INTRODUCTION
The study of parasitic species associated with economically valuable fishes is an important area of research that contributes to successful and sustainable management of fisheries and aquaculture systems throughout the world. Whether wild-caught or reared in captivity, harvested species inevitably become affected by associated environmental stressors that in turn raise the impacts of pathogenic parasites. This review documents the efforts of marine parasitologists throughout the sub-Saharan Africa region and aims to provide a useful synopsis of publications that may be used by researchers when identifying gaps in knowledge or future research planning.
In most of sub-Saharan Africa it is commonly acknowledged that fundamental research on parasites and their associated fish hosts (both commercial and not) requires more specific attention, especially in recognition of the substantial aquaculture and wild-caught fisheries that exist throughout the continent. During the 1980s, when inland aquaculture facilities were being rapidly established throughout sub-Saharan Africa, Hecht and Endemann (Reference Hecht and Endemann1998) published a review that identified parasites and diseases that may impact on the development of aquaculture in this region. Their listings included mostly freshwater species and they identified ectoparasites as being the most important group of pathogenic organisms that had the capacity to cause large-scale mortalities in fish farms.
Hardly any mention was made of parasite species that may be problematic for marine aquaculture systems in sub-Saharan Africa. Hecht and Endemann (Reference Hecht and Endemann1998) mentioned only two important marine polychaete ectoparasites: the mud blister worm, Polydora hoplura, which infects oysters (Crassostrea gigas) in Namibia and South Africa, and indigenous South African sabellid polychaetes that have become problematic in abalone (Haliotus midae) farms in South Africa. Polydora hoplura is known to form blisters in the nacre of oysters that, when punctured, release a pungent smell that affects product quality. Although infection by P. hoplura does not cause severe mortalities, the oysters do expend excessive energy secreting nacre and concholin during mud blister formation, which leads to a reduction in the oyster quality and growth rate (Nel et al. Reference Nel, Coetzee and van Niekerk1996). Other sabellid polychaete worms (Dipolydora capensis and Boccardia sp.) as well as P. hoplura are also known to attach themselves to the growing edge of abalone, where they embed and ultimately deform the shells (Hecht and Endemann, Reference Hecht and Endemann1998). Several parasite species have been recorded from abalone and oysters in South Africa and numerous studies have examined treatments and prevention of these infections (Nel et al. Reference Nel, Coetzee and van Niekerk1996; Botes, Reference Botes1999; Simon et al. Reference Simon, Ludford and Wynne2006; Mouton and Gummow, Reference Mouton and Gummow2011) (Table 1). Above all, the major concern associated with the importation of oysters and abalone is the constant threat of new introductions of pathogenic species that have as yet not been detected (Haupt et al. Reference Haupt, Griffiths, Robinson and Tonin2009).
Table 1. Parasite species known to infect abalone (Haliotus midae) and oysters (Crassostrea gigas) in southern Africa

In wild-caught marine fisheries, knowledge of associated parasite species is equally important. The potential of certain parasites to, for example, devastate stocks by impairing spawning (e.g. Eimeria sardinae in Portuguese sardine; Pinto, Reference Pinto1956); influence product quality through myoliquefaction (e.g. Kudoa thyrsites); have effects on human health such as inducing allergies (Kirstein et al. Reference Kirstein, Horsnell, Nieuwenhuizen, Ryffel, Lopata and Brombager2010) or through accidental infections by notorious species (e.g. Anisakis sp.) are well known (Lima dos Santos and Howgate, Reference Lima dos Santos and Howgate2011). Perhaps what is less obvious is the usefulness of such parasite data in ecosystem-based fisheries studies where understanding host–parasite relationships can help to predict changes in environmental conditions (such as acting as sensitive indicators of pollution and heavy metal accumulation; Sures, Reference Sures2004) or where they may be used in applied research (such as the use of parasites as biological tags for fish stock discrimination studies; Baldwin et al. Reference Baldwin, Banks and Jacobson2012), ultimately enhancing sustainable fisheries management practices.
