fish Dr František Moravec (e.g. Moravec et al., Reference Moravec, Sasal, Würtz and Taraschewski2005, Reference Moravec, de Buron and Roumillat2006, Reference Moravec, Montoya-Mendoza and Salgado-Maldonado2008a, Reference Moravec, Bakenhaster and Fajer-Avila2010, Reference Moravec, Bakenhaster and de Buron2013, Reference Moravec, Bakenhaster and Fajer-Ávila2014, Reference Moravec, Bakenhaster and Adams2016, Reference Moravec, Bakenhaster and Switzer2020, Reference Moravec, Bakenhaster, Seyoum and Tringali2021). Such increase on species descriptions as a result of actuation of these research groups (see also Moravec et al., Reference Moravec, Santana-Piñeros, González-Solís and Torres-Huerta2007, Reference Moravec, de Buron, Baker and González-Solís2008b, Reference Moravec, Novacovsky and Hernández-Orts2018) is strongly related to the results observed in Fig. 1B.
As a conclusion, regional patterns of nematode diversity in marine fish off the American continent are related to 2 main factors: the stronger activity of research groups and the higher host diversity along the Atlantic coast in comparison to that of Pacific, as well as fish LGD. It should be highlighted that research groups actuating in ecoregions from the Pacific Ocean, for example, from Chile (e.g. Muñoz and George-Nascimento, Reference Muñoz and George-Nascimento2007; Muñoz, Reference Muñoz2010; Cerna et al., Reference Cerna, Torres and Silva2019) have been also describing nematode species in the last 2 decades. However, the number of studies is considerably less numerous in comparison with those in the Atlantic, and most of the species were described from 1950 to 1990 (see Olsen, Reference Olsen1952; Mudry and Dailey, Reference Mudry and Dailey1969; Mateo, Reference Mateo1972a, Reference Mateo1972b; Caballero-Rodríguez, Reference Caballero-Rodríguez1974; Moravec and McDonald, Reference Moravec and McDonald1981; Moravec et al., Reference Moravec, Margolis and McDonald1981; Moravec, Reference Moravec1987). Therefore, evidence indicated that Pacific ecoregions of the American continent, including the Hawaiian Indo-Pacific coast, remain understudied for nematode parasites of fish.
Life cycles and biological considerations
The most comprehensive compilation of life cycle information pertaining to nematode parasites of vertebrates was provided by Anderson (Reference Anderson2000). However, proportionally to the biodiversity of nematode parasites in marine fish, our knowledge regarding their biology and development is rather limited. A common characteristic shared by almost all taxa of parasitic nematodes in marine fish is the indirect transmission (heteroxeny), mediated by trophic relationships, in which both invertebrates and vertebrates are used as intermediate or paratenic hosts (Anderson, Reference Anderson2000; Lafferty et al., Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marrcogliese, Martínez, Memmott, Marquet, McLaughlin, Mordecal, Pascual, Poulin and Thieltges2008; Lafferty, Reference Lafferty2013).
Most literature dealing with the life cycles of nematode parasites in marine fish is focused on the species with foodborne zoonotic potential, mainly those of the family Anisakidae (Anderson, Reference Anderson2000). However, those zoonotic anisakids (e.g. Anisakis, Pseudoterranova) only occur as larval stages in fish, which are used as intermediate or paratenic hosts (Anderson, Reference Anderson2000). The general life cycle aspects of the most representative nematode families, reported in the present study, are presented briefly below.
The biology of trichinelloids in general and, especially regarding those species parasitic in marine fish, remains little known (Worsham et al., Reference Worsham, Huffman, Moravec and Gibson2016), similar to the scenario observed for the less common taxa reported below (e.g. Gnathostomatoidea, Habronematoidea, Oxyuroidea). Eggs of trichinelloids are generally thick-walled, barrel-shaped and provided with polar plug-like structures or filaments, which are laid unembryonated or fully larvated and released to the external environment with host's urine or feces (Moravec, Reference Moravec2001). It is still controversial if hosts became infected by the first-, second- or third-stage larvae, although Worsham et al. (Reference Worsham, Huffman, Moravec and Gibson2016) found the second-stage larva to be infective to the definitive host. The most recent experimental life cycle known of a parasitic nematode related to the marine environment was that of Huffmanela huffmani (Trichinelloidea, Trichosomoididae) (Worsham et al., Reference Worsham, Huffman, Moravec and Gibson2016), thus representing the first elucidated cycle in this genus. This nematode species was found in a freshwater locality (upper San Marcos River, Texas) and seems to be restricted to that area, so it is believed that H. huffmani is a marine-relict, since all other congeners infect marine fish (see Huffman and Moravec, Reference Huffman and Moravec1988). Species of Huffmanela uses aquatic invertebrates (e.g. amphipods) as intermediate hosts, which ingest nematode eggs; first-stage larvae hatch from the eggs and develop in the invertebrate haemocoel into a second stage is infective to the definitive host; such features are commonly observed in trichinelloid nematodes (Anderson, Reference Anderson2000; Worsham et al., Reference Worsham, Huffman, Moravec and Gibson2016).
Members of the Camallanoidea are frequently found in the digestive tract of marine fishes. The life cycle of a number of camallanid species has been investigated (see Anderson, Reference Anderson2000). These parasites are haematophagous and ovoviviparous, as the first-stage larvae hatch in utero and are passed into water, where they are ingested by copepods that act as intermediate hosts (Anderson, Reference Anderson2000). Infective third-stage larvae in the copepod haemocoel will persist if ingested by planktivorous fish, but do not develop beyond the fourth stage in these paratenic hosts. The development will be completed only when larvae are ingested by piscivorous fish through the food chain (Anderson, Reference Anderson2000).
The biology of the Thelazioidea is highly diverse among their families, and rhabdochonids are not the exception, since they are found in the digestive tract and pancreatic ducts of their fish hosts. The life cycle of most species occurring in marine fishes is unknown but like those parasitizing freshwater forms have smooth-shelled eggs with or without floats and filaments, use insects or crustaceans as intermediate hosts and sometimes teleosts may act as intermediate or paratenic hosts (McVicar and Gibson, Reference McVicar and Gibson1975; Anderson, Reference Anderson2000).
