INTRODUCTION
The host immune response is a significant feature of the environment of parasitic nematodes. Because hosts develop an adaptive immune response, the immune environment to which parasitic nematodes are subject can vary both during the lifetime of a nematode within a host and (for different individual nematodes) between different infective episodes of the same host. For many parasitic nematodes it has been shown that the host immune response reduces both the survival of parasitic stages and their fecundity. This has been examined in detail with Strongyloides ratti, a parasite of rats. The parasitic stages of S. ratti are parthenogenetic female worms only, which occur in the mucosa of the small intestine. As an infection progresses and the rats' immune response develops, the length and per capita fecundity of parasitic female worms are reduced and the distribution of parasitic females in the small intestine is altered, so that they have a greater posterior extent (Moqbel and McLaren, 1980; Kimura et al. 1999; Wilkes et al. 2004). The host immune response is also responsible for density-dependent reductions in survival of parasitic females (Paterson and Viney, 2002). All of these immune-dependent effects either do not occur in immune-deficient rats or are reversed if either the parasitic females are transplanted to naïve rats or if immune rats are artificially immunosuppressed (Moqbel and McLaren, 1980; Moqbel et al. 1980; Paterson and Viney, 2002; Wilkes et al. 2004). Immunization of rats with an abbreviated S. ratti infection reduces the survival, size and per capita fecundity and results in an increase in the proportion of worms that are located in the distal sections of the small intestine, compared with worms from non-immunized rats (Gemmill et al. 1997; Paterson and Viney, 2002; Wilkes et al. 2004). Analysis of the host immune response against S. ratti has shown that the systemic response against migrating larvae and the intestinal immune response against intestinal larvae and parasitic females are different (Bell et al. 1981).
In S. ratti infections in naïve rats, parasitic females are approximately 2·4 mm in length at the start of an infection but, as an infection progresses these worms become shorter, such that by 20 days post-infection (p.i.) they are approximately 1·5 mm in length (Moqbel et al. 1980; Wilkes et al. 2004). Such reductions in length can be completely reversed if the worms are transplanted to naïve hosts (Moqbel et al. 1980) or if the rats are immunosuppressed (Wilkes et al. 2004). Worms that develop in rats that have been immunized against S. ratti are approximately 1·7 mm at the start of an infection, and they become progressively shorter as the infection progresses (Wilkes et al. 2004).
In the S. ratti life-cycle, infective third-stage larvae (iL3s) infect hosts by skin penetration and then migrate via the naso-frontal region of the host before being swallowed and settling in the small intestine (Tindall and Wilson, 1988). Stages are first found in the intestine at 36–38 h p.i., with patency being achieved at 4-5 days p.i. Infective L3s are approximately 500 μm in length and thus they increase in length by approximately 5-fold in their growth to adulthood. In hosts that have been immunized against S. ratti, one can envisage that the consequent immune response imposes a stress (e.g. limitation of available food, redirection of resources to immune protection) on the larvae as they develop and grow into parasitic females, that prevents the worms from growing as they would in S. ratti-naïve hosts (Moqbel and McLaren, 1980; Viney, 2002). Such a scenario raises the question (for S. ratti in particular and nematodes in general) of whether or not the occurrence of these effects during the growth and development of the juvenile stages have life-long consequences? Two possibilities, at least, exist. Firstly, maximum size and fecundity, may be fixed at a certain point of development; for example, the size that is achieved at the start of reproduction. Alternatively, a species-specific maximum size and fecundity may exist, to which any individual will grow, given the opportunity. A previous study has shown the complete reversibility of immune damage in S. ratti (Moqbel et al. 1980). However, in these cases the worms developed in naïve hosts, such that they were able to achieve maximum size and fecundity, before becoming subject to the effect of the host immune response. This previous study transplanted worms from secondary infections (and therefore, presumably, subject to the host immune response during their larval development) to naïve rats, which resulted in the partial recovery of the size and fecundity of the 20–40% of parasitic females that survived the transplant (Moqbel et al. 1980).