Fishing has clearly stressed wild populations of many commercially valuable species across the globe (Jennings and Kaiser, Reference Jennings and Kaiser1998; Jackson et al. Reference Jackson, Kirby, Berger, Bjorndal, Botsford, Bourque, Bradbury, Richard Cooke, Erlandson, Estes, Hughes, Kidwell, Lange, Lenihan, Pandolfi, Peterson, Steneck, Tegner and Warner2001). The coastal region of sub-Saharan Africa is no exception. Stretching from Mauritania in the west to Eritrea in the east and covering ±48 000 km of coastline, it includes five of the world's most productive large ocean currents (Fig. 1). This extensive stretch of coastline harbours an intensive fishing culture ranging from small subsistence and artisanal fisherman to large commercial industries. Marine aquaculture and fisheries production in this area amounted to 4 220 412 t (for fish, crustaceans and molluscs) in 2010 (FAO, 2010), an amount far less than the global production total of 148 476 426 t for the same commodities in that year, but still significant enough to have potential effects on wild fish populations.

Fig. 1. Sea surface temperature composite map showing the major ocean currents around Africa (Global Odyssea SST at 0.1 degree resolution derived by the CERSAT. Data from http://www.ifremer.fr/cersat1/exp/productscatalogdetails/)?id=CER-SST-GLO-1D-010-ODY-MGD).
Despite the importance of fishery resources throughout this region, very little research has been conducted on the effects of parasitic species associated with commercially harvested hosts, and even less work has been done using parasite data in applied studies to enhance fisheries management. Most parasitological research outputs stem from just a few countries (Senegal, Nigeria, Namibia, South Africa and Kenya) where mostly taxonomists have made concerted efforts to document parasitic species associated with hosts of both commercial and non-commercial importance.
West coast of sub-Saharan Africa
Environmental conditions along the western coast of sub-Saharan Africa are driven by two major ocean currents (Gyory et al. Reference Gyory, Bischof, Mariano and Ryan2005). The Canary Current (off the coasts of Mauritania, Senegal, the Gambia, Guinea Bissau and Guinea) is a wind-driven cold-water surface current that forms part of the North Atlantic Gyre and flows in a south-westerly direction (Fig. 1), and the Guinea Current (off the coasts of Sierra Leone, Liberia, Cote d’ Ivoire, Ghana, Benin, Nigeria, Cameroon, Equatorial Guinea, Gabon, Congo and Democratic Republic of Congo) is fed by the North Equatorial Counter-Current and flows in an easterly direction (Fig. 1). The most abundant commercial species in these regions are the clupeids (Sardina pilchardus), jack mackerel (Trachurus spp.) and chub mackerel (Scomber japonicus), as well as several large pelagic (Thunnus spp.) and demersal species.
Research on parasite species associated with marine fishes in this region includes mostly surveys of parasite faunas and descriptions of new species. Parasitologists in Senegal, in particular, have made a tremendous contribution to the known diversity of certain parasitic taxa (esp. Myxozoa, Coccidia and Microsporidia) in both commercial and non-commercial marine fishes (Table 2). Nearly 100 parasite species from these groups have been recorded or described from marine fishes in Senegal since the 1990s. Similarly parasitologists in Nigeria have made a substantial contribution to the known diversity of parasite species infecting specifically commercially important marine and estuarine fishes (Table 3).
Table 2. Some of the major taxonomic contributors to marine parasitology in west and central sub-Saharan Africa

Table 3. Studies of parasites infecting commercial marine fishes in west and central sub-Saharan Africa (‘Numerous’ = more than five fish hosts examined)
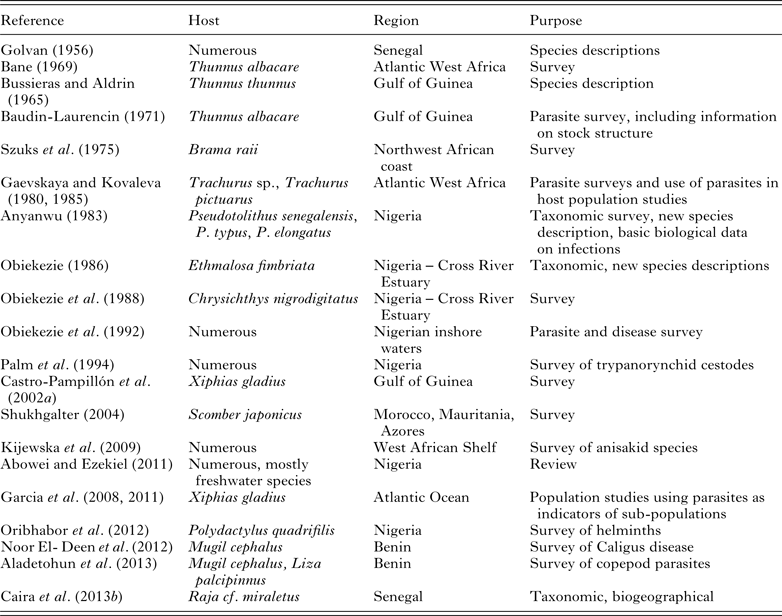