The Habronematoidea is another highly biologically diverse superfamily of nematodes and includes economically important groups of tetramerids of the proventiculus of birds and habronematids in horses and certain ruminants (Anderson, Reference Anderson2000). The family Cystidicolidae is the best represented with 27 genera (Moravec, Reference Moravec2007; Moravec and Justine, Reference Moravec and Justine2010; Moravec and Sobecka, Reference Moravec and Sobecka2012), among which several species infect the digestive tract of marine fish. Given the high biodiversity of cystidicolid nematodes, their life cycles are almost unknown; only 9 species of more than 140 have been investigated (Moravec, Reference Moravec2007; Appy and Butterworth, Reference Appy and Butterworth2011). The main intermediate hosts of cystidicolids in marine environments are crustaceans of different types, where nematode larvae attain the infective third-stage prior to ingestion by the fish definitive host (Moravec, Reference Moravec2007). Interestingly, in some cases these larvae can develop early into adulthood and become gravid, thus representing a source of environmental contamination via egg production, as well as being infective to the definitive host (Moravec, Reference Moravec2007). Some small fish are also reported as paratenic hosts (Moravec, Reference Moravec2007), but their role in the transmission of these parasites appears not to be as important as observed for anisakids and raphidascaridids.
The Dracunculoidea is characterized by having large females filled with huge number of first-stage larvae that must be dispersed into the environment and be available to copepod intermediate hosts. One important family is Philometridae, which is highly speciose in marine and freshwater environments and exclusive to fish (Anderson, Reference Anderson2000; Moravec and de Buron, Reference Moravec and de Buron2013). However, life cycles of these parasites have received attention only recently (Perez et al., Reference Perez, Roumillat, Levesque, Connors and de Buron2009; Chávez and Oliva, Reference Chávez and Oliva2011; de Buron et al., Reference de Buron, France, Connors, Roumillat and Tsoi2011; Séguin et al., Reference Séguin, Bouchard, Measures, Uhland and Lair2011; Williams et al., Reference Williams, Moravec, Turnbull and Ferguson2012). Philometrids are tissue-dwelling parasites commonly haematophagous, which may negatively impact their hosts, causing economic losses in economically important fish (Moravec, Reference Moravec2006; Moravec and de Buron, Reference Moravec and de Buron2013). Females of philometrids are ovoviviparous giving birth to first-stage larvae, through bursting their bodies when in contact with water (except Alinema) (Anderson, Reference Anderson2000; Moravec and de Buron, Reference Moravec and de Buron2013). Evidence indicates that the development of philometrids can be seasonal, and that the copepod component of the zooplankton and some prey fish may act as intermediate and paratenic hosts, respectively, for these parasites (see Molnár and Fernando, Reference Molnár and Fernando1975; Perez et al., Reference Perez, Roumillat, Levesque, Connors and de Buron2009; Chávez and Oliva, Reference Chávez and Oliva2011; de Buron et al., Reference de Buron, France, Connors, Roumillat and Tsoi2011; Séguin et al., Reference Séguin, Bouchard, Measures, Uhland and Lair2011; Williams et al., Reference Williams, Moravec, Turnbull and Ferguson2012).
The biology of most members of Gnathostomatoidea have not been studied extensively, except the species of the genera Gnathostoma due to their significance to human and animal health (Anderson, Reference Anderson2000). The genus Echinocephalus is the only one represented in marine fishes and uses probably marine crustaceans (copepods) as intermediate hosts, and molluscs, echinoderms and other marine organisms as paratenic or second intermediate hosts in which growth occurs (Anderson, Reference Anderson2000).
The Oxyuroidea is also a highly diverse superfamily, with families parasitizing herbivorous lower vertebrates, mammals and reptiles (Anderson, Reference Anderson2000). The only genus occurring in marine fishes is Echinocephalus, whose life cycle is unknown, but as other pharyngodonid genera the transmission seems to be direct, without any intermediate hosts (Moravec, Reference Moravec1998).
The Ascaridoidea mostly includes nematode families parasitizing aquatic mammals and fishes. The most remarkable are Anisakidae and Raphidascarididae, the former for including species with zoonotic potential. Anisakids have mostly mammals as definitive hosts and use fish as intermediate and/or paratenic hosts, while raphidascaridids use invertebrates as intermediate hosts (e.g. mysids, copepods, isopods) and fish as paratenic and/or definitive hosts (Anderson, Reference Anderson2000). It is known that the transmission of the most studied raphidascaridid genera (i.e. Goezia, Hysterothylacium and Raphidascaris) involves small aquatic invertebrates (mainly arthropods), fry or other smaller foraging fish as paratenic or intermediate hosts, in which the biological strategy depends upon the parasite species (see Anderson, Reference Anderson2000; Klimpel and Rückert, Reference Klimpel and Rückert2005). Life cycles of some anisakid (e.g. genera Anisakis and Contracaecum) and raphidascaridid (e.g. genera Hysterothylacium) nematodes are known (Anderson, Reference Anderson2000), although all for other genera within these families are still scarce or absent.
The Seuratoidea represents a diparate group of genera, whose transmission and development are very limited for most of the species. Cucullanidae is one of the most common families of parasitic nematodes occurring in fish. However, our knowledge relating to the life cycle of these species is still imperfect (Anderson, Reference Anderson2000). These intestinal parasites are primarily heteroxenous using vertebrates (e.g. prey fish) or invertebrates (e.g. polychaetes) as intermediate hosts, but there is also evidence that, in some species, the intermediate host was replaced by a histotropic phase in the definitive host (see Anderson, Reference Anderson2000; Køie, Reference Køie2000, Reference Køie2001; Pronkina et al., Reference Pronkina, Dmitrieva, Polyakova and Popyuk2017).
Advances in the taxonomic and phylogenetic knowledge
Even though several taxa of nematode parasites in marine fishes from off the Americas require taxonomic revision (see the following section), relatively recent technological advances have been very important for improving our knowledge concerning these organisms. Scanning electron microscopy (SEM) and genetic characterization appear to be the most important new tools in this process.