Here we have investigated whether the reduced size and per capita fecundity of worms that have developed in the presence of the host immune response, are reversed if those hosts are immunosuppressed. This was done by the use of immunization and immunosuppression regimes. We have observed the full recovery of the per capita fecundity and partial recovery of size, of parasitic females from immunized and immunosuppressed hosts. We also observed that the host immune response affects the allometry of the parasitic female.
MATERIALS AND METHODS
Overview and rationale
The principal purpose of this experiment was to determine whether the reduction in size and per capita fecundity of parasitic females of S. ratti that have developed in rats that have been immunized with S. ratti, was reversed if those hosts are immunosuppressed. Four treatment groups were used: Group A were immunized and then immunosuppressed; group B were immunized only; control group C were naïve (i.e. non-immunized) but then immunosuppressed and control group D were naïve (i.e. non-immunized) only (Table 1).
Table 1. A summary of the four treatment regimes (The details of the treatments are given in the accompanying text.)

Parasites and infections
S. ratti isofemale line ED321 Heterogonic was used and was maintained as previously described (Wilkes et al. 2004). Twenty-eight female Wistar rats weighing approximately 100 g were used for each of the 4 groups. All rats from Groups A and B were administered subcutaneously with 100 iL3s (Wilkes et al. 2004) on day 0 p.i. rats in Groups C and D were given a sham infection of an equal volume of PBS. To remove this immunizing infection, all rats (Groups A–D, inclusive) were treated with the anthelminthic thiabendazole on days 11 and 12 p.i. as previously described (Paterson and Viney, 2002). On day 14 p.i., (that corresponds to day 0 post-challenge infection (p.c.i.)) all animals were administered 1000 iL3s. Daily, on days 5–9 p.c.i. inclusive, all rats in Groups A and C were administered intraperitoneally 0·4 mg dexamethasone/kg body weight; Groups B and D were contemporaneously given a sham inoculation of an equal volume of PBS. These treatment regimes are summarized in Table 1. On days 4, 5, 8, 11, 14 and 21 p.c.i. faeces were collected from 4 randomly selected animals from each group, the faeces cultured, maintained at 19 °C and the total reproductive output of each infection determined, as previously described (Paterson and Viney, 2002). These same animals were sacrificed on days 5, 6, 9, 12, 15 and 22 p.c.i., respectively, and the parasitic females were recovered from each animal and fixed, as previously described (Wilkes et al. 2004). Four animals from each group were sacrificed on day 4 p.c.i. and parasitic females similarly recovered and fixed. In all cases the number of parasitic females from each rat was determined. For each rat the lengths of at least 10 worms (when available) were determined, as previously described (Wilkes et al. 2004). To investigate any allometric changes that occurred in parasitic females recovered from rats on days 6, 9 and 12 p.c.i. in response to these treatments, the positions of the oesophagus-intestine junction, of the vulva (both measured from the worm's anterior end), the anterior extremity of the anterior gonad arm, the posterior extremity of the posterior gonad arm (both measured from the vulva), and the body width at the vulva, were determined as a proportion of the total length of each worm. For each rat, the parasitic female per capita fecundity was determined as the total reproductive output per rat on day x, divided by the number of parasitic females recovered on day x+1 (Wilkes et al. 2004). The parasitic females recovered from 1 rat in Group D and 1 rat in Group C sacrificed on day 12 p.c.i. were thought to be muddled during processing, and therefore have been excluded.
Statistical analyses
The per capita fecundity data were log(x+1) transformed to ensure normality of residuals and homogeneity of variance; the worm length data required no transformation. The worm length data were analysed as a two-by-two factorial design of IMMUNIZATION and IMMUNOSUPPRESSION with DAY (or PERIOD, see below) as an additional factor using a mixed model GLM. Because groups of worms were obtained from individual rats, to avoid pseudoreplication, RAT was included as a random factor, nested within IMMUNIZATION, IMMUNOSUPPRESSION and DAY (or PERIOD). The per capita fecundity data were analysed by a three-way ANOVA with a factorial design of IMMUNIZATION, IMMUNOSUPPRESSION and DAY, which was possible because there was no pseudoreplication of RAT.