Studies on parasites infecting commercially important marine fishes are thus scattered throughout this region, several of which comprise surveys of entire parasite faunas of hosts of commercial value (Table 3). Gaevskaya and Kovaleva (Reference Gaevskaya and Kovaleva1980, Reference Gaevskaya and Kovaleva1985) surveyed the parasites of jack mackerel (Trachurus spp.) off the coast of West Africa, emphasizing the importance of using parasites in population studies and the use of parasites to distinguish sub-populations of the Trachurus spp. they studied. Shukhgalter (Reference Shukhgalter2004) surveyed parasites of chub mackerel (S. japonicus) in the same region, showing the occurrence of differences in parasite assemblages infecting these fish off the coasts of Morocco and Mauritania, and thus the possible existence of two sub-populations of this fish species in those regions. The presence of Kudoa histolytica in the musculature of S. japonicus was shown to have negative effects on the commercial value of this species. Several parasites that were of concern for human health were also recorded (larvae of Bolbosoma sp. that occurred off the Azores Archipelago bank; Anisakis simplex and Contracaecum sp. that occurred in all the areas sampled). Kijewska et al. (Reference Kijewska, Dzido, Shukhgalter and Rokicki2009) surveyed the anisakid parasites of six commercially important fish species caught over the West African Shelf (Table 3).
In an extensive study by Spanish parasitologists, Castro-Pampillón et al. (Reference Castro-Pampillón, Rodríguez-Domínguez, Soto-Búa, Mejuto-García, Arias-Fernández and García-Estévez2002a , Reference Castro-Pampillón, Soto-Búa, Rodríguez-Domínguez, Mejuto-García, Arias-Fernández and García-Estévez b ) surveyed the parasites of swordfish (Xiphias gladius) in the Gulf of Guinea for the purposes of identifying species that may act as suitable biological tags (Table 3). Subsequently, Garcia et al. (Reference Garcia, Mattiucci, Damiano, Santos and Nascetti2011) compared the metazoan parasite faunas of X. gladius from four regions in the Atlantic, finding that certain parasite taxa showed varying levels of infection from these different regions. Hysterothylacium corrugatum (s.l.) and Rhadinorhynchus pristis were more abundant in fish caught in the northwest, and Anisakis paggiae and Hysterothylacium incurvum in fish caught in the southern areas, while A. simplex (s.s.) was common in fish from all northern localities. The results of this study supported the existence of at least two distinct sub-populations of X. gladius in the Atlantic, which corresponds to the results of genetic stock structure of this species.
In Nigeria, efforts to document parasites infecting fishes of commercial value has led to numerous publications on surveys of parasite faunas (Table 3). Anyanwu (Reference Anyanwu1983) described the nematode Philometra translucida from the ovaries of commercially harvested croaker species (Pseudotolithus senegalensis, P. typus and P. elongatus), and Obiekezie (Reference Obiekezie1986) described a coccidian, Goussia ethmalotis, from west African shad, Ethmalosa fimbriata. Obiekezie et al. (Reference Obiekezie, Möller and Anders1988) published data on parasitic infections and gross external lesions of commercially harvested bagrid catfish, Chrysichthys nigrodigitatus from the Cross River Estuary and Obiekezie et al. (Reference Obiekezie, Anders, Lick, Möller and Palm1992) conducted a large-scale survey of parasites infecting commercial fishes from inshore off Nigeria. A large-scale survey of trypanorhynchid cestodes infecting commercial inshore fishes off the Nigerian west African coast was conducted by Palm et al. (Reference Palm, Obiekezie and Möller1994) recording nine species and reporting that the low incidence of infection of all species did not pose a threat to the marketability of commercial fishes in this region.