For example, in the Cystidicolidae that allocates 26 genera with complicated taxonomy and the generic diagnosis is mainly based on minute cephalic structures, SEM observations have proven to be crucial for the resolution of taxonomic problems and the improvement of morphological knowledge (Moravec, Reference Moravec2007; Moravec and Justine, Reference Moravec and Justine2010). Similarly, in the Philometridae, which includes commonly very small male specimens and minute cephalic structures, the use of SEM has been very important (Moravec and de Buron, Reference Moravec and de Buron2013) especially for those parasites of marine fish off the American continent, which have been studied intensively during the last decade (see Moravec and de Buron, Reference Moravec and de Buron2013; Moravec et al., Reference Moravec, Bakenhaster and Fajer-Ávila2014, Reference Moravec, Bakenhaster and Adams2016, Reference Moravec, Bakenhaster and Switzer2020, Reference Moravec, Bakenhaster, Seyoum and Tringali2021). The application of SEM is not restricted to few taxa of nematode parasitizing marine fish in the Americas; it has been practically mandatory for taxonomic studies regarding these parasites during the last 20 years, proving to be very important for the description of new species (e.g. Vieira et al., Reference Vieira, Pereira, Pantoja, Soares, Pereira, Timi, Scholz and Luque2015; Irigoitia et al., Reference Irigoitia, Braicovich, Farber and Timi2017; Aguilar-Aguilar et al., Reference Aguilar-Aguilar, Ruiz-Campos, Martorelli, Montes and Martínez-Aquino2019; Moravec et al., Reference Moravec, Bakenhaster and Switzer2020, Reference Moravec, Bakenhaster, Seyoum and Tringali2021), as well as for improving the knowledge of poorly known taxa and resolution of taxonomic issues (e.g. Mejía-Madrid and Aguirre-Macedo, Reference Mejia-Madrid and Aguirre-Macedo2011a; Pereira et al., Reference Pereira, Vieira and Luque2015; Moravec et al., Reference Moravec, Bakenhaster and Leone2017; Sardella et al., Reference Sardella, Pereira and Luque2017). Even though the use of SEM has been increased exponentially in studies pertaining to nematodes parasitizing marine fish in the American continent, certain species from different families still remain poorly known regarding their morphology, and/or have never been observed using SEM, especially those that are reported less frequently, such as Caballeronema wardlei (Smedley, 1934), Spinitectus beaveri Overstreet, 1970, S. cristatus Railliet et Henry, 1915 (all Cystidicolidae), Cucullanus longipapillatus Olsen, Reference Olsen1952 and C. elongatus Smedley, 1933 (both Cucullanidae), Dollfusnema piscicola Caballero-Rodríguez, Reference Caballero-Rodríguez1974 (Spiruridae), Echinocephalus pseudouncinatus Millemann, 1951 (Gnathostomatidae), Heliconema heliconema Travassos, 1919 (Physalopteridae), Lappetascaris lutjani Rasheed, 1965 (Raphidascarididae), Laurotravassoxyuris travassosi Vigueras, 1938 (Pharyngodonidae), Oncophora melanocephala (Rudolphi, Reference Rudolphi1819) (Rhabdochonidae) and Phlyctainophora squali Mudry et Dailey, 1969 (Micropleuridae).
Genetic characterization of nematodes infecting marine fish from the Americas is a more recent tool that has also been very valuable for the better comprehension of different aspects related to these parasites. However, genetic database is rather scarce, since only about 18% (38/209) of the species have genetic sequences available in GenBank. Most genetic markers used are nuclear, in which 18S rDNA sequences are the most common type. Sequences of cox1 mtDNA are the second most common, followed by the nuclear 28S rDNA and ITS1-5.8S-ITS2. Cox2 and 12S mtDNA sequences are also available, but they are very rare. According to the database, only representatives of the following nematode families have been genetically characterized: Anisakidae (3 species), Camallanidae (3), Capillariidae (1), Cucullanidae (6), Cystidicolidae (2), Daniconematidae (1), Gnathostomatidae (2), Philometridae (11), Physalopteridae (1) and Raphidascarididae (8). Genetic markers used for raphidascaridids are the most diverse, followed by those used for cucullanids and anisakids (see Supplementary Material 2 for details). Raphidascaridid and anisakid larval forms are commonly found in marine fish and, in addition to their zoonotic potential (Mattiucci et al., Reference Mattiucci, Garcia, Cipriani, Santos, Nascetti and Cimmaruta2014; Macchioni et al., Reference Macchioni, Tedesco, Cocca, Massaro, Sartor, Ligas, Pretti, Monni, Cecchi and Caffara2021), specific diagnosis based upon larval morphology is almost impossible (Moravec, Reference Moravec1994, Reference Moravec1998), facts that can boost the genetic characterization of these parasites and, increasingly, the search for new markers. Cucullanids and philometrids currently are the most specious taxa of nematode parasites in marine fish from the Americas (see Fig. 1C). Consequently, it is expected that genetic studies focused on these groups will also stand out.
In addition to the fragmentary genetic database, a great part of genetic sequences in GenBank comes from direct submissions, i.e. in the absence of a supporting scientific publication. Such submissions limit checks on the accuracy of taxonomic identifications, and some likely misidentifications have been noted by some authors (see Černotíková et al., Reference Černotíková, Horák and Moravec2011; Ailán-Choke et al., Reference Ailán-Choke, Davies, Tavares and Pereira2019, Reference Ailán-Choke, Tavares, Luque and Pereira2020). However, despite the limited and sometimes inaccurate data, the genetic characterization of nematodes reported herein has been crucial for supporting species validity and the evaluation of their phylogenetic positions (Mejía-Madrid and Aguirre-Macedo, Reference Mejia-Madrid and Aguirre-Macedo2011a, Reference Mejía-Madrid and Aguirre-Macedo2011b; Sardella et al., Reference Sardella, Pereira and Luque2017; Aguilar-Aguilar et al., Reference Aguilar-Aguilar, Ruiz-Campos, Martorelli, Montes and Martínez-Aquino2019; Sokolov and Gordeev, Reference Sokolov and Gordeev2021), genetic variability (Li et al., Reference Li, Du, Xu, Guo, Wang and Zhang2014), broad phylogenetic studies of higher taxa (Nadler and Hudspeth, Reference Nadler and Hudspeth1998, Reference Nadler and Hudspeth2000; Nadler et al., Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007; Li et al., Reference Li, Lü, Nadler, Gibson, Zhang, Chen, Zhao and Guo2018; Barton et al., Reference Barton, Moravec, Zhu and Shamsi2022), specific identification of larval forms (Palesse et al., Reference Palesse, Meadors, de Buron, Roumillat and Strand2011; Haarder et al., Reference Haarder, Kania and Buchmann2013; Roca-Geronès et al., Reference Roca-Geronès, Montoliu, Godínez-González, Fisa and Shamsi2018) and even the elucidation of life cycles (May-Tec et al., Reference May-Tec, Martínez-Aquino, Aguirre-Macedo and Vidal-Martínez2018). It should be mentioned that some of these works were not specifically carried out in marine waters off the American continent (Nadler and Hudspeth, Reference Nadler and Hudspeth1998, Reference Nadler and Hudspeth2000; Nadler et al., Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007; Haarder et al., Reference Haarder, Kania and Buchmann2013; Li et al., Reference Li, Du, Xu, Guo, Wang and Zhang2014, Reference Li, Lü, Nadler, Gibson, Zhang, Chen, Zhao and Guo2018; Roca-Geronès et al., Reference Roca-Geronès, Montoliu, Godínez-González, Fisa and Shamsi2018), but included nematode species that have been reported in this region.