Southern Africa (Angola, Namibia, South Africa, Mozambique, Madagascar)
There are several ocean currents that drive environmental conditions around the coast of southern Africa (Fig. 1). On the west coast, cold, nutrient-rich water is carried to the surface by the wind-driven Benguela current that originates to the west of the South Atlantic current and transports this water northwards past the west coast of South Africa and the coasts of Namibia and Angola. The Benguela Current is one of four major upwelling systems in the world, but unlike other upwelling systems, the Benguela is flanked on either side (north and south; 19–34°S) by two stratified sub-tropical or warm-temperate boundary regions (Hutchings et al. Reference Hutchings, van der Lingen, Shannon, Crawford, Verheye, Bartholomae, van der Plas, Louw, Kreiner, Ostrowski, Fidel, Barlow, Lamont, Coetzee, Shillington, Veitch, Currie and Monteiro2009) and is itself divided into the northern and southern Benguela by the powerful Lüdertiz upwelling cell, which is present at 26°S (Fig. 1). This wind-driven upwelling system supports an intensive fishing industry dominated by small pelagic species such as sardine (Sardinops sagax), anchovy (Engraulis encrasicolus) and horse mackerel (Trachurus capensis) as well as larger pelagics such as the Cape hakes (Merluccius capensis and Merluccius paradoxus).
On the eastern coast of southern Africa, conditions are entirely different. Here the warm Agulhas Current, fed by the Mozambique and East Madagascar Currents (Fig. 1), transports sub-tropical water from the equator southwards past Madagascar, Mozambique and the eastern shores of South Africa, eventually forming a retroflection to re-join the Indian Ocean Gyre near Cape Agulhas (Fig. 1) (Heileman et al. Reference Heileman, Lutjeharms and Scott2009). Warmer waters, lower nutrient levels and high species diversity dominate the eastern shores of southern Africa. The existence of these very different environmental conditions around the coast of southern Africa makes this, the southern tip of the African continent, one of the most species-rich marine regions in the world.
Large-scale marine fisheries in Namibia and South Africa are well developed and in both these countries, demersal Cape hake (Merluccius capenis and M. paradoxus) fisheries are the most valuable. In Namibia, the second most important fishery is that for horse mackerel (Trachurus capenis and Trachurus trecae), followed by small pelagic species such as sardine (S. sagax). In South Africa, the largest fishery in terms of annual tonnage landed is that for small pelagics (sardine, S. sagax; anchovy, E. encrasicolus; round herring, Etrumeus whiteheadi), followed by the fishery for horse mackerel (T. capensis). Other important fisheries off Namibia and South Africa include the line fish, netfish such as Liza richardsonii (South African mullet; harder) and Callorhynchus capensis (St Joseph shark) as well as several species of shark (DAFF, 2012).
In Mozambique, small-scale artisanal fishermen dominate the fishing industry and contribute around 80% of the annual catch landings which are on average about 120 000 t (FAO, 2007). Unlike other coastal African countries, the most important marine species harvested in Mozambique are crustaceans (especially prawns, deepwater shrimp, crayfish, lobsters and crabs). Marine finfish (demersal) and pelagic species such as grouper (Serranidae), snapper (Lutjanidae), emperor (Pomacanthidae) and sea bream (Sparidae), migratory tuna species (Thunnus alalunga, Thunnus albacares, Thunnus obesus), swordfish (X. gladius) and shark are amongst the harvested species (FAO, 2007). An extensive aquaculture industry has recently been established (in both freshwater and marine environments). The main marine species farmed in Mozambique include black tiger prawn (Penaeus monodon), Indian white prawn (Fenneropenaeus indicus, F. japonicus), pink prawn (Macrobrachium monocerosi), kuruma prawn (Modiolus philippinarum), bivalves (Perna perna, Meretrix meretris, Modiolous philippinarum, Eumarcia pauperculata, Sacrostrea cucullata, Cassostrea gigas and Venerupis japonica) and mud crab (Scylla serrata).
Research on marine parasitic species in southern Africa is probably the best documented out of the entire sub-Saharan African region. Taxonomic studies outnumber applied research by far because of the concerted efforts by taxonomists dating back to the early 1900s. Some of the earliest records of parasite species described from marine fishes in southern Africa were by naturalists such as K.H. Barnard, H.B. Fantham and J.D. Gilchrist who described large numbers of new species of parasites from marine fishes during general surveys of marine life in South Africa during the early 1900s. Subsequently specialist taxonomists, in South Africa in particular, have made tremendous progress in describing new parasitic species and recording new host records (Table 4). Although some parasite taxa have received more attention than others, all groups have been investigated in some way by passionate taxonomists.
Table 4. Some of the major taxonomic contributors to marine parasitology in southern Africa (see reference list for details of these studies).

Recently, attention has turned to parasites of commercially important species in southern Africa. Current research by scientists from South Africa's Department of Agriculture, Forestry and Fisheries and the University of Cape Town aims to include the use of parasite data in fisheries research for the purposes of improving management strategies for target species. Some of these target species currently being investigated are discussed below, while other studies relating to parasite faunas of commercially valuable fishes in southern Africa are listed in Table 5.
Table 5. Studies of parasites infecting commercial marine fishes in southern Africa (‘Numerous’ = more than five)

Sardine (S. sagax) are the target species caught in Namibian and South African pelagic purse-seine fisheries and are the subjects of the first biological tagging project to be attempted in southern Africa. These fish are distributed around the southern African coastline from southern Namibia in the west to Richards Bay in the east (Coetzee et al. Reference Coetzee, van der Lingen, Hutchings and Fairweather2008). Within this distribution range, the Lüderitz upwelling cell (26°S) off Namibia acts as a powerful environmental barrier for sardine movement, hence it is accepted that a separate sub-population of S. sagax exists off the Namibian coast. In South Africa, the sardine specific fishery is managed as a single population around the entire coastline. There are however several indications that the sardine population in South Africa could be separated into two or even three sub-populations (van der Lingen et al. Reference van der Lingen, Weston, Ssempa and Reed2014). A survey of parasite species infecting S. sagax by Reed et al. (Reference Reed, MacKenzie and van der Lingen2012) identified seven parasite taxa (Table 5), two of which (a digenean ‘tetracotyle’-type metacercariae infecting the eyes and a coccidian, E. sardinae infecting the testes) met the criteria for suitable biological tags as set out by MacKenzie and Abaunza (Reference MacKenzie, Abaunza, Cadrin, Kerr and Mariani2013). Subsequent studies (Ssempa, Reference Ssempa2013; Weston, Reference Weston2013) have further supported the existence of multiple sub-populations of S. Sagax around the coast of southern Africa (van der Lingen et al. Reference van der Lingen, Weston, Ssempa and Reed2014).
Amongst the medium-sized pelagics, Cape horse mackerel (T. capensis) is an important mid-water species found around the coast of southern Africa from northern Namibia/southern Angola to the east coast of South Africa, where they shoal over the continental shelf (DAFF, 2012). A second species, Cunene horse mackerel (Trachurus trecae) occurs off the north coast of Namibia and Angola. Despite the economic importance of these species in both South Africa and Namibia, only a few studies on parasites infecting T. capensis have been documented (Table 5). Some of these are incidental accounts of infection such as Hecht (Reference Hecht1976) who, whilst investigating the biology of six trawl species in the southern Benguela, noted that the testes and liver of T. capenis harboured an unusually high intensity of infection by a nematode from the genus Anisakis. This infection was present throughout the year, with highest intensities peaking from December to April, a time that corresponded with the gonads being in early stages of seasonal development. During this peak level of infection intensity the Anisakis sp. contributed up to 65·8% of the total gonad mass of T. capensis (Hecht, Reference Hecht1976, Reference Hecht1990), raising concern of parasitic castration. No further research took place following these observations. Several years later Gaevskaya and Kovaleva (Reference Gaevskaya and Kovaleva1980) conducted a full parasitological survey of T. capensis specimens collected off the coast of Namibia. These authors recorded numerous species of helminths and two species of protozoan parasites infecting T. capensis off Namibia (Table 5).
Recently Le Roux (Reference Le Roux2013) investigated parasite assemblages of two sub-populations of T. capensis from the northern (Namibian) and southern (South African) Benguela ecosystem, respectively (Table 5). These two sub-populations are believed to be divided by the powerful Lüderitz upwelling cell. Some uncertainty exists regarding the degree of mixing that may occur between these northern and southern Benguela sub-populations of T. capensis. Le Roux (Reference Le Roux2013) found that fish from the northern and southern Benguela sub-populations hosted very similar parasite assemblages, but showed significant differences in infection intensity, prevalence and abundance of four parasite species in particular (the liver coccidian, Goussia cruciata, the gill monogenean Gastrocotyle trachuri, a visceral nematode, Anisakis sp., and a gill copepod Lernanthropus trachuri), reflecting the distribution of these two sub-populations. In a first ever survey of parasites of T. trecae collected off the northern coast of Namibia and southern Angola, Bowker (Reference Bowker2013) recorded six parasite taxa of which four were identifiable to species, two to genus and one to class level (Table 5). The parasite assemblages recorded by Bowker (Reference Bowker2013) were very similar to those for T. capensis (Le Roux, Reference Le Roux2013), yet significant interspecific differences were seen in Anisakis sp. and G. cruciata intensity of infection between these two hosts.