As previously commented, phylogenetic relationships within the Nematoda have changed considerably with the use of genetic analyses (see the reviews by Mitreva et al., Reference Mitreva, Blaxter, Bird and McCartes2005; Blaxter, Reference Blaxter2011; Blaxter and Koutsovoulos, Reference Blaxter and Koutsovoulos2015). Broad and pioneer phylogenetic works on nematode parasites included very few representatives that infect marine fish and have been reported in the American continent (Nadler and Hudspeth, Reference Nadler and Hudspeth1998, Reference Nadler and Hudspeth2000). Wijová et al. (Reference Wijová, Moravec, Horák and Lukeš2006) published the first phylogenetic work including a representative number of samples from nematode parasites of fish; the study emphasized on the superfamily Dracunculoidea. However, it included only 1 parasite of marine fish from the American continent, namely Dentiphilometra sp. from Mexico. Posteriorly, Černotíková et al. (Reference Černotíková, Horák and Moravec2011) investigated the phylogenetic relationships within Spirurina specifically in regards to parasites of fish including and providing sequences for several nematode species from the tropical and temperate zones of the western Atlantic. Subsequent studies including nematode species herein listed have been focused on phylogenetic and systematic aspects of the most specious families Cucullanidae (Choudhury and Nadler, Reference Choudhury and Nadler2018) and Philometridae (Negreiros et al., Reference Negreiros, Tavares-Dias, Elisei, Tavares and Pereira2019; Barton et al., Reference Barton, Moravec, Zhu and Shamsi2022), as well as Raphidascarididae and Anisakidae (Pereira and Luque, Reference Pereira and Luque2017a), Camallanidae (Ailán-Choke and Pereira, Reference Ailán-Choke and Pereira2021) or a combination of these families (Holterman et al., Reference Holterman, Schratzberger and Helder2019). The approaches used in the remaining phylogenetic studies are narrower and focused on reduced taxonomic groups (i.e. genus or restricted to some species) (see Mejía-Madrid and Aguirre-Macedo, Reference Mejia-Madrid and Aguirre-Macedo2011a, Reference Mejía-Madrid and Aguirre-Macedo2011b; Sardella et al., Reference Sardella, Pereira and Luque2017; Roca-Geronès et al., Reference Roca-Geronès, Montoliu, Godínez-González, Fisa and Shamsi2018; Aguilar-Aguilar et al., Reference Aguilar-Aguilar, Ruiz-Campos, Martorelli, Montes and Martínez-Aquino2019).
Even though the knowledge on the phylogeny of nematode parasitic in marine fish from the American continent remains superficial, since only few representatives have been genetically characterized and included in broad phylogenetic studies, evidence supports the following conclusions. The monophyly of the families Camallanidae, Cucullanidae, Philometridae and Raphidascarididae is strongly supported (Černotíková et al., Reference Černotíková, Horák and Moravec2011; Pereira and Luque, Reference Pereira and Luque2017a, Reference Pereira and Luque2017b; Choudhury and Nadler, Reference Choudhury and Nadler2018; Negreiros et al., Reference Negreiros, Tavares-Dias, Elisei, Tavares and Pereira2019; Ailán-Choke and Pereira, Reference Ailán-Choke and Pereira2021; Barton et al., Reference Barton, Moravec, Zhu and Shamsi2022), whereas in Anisakidae and Cystidicolidae, evidence showed lack of monophyly and, consequently, suggests their artificiality (Pereira et al., Reference Pereira, Pereira and Luque2018). Moreover, monophyly is not supported for the genera Procamallanus and Spirocamallanus (Camallanidae) (sensu Ailán-Choke and Pereira, Reference Ailán-Choke and Pereira2021), Cucullanus and Dichelyne (Camallanidae), Philometra and Philometroides (Philometridae), Hysterothylacium (Raphidascarididae), Contracaecum (Anisakidae) and Ascarophis (Cystidicolidae) (Černotíková et al., Reference Černotíková, Horák and Moravec2011; Pereira and Luque, Reference Pereira and Luque2017a, Reference Pereira and Luque2017b; Choudhury and Nadler, Reference Choudhury and Nadler2018; Pereira et al., Reference Pereira, Pereira and Luque2018; Negreiros et al., Reference Negreiros, Tavares-Dias, Elisei, Tavares and Pereira2019; Mata et al., Reference Mata, Paiva, Elisei, Tavares and Pereira2020a, Reference Mata, Paiva, Elisei, Tavares and Pereira2020b; Ailán-Choke and Pereira, Reference Ailán-Choke and Pereira2021; Barton et al., Reference Barton, Moravec, Zhu and Shamsi2022). Thus, it is pertinent that the classification system of the previously mentioned taxa, which includes the most representative ones, should be revised in the future with the improvement of both genetic and morphological databases. Extra efforts in the genetic characterization of most of the species listed herein are sorely needed.