Amongst commercial species in southern Africa, the Cape hakes have been the best studied in terms of their parasite faunas, most likely driven by the tremendous value of these species in this region (Table 5). Two species of Cape hake, M. capensis and M. paradoxus are commercially harvested off the coasts of Namibia and South Africa (Kainge et al. Reference Kainge, Kjesbu, Thorsen and Salvanes2007). Both species are distributed around the southern African coastline from roughly southern Angola in the west to southern Mozambique in the east, varying only in average distribution with depth and distance from the coast. Within South African waters it is assumed that a single population of each species persists, but the number of populations within the entire Benguela Ecosystem is not entirely confirmed. Several indications are that M. paradoxus may comprise a single stock, shared by South Africa and Namibia, while M. capensis appears to consist of two stocks separated by the powerful Lüderitz upwelling cell at 26°S. Studies have shown that M. paradoxus spawns in both Namibian and South African waters, while M. capensis spawns only in South African waters (Burmeister, Reference Burmeister2005; Kainge et al. Reference Kainge, Kjesbu, Thorsen and Salvanes2007). Furthermore, M. capensis show morphological variation (colour of the anal fin and iris) between these two regions (Durholtz et al. Reference Durholtz, Singh, Fairweather, Leslie, van der Lingen, Bross, Hutchings, Rademeyer, Butterworth, Payne and Yáñez-Aranciba2014). Yet, recent genetic analyses (von der Heyden et al. Reference von der Heyden, Lipiński and Matthee2007, Reference von der Heyden, Lipiński and Matthee2010) have not successfully clarified the current perceptions of stock structure of these fishes by showing no differences in mitochondrial DNA between M. capensis from Namibia and South Africa, but showing significant genetic differentiation between adult M. paradoxus from these two regions. Currently both species are managed separately in South Africa and Namibia (Durholtz et al. Reference Durholtz, Singh, Fairweather, Leslie, van der Lingen, Bross, Hutchings, Rademeyer, Butterworth, Payne and Yáñez-Aranciba2014), but were it the case that the M. paradoxus population is shared between these countries, serious re-evaluation of management strategies should be considered.
Parasite studies of these fishes date back to Davies and Beyers (Reference Davies and Beyers1947) who recorded a high incidence of the myxozoan K. thyrsites (previously Chloromyxum) in M. capensis and M. paradoxus. Barnard (Reference Barnard1955a ) described two ectoparasitic copepods from the buccal cavity of these fish (Chondracanthus merluccii and Parabrachiella australis). A further three helminth parasites were identified by Meyer-Rochow (Reference Meyer-Rochow1972): Dibothriorhynchus grossum, Tetrarhynchus sp. and Livoneca reynaudii. Krzeptowski (Reference Krzeptowski1980) recorded larval A. simplex and Hepatoxylon trichiuri in the body cavities of both species collected off Namibia. Aleshkina (Reference Aleshkina1982) surveyed the parasite fauna of both M. capensis and M. paradoxus off Namibia and determined that the parasitic species composition was dependent on the age of the hosts. Subsequently Botha (Reference Botha1986) examined the major endoparasites of both M. capensis and M. paradoxus. Botha (Reference Botha1986) compared the dominant helminth fauna between the two species, while Reimer (Reference Reimer1993) showed that the parasite fauna of both species differed remarkably above and below the 25°30′S latitude, suggesting that fish from each of these regions could originate from separate sub-populations. This study was not a dedicated biological tagging project, but provided some insight into possible differences in these fish populations based on parasite assemblages. Most recently, parasitic species infecting Cape hakes, M. paradoxus and M. capensis, from South African waters were examined (Reed et al. unpublished data) revealing the regular occurrence of nine different parasite taxa (Table 5). This study aims to specifically collect parasite data for use as biological tags to contribute to understanding the number of Cape hake sub-populations in southern Africa.
Cape ‘snoek’, Thyrsites atun, is a valuable large pelagic commercial species that occurs predominantly in the Benguela Ecosystem from the Cunene River mouth in Namibia to Cape Agulhas in South Africa. This species is an important predator of small pelagics in the Benguela (Griffiths, Reference Griffiths2002) and is also traditionally one of the most valued food species caught by local fisherman in the Western Cape Province of South Africa. One of the oldest and most significant records of a parasitic species from South Africa is the original description of the myxozoan K. thyrsites from T. atun. Gilchrist (Reference Gilchrist1924) described Kudoa (Chloromyxum) thyristes from the muscles of Cape ‘snoek’, reporting that the flesh of those fish infected with this myxozoan became soft and liquid after death. Several studies subsequently recorded the presence of this parasite in fish musculature from this region (Davies and Beyers, Reference Davies and Beyers1947; Dubina and Isakov, Reference Dubina and Isakov1976, Gaevskaya and Kovaleva, Reference Gaevskaya and Kovaleva1979; Schulman et al. Reference Schulman, Kovaleva and Dubina1979) and K. thyrsites is today known as a notorious cosmopolitan species negatively affecting the quality of fish fillets in many parts of the world.
The widespread occurence of this parasite in other commercially valuable fish species in South Africa, such as S. sagax, led to several studies examining aspects of its infection by scientists at South Africa's Department of Agriculture, Forestry and Fisheries (Lamprecht et al. Reference Lamprecht, Avery, Fick and de Korte1989; Avery et al. Reference Avery, Lamprecht and de Korte1990; Webb, Reference Webb1990, Reference Webb1993; Matta and Cloete, Reference Matta and Cloete1992). In acknowledgement of the economic concerns of K. thyrsites infections, Henning et al. (Reference Henning, Hoffman and Manley2013) reviewed the occurrence of Kudoa-induced myoliquefaction in South Africa and other parts of the world. These authors recognized the need for an early detection mechanism for Kudoa infection in commercially harvested hosts, such as T. atun, and suggest the use of technologies such as ionizing irradiation of fish fillets to prevent the effects of myoliquefaction. These technologies still need to be developed in South Africa, and hence an opportunity exists for food scientists such as the authors of Henning et al. (Reference Henning, Hoffman and Manley2013) to make significant contributions towards reducing potential food wastage.
Recent medical research involving parasites of T. atun (and other species such as M. capensis and M. paradoxus) from South Africa includes that of Nieuwenhuizen et al. (Reference Nieuwenhuizen, Lopata, Jeebhay, Herbert, Robins and Brombacher2006, Reference Nieuwenhuizen, Herbert, Brombacher and Lopata2009, Reference Nieuwenhuizen and Lopata2013), Kirstein et al. (Reference Kirstein, Horsnell, Nieuwenhuizen, Ryffel, Lopata and Brombager2010) and Nieuwenhuizen and Lapata (Reference Nieuwenhuizen and Lopata2013) who examined, amongst other things, the effects of exposure of fish workers to anisakid nematodes and the associated upper respiratory tract allergies that are manifested in these fish workers who handle species such as T. atun in fish factories in the Western Cape Province of South Africa. A current project (Nunkoo, unpublished data) is investigating temporal and spatial variation in parasite assemblages of T. atun off the coast of South Africa.
More than 180 species of sharks, skates, rays and chimaeras are known to live in coastal inshore waters of southern Africa, 98 of which are harvested commercially (DAFF, 2012). Limited, and again mostly taxonomic research has been conducted on the parasite species associated with numerous shark species off southern Africa, with parasitic Copepoda being extremely well documented (Tables 4 and 5). Some studies, such as that of Yeld (Reference Yeld2006), examined whole parasite assemblages of endemic shyshark species on the west and south coasts of South Africa. Amongst the commercially harvested sharks, the small chondrichthyan, Callorhinchus capensis, which is caught in the demersal longline and trawl fisheries off the west and south coasts of South Africa has received more general parasitological attention than others. This species is known to harbour a unique lineage of cestodes from the genus Gyrocotyle. Linton (Reference Linton1924) was the first to describe Gyrocotyle plana from C. capensis in False Bay. Subsequently a species of monogenean (Callorhynchicola multitesticulatus) was described from the gills by Manter (Reference Manter1955) and later recorded once more by Beverly-Burton et al. (Reference Beverly-Burton, Chisholm and Allison1993). Recently, Bih-Awa (Reference Bih-Awa2012) conducted a survey of parasites infecting this species on the west coast of South Africa recording five species, including the two previously mentioned (Table 5). A current project (Morris, unpublished data) is investigating the accumulation of heavy metals in parasites of C. capensis off the coast of South Africa.
Several taxonomic studies have recorded parasitic species infecting fishes, cephalopods and crustaceans off the coasts of Mozambique and Madagascar. Important contributions include the works of Reimer (Reference Reimer1984, Reference Reimer1989) who recorded parasites associated with prawns, fishes and cephalopods in Mozambique, Palm et al. (Reference Palm, Walter and Schwerdtfeger1997) who recorded numerous species from the trypanorynch (Cestoda) genus Nybelinia and Kensley et al. (Reference Kensley, Schotte and Poore2009) who recorded 12 new species of parasitic isopods from the family Gnathiidae (Tables 4 and 5).