Systematics and species list of nematodes
A total of 209 valid species, 19 species inquirenda and 6 dubious records of nematodes were reported parasitizing 504 marine fishes off the Americas. The most represented suborder was the Ascaridina (37%, 78/209), followed by the Spirurina (27%, 56/209), Dracunculina (24%, 51/209), Trichinellina (10%, 21/209), Gnathostomatina (1%, 2/209) and Oxyurina (0.5%, 1/209). The Cucullanidae was the family with the highest number of species (20%, 42/209), followed by the Philometridae and Cystidicolidae (19%, 40/209 and 12%, 25/209, respectively). More information can be found in Supplementary Material 2 and Fig. 1C.
Phylum Nematoda Cobb, 1932
Class Dorylaimea Hodda, 2007
Order Trichocephalida Spasski, 1954
Suborder Trichinellina Hodda, 2007
Superfamily Trichinelloidea Ward, 1907
This superfamily forms part of the order Trichocephalida Spasski, 1954, the latter frequently phylogenetically grouped in a strongly supported cluster formed by Dorylaimida, Mermithida and Mononchida (see Dorris et al., Reference Dorris, De Ley and Blaxter1999). The superfamily is apparently derived from ancestors that were initially tissue parasites of freshwater fishes (Anderson and Bain, Reference Anderson, Bain, Anderson, Chabaud and Willmott1982) and characterized by having members with the anterior part of the body narrowed and the posterior part considerably expanded; oesophagus divided into a shorter anterior muscular part and a longer posterior glandular part (stichosome). Trichinelloids are parasites of the digestive tract of vertebrates (amphibians, birds, fishes, mammals, reptiles) in both terrestrial and aquatic (freshwater and marine) environments; those species parasitizing fishes utilize aquatic oligochaetes as intermediate hosts. Three families are included in this superfamily: Capillariidae Railliet, 1915 (with 6 genera, 6 subgenera, 13 species), Trichosomoididae Hall, 1916 (1 genus, 7 species) and Trichuridae Ransom, 1911 (1 genus, 1 species). All these 21 species are valid, although P. (P.) hathawayi was originally described based only in females and provisionally assigned to Piscicapillaria Moravec, 1982 (see Moravec, Reference Moravec1987). Since trichinelloids are host-specific, the finding of C. (P.) gracilis in hosts of different fish orders and geographical localities requires re-confirmation. Capillaria (H.) cyprinodonticola Huffman et Bullock, 1973 was reported in fishes inhabiting marine, brackish and freshwater habitats, although its origin does not seem to be purely marine. Capillostrongyloides congiopodi Cantatore, Rossin, Lanfranchi et Timi, 2009 was listed as a part of Trichuridae by Hodda (Reference Hodda2022a) instead of Capillariidae where it was first classified.
Class Chromadorea Inglis, 1983
Order Spirurida Chitwood, 1933
Suborder Spirurina Railliet et Henry, 1915
Superfamily Camallanoidea Railliet et Henry, 1915
The order Spirurida Railliet, 1915 includes the superfamilies Camallanoidea, Thelazioidea and Habronematoidea and formed a phylogenetic clade along with the zooparasitic orders Ascaridida, Oxyurida and Rhigonematida (Blaxter et al., Reference Blaxter, De Ley, Garey, Liu, Scheldeman, Vierstraete, Vanfleteren, Mackey, Dorris, Frisse, Vida and Thomas1998; Dorris et al., Reference Dorris, De Ley and Blaxter1999). The Camallanoidea is monophyletic, forms a sister group to Dracunculoidea (Černotíková et al., Reference Černotíková, Horák and Moravec2011) and is represented by spirurid nematodes occurring in the digestive tract of fishes, amphibians and reptiles all over the world. These nematodes have an oral opening with rudimentary or without lips, pseudolabia absent, oesophagus divided into muscular and glandular sections and the body size of females is rarely double the size of males. Camallanoids use copepods as intermediate hosts, small fish as paratenic hosts and larger fish as definitive hosts. Paratenesis and precocity during transmission may help to explain the wide host distribution of many camallanoids (Anderson, Reference Anderson1988). The superfamily includes 2 families: Camallanidae Railliet et Henry, 1915 (2 genera, 1 subgenus and 15 species) and Physalopteridae Railliet, 1893 (3 genera and 9 species); of these, Procamallanus (Spirocamallanus) is valid and the fourth most speciose in marine fishes off America (14 species). All these taxa are valid, although some records of O. melanocephala (Rudolphi, Reference Rudolphi1819) Baudin-Laurencin, 1971, Mooleptus rabuka (Machida, Ogawa et Okiyama, 1982) Özdikmen, 2010 and Proleptus obtusus Dujardin, 1845 should be confirmed, since they are from very distant geographical areas and hosts. Procamallanus (Spirocamallanus) cruzi (Guimarães, Cristofaro et Rodrigues, 1976) Moravec et Sey, 1988 and Proleptus elegans (Örley, 1885) were not included because they are species inquirendae (see Sardella et al., Reference Sardella, Pereira and Luque2017). Procamallanus (Spirocamallanus) pereirai (Annereaux, 1946) Olsen, Reference Olsen1952 was commonly reported in different regions off the Americas, but according to Sardella et al. (Reference Sardella, Pereira and Luque2017), all specimens reported in the South Atlantic belong to P. (S.) macaensis. Bashirullah and Williams (Reference Bashirullah and Williams1980) poorly described P. (S.) garnotus (Bashirullah et Williams, Reference Bashirullah and Williams1980) Moravec et Sey, 1988, P. (S.) papillicaudatus (Bashirullah et Williams, Reference Bashirullah and Williams1980) Moravec et Sey, 1988, P. (S.) partitus (Bashirullah et Williams, Reference Bashirullah and Williams1980) Moravec et Sey, 1988, P. (S.) plumierus (Bashirullah et Williams, Reference Bashirullah and Williams1980) Moravec et Sey, 1988 and P. (S.) spinicaudatus (Bashirullah et Williams, Reference Bashirullah and Williams1980) Moravec et Sey, 1988, from Puerto Rico and Venezuela, which are morphologically similar and a deep revision of the type material is required. Proleptus carvajali Fernández et Villalba, 1985 was reported in Anisotremus scapularis (Tschudi), Isacia conceptionis (Cuvier) (both Haemulidae), Cilus gilberti (Abbott) (Sciaenidae), Labrisomus philippii (Steindachner) (Labrisomidae) (all Eupercaria) from Perú (see Chero et al., Reference Chero, Cruces, Iannacone, Saez and Alvariño2014a, Reference Chero, Iannacone, Cruces, Flores and Flores2014b; Cruces et al., Reference Cruces, Chero, Iannacone, Sáez and Alvariño2015; Iannacone et al., Reference Iannacone, Alvariño, Chero and Sáez2015), but they could be misidentifications. Whereas Torres et al. (Reference Torres, Iannacone, Romero, Guabloche, Alvariño, Chero, Cruces, Minaya, Cárdenas-Callirgos and Naupay-Naupay2018) and Chero et al. (Reference Chero, Ortega, Cruces, Sáez and Iannacone2019) reported larval nematodes such as P. carvajali in Emerita analoga (Decapoda) and Cheilodactylus variegatus (Cheilodactylidae), respectively, from Perú, but the identity of the nematode should be verified.
Superfamily Thelazioidea Sobolev, 1949
This superfamily is paraphyletic and was grouped with the taxa of Acuaroidea, Diplotriaenoidea, Filaroidea, Habronematoidea, Physalopteroidea and Spiruroidea (see Černotíková et al., Reference Černotíková, Horák and Moravec2011; Choudhury and Nadler, Reference Choudhury and Nadler2018). Members of this superfamily have an oral opening round or hexagonal (not compressed laterally), stoma variable in size and are viviparous. Thelazioids are represented by 2 families: Rhabdochonidae Travassos, Artigas, et Pereira, 1928 (3 genera, 3 species) and Thelaziidae Railliet, 1910 (1 genus, 1 species), which are all valid, although the rhabdochonid genus Megachona Mejía-Madrid et Pérez-Ponce de León, Reference Mejía-Madrid and Pérez-Ponce de León2007 was not listed by Hodda (Reference Hodda2022a), but no reason to be invalid. Most of rhabdochonid genera (e.g. Heptochona Rasheed, 1965; Rhabdochona Railliet, 1916) are of freshwater origin, although there are others, such as Johnstonmawsonia Campana-Rouget, 1955, Megachona Mejía-Madrid et Pérez-Ponce de León, Reference Mejía-Madrid and Pérez-Ponce de León2007, Pancreatonema McVicar et Gibson, Reference McVicar and Gibson1975 and Vasorhabdochona Martin et Zam, 1967, that were found in marine or freshwater fish.
Superfamily Habronematoidea Chitwood et Wehr, 1932
This superfamily along with Thelazioidea are not natural groups (paraphyletic) as stated by Černotíková et al. (Reference Černotíková, Horák and Moravec2011) and Choudhury and Nadler (Reference Choudhury and Nadler2018). Habronematoids bear pseudolabia, with or without cephalic cuticular ornamentation, caudal alae in male and include 3 families: Cystidicolidae Skryabin, 1946 (9 genera, 25 species), Habronematidae Chitwood et Wehr, 1932 (1 genus, 1 species) and Hedruridae Petter, 1971 (1 genus, 2 species). All these are valid, although Similascarophis is considered as genus by Hodda (Reference Hodda2022a) and not a subgenus of Ascarophis as proposed by Moravec and Justine (Reference Moravec and Justine2007). Despite the high number of genera, they are monotypical and restricted to certain regions of the continent, such as Caballeronema Margolis, 1977 in Canada and Comephoronema Layman, 1933 in Brazil. On the other hand, the genus Ascarophis van Beneden, 1871 (Cystidicolidae) has the higher number of species within this superfamily (10 species), of which some show narrow geographical distribution (A. brasiliensis Pinto, Vicente et Noronha, 1984 in Brazil, A. carvajali Muñoz et George-Nascimento, Reference Muñoz and George-Nascimento2007 and A. draconi Muñoz et George-Nascimento, Reference Muñoz and George-Nascimento2007 in Chile, A. cestus Chitwood, 1934 in Puerto Rico, A. extalicola Appy, 1981 in Canada and A. morronei Aguilar-Aguilar, Ruiz-Campos, Martorelli, Montes et Martínez-Aquino, 2019 in Mexico). Meanwhile, A. arctica Polyanskiy, 1952, A. filiformis Polyanskiy, 1952, A. morrhuae van Beneden, 1871 and A. sebastodis Olsen, Reference Olsen1952 were reported in fishes of different families and orders, as well as from distant localities, thus the identity of the specimens from each host and geographical region should be corroborated. Ascarophis helix Cobb, 1928 was not included since Dollfus and Campana-Rouget (Reference Dollfus and Campana-Rouget1956) considered it as a species inquirenda.
Suborder Dracunculina Stiles, 1907
Superfamily Dracunculoidea Stiles, 1907
This superfamily is monophyletic, with Camallanoidea as sister group, thus showing some remote affinities between them (see Černotíková et al., Reference Černotíková, Horák and Moravec2011). Its members are parasites of a variety of host tissues and cavities in both cold- and warm-blooded vertebrates (Anderson, Reference Anderson2000), including freshwater, brackishwater and marine fishes. Dracunculoid nematodes are represented by 5 families: Daniconematidae Moravec et Køie, 1987 (1 genus, 1 species), Guyanemidae Petter, 1974 (2 genera, 5 species), Micropleuridae Baylis et Daubney, 1926 (2 genera, 3 species), Philometridae Baylis et Daubney, 1926 (8 genera, 40 species) and Skrjabillanidae Shigin et Shigina, 1958 (1 genus, 2 species). All these taxa are valid, although Caranginema Moravec, Montoya-Mendoza et Salgado-Maldonado, 2008, which was not listed by Hodda (Reference Hodda2022a) but no reason to be considered invalid. Moreover, family Dracunculidae Stiles, 1907 is not herein listed because the genus Lockenloia Adamson et Caira, 1991, represented in marine fishes off America, is considered as incertae sedis within Dracunculoidea (see Moravec, Reference Moravec2004). The genus Margolisianum Blaylock et Overstreet, 1999 and the species Philometra fariaslimai Fortes, 1981 were not included because the former is considered as a genus inquirendum (see Moravec and de Buron, Reference Moravec and de Buron2006) and the latter as a species inquirenda (see Moravec, Reference Moravec2006). Clavinema mariae (Layman, 1930) Margolis et Moravec, 1987 probably represents a complex of related species with almost identical female morphology (see Moravec et al., Reference Moravec, Nagasawa, Nitta and Tawa2019); whereas Philometra chilensis Moravec, Chávez et Oliva, Reference Chávez and Oliva2011 reported by Chávez and Oliva (Reference Chávez and Oliva2011) represents a nomen nudum and refers to P. genypteri Moravec, Chávez et Oliva, Reference Chávez and Oliva2011. The philometrid genus Philometra Costa, 1845 is the most speciose in the whole continent (40 species), while other genera are represented by much less species, such as Caranginema, Clavinema Yamaguti, 1935, Dentiphilometra Moravec et Wang, 2002, Digitiphilometroides Moravec et Barton, 2018, Mexiconema Moravec, Vidal et Salgado-Maldonado, 1992, Moravecia Ribu et Lester, 2004, Phlyctainophora Steiner, 1921, Barracudia Moravec et Shamsi, 2017 (1 each) and Granulinema Moravec et Little, 1988 (2 species). Philometra globiceps (Rudolphi, Reference Rudolphi1819) Railliet, 1916 and Philometra lateolabracis Yamaguti, 1935 only parasitize Uranoscopus scaber Linnaeus (see Moravec and Tedesco, Reference Moravec and Tedesco2015) and Lateolabrax japonicus (Cuvier), respectively (see Quiazon et al., Reference Quiazon, Yoshinaga and Ogawa2008), which do not occur in American waters, thus both nematode species are absent from the Americas.