East coast of sub-Saharan Africa
Environmental conditions off the east African coastal countries of Tanzania, Kenya, Somalia and Comores are driven by the southwards flowing Somali Coastal Current and the northwards flowing East African Coastal Current that meet and then diverge off the coast of Kenya (Fig. 1). This region boasts a rich diversity of important habitats such as mangroves, coral reefs, seagrass beds and estuaries, even including the presence of several endangered marine turtle and whale species, dugong and the CITES-listed coelacanth, Latimeria chalumnae. The majority of fisheries along this coastline consist of subsistence and artisanal fisheries that are confined to inshore areas due to the ease of access and lack of technology to fish in offshore waters. More than 96% of marine fisheries in Tanzania and 80% in Kenya are due to small-scale artisanal fishers. In Somalia, a larger industrial fishing sector exists that contributes about 40% to the annual landings, with an artisanal sector operating in inshore areas and accounting for most of the landings (60%). More than 500 species of fish are caught for food in this region, with reef fishes being the most important category including emperors (Lethrinidae), snappers (Lutjanidae), sweetlips (Plectorhinchus sp.), parrotfish (Scaridae), surgeonfish (Acanthuridae), rabbitfish (Siganidae), groupers (Serranidae) and goatfish (Mulidae) (Jiddawi and Ohman, Reference Jiddawi and Ohman2002). Off shore fisheries are dominated by distant fleets from Europe and East Asia.
Several taxonomic reports (such as Bassett-Smith, Reference Bassett-Smith1903; Parukhin, Reference Parukhin1976a , Reference Parukhin b , Reference Parukhin1978; Moravec et al. Reference Moravec, Orecchia and Paggi1988; Bruce and Bowman, Reference Bruce and Bowman1989; Hadfield et al. Reference Hadfield, Bruce and Smit2011) are known from this region as well as a few studies that describe the parasite assemblages associated with single host species, such as Martens and Meons (Reference Martens and Meons1995) who described the ecto- and endo-parasites infecting rabbitfish, Siganus sutor off the Kenyan Coast (Table 6) and Aloo et al. (Reference Aloo, Anam and Mwangi2004) who conducted a survey of parasitic species infecting commercially important marine fishes in Kenya (Table 6).
Table 6. Studies of parasitic species infecting commercial marine fishes in east Africa. (‘Numerous’ = more than five fish hosts examined)

Some studies from this region of particular interest include that of Kamegai (Reference Kamegai1971) who described a new genus and species of monogenea (Dactylodiscus latimeris) from the gills of L. chalumnae caught off Anjouan Island, Comoros. Two larval helminths, Tentacularia sp. (Cestoda) and Anisakis sp. (Nematoda), were also recorded from this specimen of coelacanth. Thoney and Hargis (Reference Thoney and Hargis1991) subsequently recorded juvenile anisakid nematodes from two coelacanths captured in waters off the Comoros Islands. Eight third-stage anisakines of the genus Terranova or Pulchrascaris were collected from the spiral valve and rectum of the two coelacanths examined. Anderson (Reference Anderson1993) also reported on 3rd stage larval anisakids coiled in the mesenteries of a female coelacanth examined at Guelf.
Future prospects for applied marine parasitology research in sub-Saharan Africa
Despite the lack of applied parasitological studies relating directly to marine fisheries management in sub-Saharan Africa, there exists a tremendous amount of information on parasitic species infecting certain fishes of commercial value due to the efforts of taxonomists. The availability of these data provides a foundation for future applied parasitological studies of economically important fishes that could aid in sustainable management strategies and will provide an added biological understanding of these important natural resources in this region. Aquatic parasitologists (marine and freshwater) in Africa have a tremendous opportunity to rapidly advance this field of research by documenting new species and also recording species assemblages associated with certain hosts in different regions. There is also an urgency to begin collecting long-term datasets on key commercial species. Inferring information from single-year datasets is not ideal in a constantly changing environment influenced by El Niño, La Niña and numerous other anthropogenic pressures such as over-fishing and global climate change.
ACKNOWLEDGEMENTS
The University of Cape Town Research Committee and the National Research Foundation of South Africa. Many thanks to Dr Marjolaine Krug (CSRI, Earth Observation Research Group, Cape Town) for assistance in creation of the SST map for Africa as seen in Fig. 1.