Suborder Gnathostomatina Skryabin et Ivaschkin, 1973
Superfamily Gnathostomatoidea Railliet, 1895
This superfamily, along with Anguillicoloidea, formed a well-supported sister group with the clade III composed by the orders Ascaridida, Oxyurida and Rhigonematida (see Černotíková et al., Reference Černotíková, Horák and Moravec2011). Gnathostomatoids have a large, trilobed pseudolabia, 4–6 large unicellular lemniscus-like cephalic glands, and are parasites of fishes, amphibians, reptiles and mammals. Most of its genera (e.g. Gnathostoma Owen, 1836, Spiroxys Schneider, 1866) use fishes as intermediate hosts, while adults are represented by the family Gnathostomatidae Railliet, 1895 and genus Echinocephalus Molin, 1858 (2 species). This is one of the less represented taxa in marine fishes off the Americas.
Suborder Oxyurina Railliet, 1916
Superfamily Oxyuroidea Cobbold, 1864
This superfamily is monophyletic (see Sata and Nakano, Reference Sata and Nakano2020) and represented by small parasitic nematodes of all classes of vertebrates and invertebrates. They are also called ‘pinworms’ and characterized by the presence of a bulb in the posterior end of the oesophagus and a prolonged caudal extremity of the female. It is represented in marine fishes off America by the family Pharyngodonidae Travassos, 1919 and genus Laurotravassosoxyuris Vigueras, 1938 (1 species). Like the latter superfamily, this is also one of the less represented taxa.
Suborder Ascaridina Inglis, 1983
Superfamily Ascaridoidea Railliet et Henry, 1915
This superfamily is monophyletic (see Nadler and Hudspeth, Reference Nadler and Hudspeth1998) and its members are medium-sized to large nematodes with 3 lips (sometimes separated by interlabia) and inhabiting the stomach and intestine of all classes of vertebrates, including species that are of medical end economic importance. Out of the approximately 54 genera within Ascaridoidea, at least 11, belonging to 3 families, are represented in marine fishes off America, namely Anisakidae Railliet et Henry, 1912 (5 genera, 12 species), Ascarididae Baird, 1853 (1 genus, 2 species) and Raphidascarididae Hatwich, 1954 (5 genera, 1 subgenus, 22 species). All are valid, although the genera Euterranova Moravec et Justine, Reference Moravec and Justine2020 and Iheringascaris Pereira, 1935 were not listed by Hodda (Reference Hodda2022a), but there is no reason to consider them invalid. The genus Terranova Leiper et Atkinson, 1914 (and 3 species) was not included because it is considered as genus inquirendum (see Moravec and Justine, Reference Moravec and Justine2020). Hysterothylacium Ward et Magath, 1917 is the third most speciose genus represented in off the Americas (17 species), although also the most problematic because several species have been reported in various fish hosts and even distant locations. Similarly, the species Acanthocheilus rotundatus (Rudolphi, Reference Rudolphi1819) Hartwich, 1957, Euterranova galeocerdonis (Thwaite, 1927) Moravec et Justine, Reference Moravec and Justine2020 and Pseudanisakis tricupola Gibson, 1973 were reported in several elasmobranchs in different regions of the world, thus its identity should be corroborated in all of them. The systematic status of Hysterothylacium deardorffoverstreetorum Knoff, Felizardo, Iniguez, Maldonado Jr., Torres, Pinto et Gomes, 2012, H. magnum (Smedley, 1934) Deardorff et Overstreet, 1981, H. melanogrammi (Smedley, 1934) Deardorff et Overstreet, 1981 and Raphidascaris (Raphidascaris) anchoviellae (Chandler, 1935) Moravec et Nagasawa, 2002 is uncertain because they were poorly described or based on larval stages.
Superfamily Seuratoidea Chabaud, Campana-Rouget et Brygoo, 1960
The Seuratoidea was recognized as valid by Inglis (Reference Inglis1967) and Chabaud et al. (Reference Chabaud, Campana-Rouget and Brygoo1960), although phylogenetically it represents a non-natural group (see Skryabin and Ivashkin, Reference Skryabin and Ivashkin1968; Choudhury and Nadler, Reference Choudhury and Nadler2018) and more phylogenetic analyses with more extensive sampling of non-cucullanid taxa are required (see Choudhury and Nadler, Reference Choudhury and Nadler2018). Seuratoids are usually medium-sized nematodes with lips reduced or absent, a short oesophagus, precloacal sucker, and inhabit in all classes of vertebrates. The superfamily includes only 1 family: Cucullanidae Cobbold, 1864 (2 genera, 2 subgenera, 42 species). The genus Cucullanus Müller, 1777 is the second most speciose in the continent (26 species), while genus Dichelyne Jägerskiöld, 1902 subgenus Cucullanellus Törnquist, 1931 the fourth along with Procamallanus (Spirocamallanus) (14 species). Despite all 42 species are valid, Dichelyne (Cucullanellus) travassosi (Guimarães et Cristófaro, 1974) Vicente, Magalhães et Aguilera, 1989 and D. (C.) elongatus (Törnquist, 1931) Petter, 1974 should be redescribed. Apparently, the latter only occurs in the Pacific coast of South America and all previous reports from the Atlantic coast (Argentina, Brazil, Venezuela) belong to D. (C.) sciaenidicola (see Timi et al., Reference Timi, Lanfranchi, Tavares and Luque2009). Cucullanus hians (Dujardin, 1845) Petter, 1974 was reported from a non-American marine fish (C. conger) collected from its southernmost distribution range in North America (65–70 fathoms off Gay Head, Massachusetts, USA) (see Linton, Reference Linton1901), although most reports of this nematode are from the coasts of Europe and Africa, so its identity should be corroborated. There are several species that were not included because they are species inquirendae [C. lopholatilus (MacCallum, 1921) Petter, 1974, C. rougetae Vicente et Santos, 1974, D. cylindricus Chandler, 1935, D. (C.) rodriguesi (Pinto, Fábio et Noronha, 1970) Petter, 1974, D. (D.) micropogonii Pereira Jr. et Costa, 1996] or dubious records [C. chrysophrydis Gendre, 1927, D. (C.) minutus (Rudolphi, Reference Rudolphi1819) Törnquist, 1931, D (C.) tripapillatus (Gendre, 1927) Törnquist, 1931].
There are records of Ichthyostrongylus thunni Nikolaeva, Reference Nikolaeva1969 (family Trichostrongylidae Leiper, 1908) from the USA and Mexico (Nikolaeva, Reference Nikolaeva and Yankovskaya1968, Reference Nikolaeva1969), but Durette-Desset (Reference Durette-Desset, Anderson, Chabaud and Willmott1983) stated that they are insufficiently known to be classified.
Final considerations
It is assumed that parasite richness is proportional to that of the host communities (Hechinger and Lafferty, Reference Hechinger and Lafferty2005; Poulin, Reference Poulin2014). In this sense, in the tropical western and temperate south Atlantic ecoregions, fish richness easily overcomes 2000 species (Froese and Pauly, Reference Froese and Pauly2022) and they showed the highest number of nematode species reported, thus supporting this assumption. However, the present compilation clearly shows that the biodiversity of nematode parasites in marine fish from off the Americas is underestimated, since the diversity of hosts in other ecoregions (e.g. tropical eastern Pacific) is also high (see Miloslavich et al., Reference Miloslavich, Klein, Díaz, Hernández, Bigatti, Campos, Artigas, Castillo, Penchaszadeh, Neill, Carranza, Retana, de Astarloa, Lewis, Yorio, Piriz, Rodríguez, Yoneshigue-Valentin, Gamboa and Martín2011; Carrillo-Briceño et al., Reference Carrillo-Briceño, Carrillo, Aguilera and Sanchez-Villagra2018; Manel et al., Reference Manel, Guerin, Muillot, Blanchet, Velez, Albouy and Pellissier2020) and there have been fewer reports of nematodes, thus indicating that more studies are needed.
Such panorama, combined with numerous taxonomic problems, represents a real challenge when dealing with the nematode parasites of marine fish from off the Americas. Therefore, the present review may provide useful data and a practical way to follow for researches who desire to develop further studies on the subject.
Cucullanidae was the family with the highest richness of nematode species in the present survey. High diversity is commonly associated with cucullanids infecting fish in both marine and freshwater environments throughout the world (see Moravec et al., Reference Moravec, Sasal, Würtz and Taraschewski2005; Vieira et al., Reference Vieira, Pereira, Pantoja, Soares, Pereira, Timi, Scholz and Luque2015; Moravec and Justine, Reference Moravec and Justine2020), which is not different in the Americas. Philometridae was the second most species-rich family with numbers very close to those of Cucullanidae. Such representativeness of philometrids in terms of richness is related to the efforts of the taxonomists (especially Dr František Moravec) working on this group over the last 2 decades, resulting in the description of several species (see Supplementary Material 2 for all authorities and years). Cystidicolidae was also representative regarding its species richness and also showing high number of genera (Moravec, Reference Moravec2007; Moravec and Justine, Reference Moravec and Justine2010; Moravec and Sobecka, Reference Moravec and Sobecka2012). Nematodes of this family are assumed to be frequent in marine fish (Moravec and Justine, Reference Moravec and Justine2010). In the American continent, cystidicolids are particularly common in freshwater fish (especially from North America), in which the genus Spinitectus is the most frequently reported (Moravec, Reference Moravec1998; Luque et al., Reference Luque, Aguiar, Vieira, Gibson and Santos2011; Choudhury and Nadler, Reference Choudhury and Nadler2018). The present results reaffirm the high biodiversity potential of cystidicolid nematodes in marine environments of the Americas, and the real diversity of genera and species seem to be higher than in freshwater. Interestingly, the family Camallanidae, which is very specious in freshwater fish of the Americas (especially in the Neotropical Region), appeared less diverse in marine waters (see Moravec, Reference Moravec1998; Luque et al., Reference Luque, Aguiar, Vieira, Gibson and Santos2011 for a general picture).
The present review indicates that nematode parasites of marine fish in the Americas are diverse, although a higher biodiversity is still unexplored. Moreover, the current taxonomy of these organisms still has numerous problems, in which certain taxa and reports require detailed reevaluation, as well as the life cycle aspects that are practically unknown. Advances on genetic and morphological analyses of these parasites have been producing more accurate data, helping the resolution of taxonomic issues, strengthing taxa validity, revealing evolutionary relationships, as well as suggesting and linking life cycle pathways of species. However, the current knowledge related to these nematodes is far from substantial, needing much additional efforts and having challenges to be overcome, especially with regard to genetic characterization. Steps are being taken towards a better understanding, but there is still a long way to go.
Author's contributions
F. B. P. and D. G. S. conceived and designed the study, as well as both wrote the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022001287.