Immunosuppression (treatment Groups A and C) by the administration of the steroid dexamethasone occurred for 5 days (days 5–9 p.c.i.) and it was therefore desirable to compare the length and per capita fecundity of worms before, during and after this steroid administration. This was done by defining days 4 and 5 p.c.i. as ‘before’; days 6, 9 and 12 p.c.i. as ‘during’ and days 15 and 22 p.c.i. as ‘after’ this administration and data for these 3 time-period (i.e. PERIOD) categories were analysed separately. In defining ‘during’ steroid administration, it was assumed that there was a lag between the final administration of the steroid (day 9 p.c.i.) and an effect on the worms (Gemmill et al. 1997).
The data for the relative positions of the body structures were only analysed during the steroid administration, i.e. days 6, 9 and 12 p.c.i. These data were transformed by x1·5 and subsequently analysed by principal component analysis (PCA). This transformation was used to minimize the skew of the data. However, even after this transformation, the skew of the data for the relative position of the vulva was −2·13, which is more than is desirable for PCA analysis (Quinn and Keough, 2002); therefore these vulva data were excluded. A significance level of P<0·01 was used throughout. All analyses were performed using Minitab.
RESULTS
Length of parasitic females
The mean lengths of parasitic females for each treatment group through time are shown in Fig. 1. We first analysed the data including the factor PERIOD, which referred to the sample days before, during or after the administration of steroids. This showed that there were significant effects of IMMUNIZATION, IMMUNOSUPPRESSION and PERIOD, and interactions thereof (IMMUNIZATION F1,976=66·3, P<0·0001; IMMUNOSUPPRESSION F1,976=19·9, P<0·0001; PERIOD F2,976=69·7, P<0·0001; IMMUNOSUPPRESSION∗PERIOD F2,976=5·4, P=0·006; IMMUNIZATION∗IMMUNOSUPPRESSION∗PERIOD F2,976=6·4, P=0·002); there was a significant effect of rat (RAT [IMMUNIZATION∗IMMUNOSUPPRESSION∗PERIOD] F84,976=8·9, P<0·0001). Given the significant effect of PERIOD, each (i.e. before, during and after the administration of steroids) was analysed separately.

Fig. 1. The mean length of parasitic females from rats immunized and immunosuppressed (Group A) (—[bull ]—); immunized only (Group B) (—[squf ]—); naïve (i.e. not immunized) but then immunosuppressed (Group C) (---○---) and naive (i.e. not immunized) only (Group D) (---□---). The period of steroid administration is shown by ↑. Error bars are ±1 S.E.
Before the administration of steroids (days 4 and 5 p.c.i.) animals had either been immunized (Groups A and B) or not (Groups C and D) and this had a significant effect on worm length (IMMUNIZATION F1,397=27·6, P<0·0001) as does DAY (F1,397=29·0, P<0·0001). Thus, worms from immunized rats were shorter (mean±S.E.=1042±21·5; 1279±19·8 μm for days 4 and 5 p.c.i, respectively, for Groups A and B combined) than worms from naïve, non-immunized rats (1263±32·7; 1638±26·1 μm for days 4 and 5 p.c.i., respectively, for Groups C and D combined). The increase in size between days 4 and 5 is likely to reflect the fact that the worms from all groups were still growing. There was a small effect of immunization and future immunosuppression but below our accepted level of significance (IMMUNIZATION∗IMMUNOSUPPRESSION F1,397=5·14 P=0·032); there was a significant effect of rat (RAT[IMMUNIZATION∗IMMUNOSUPPRESSION∗DAY] F24,397=6·4, P<0·0001).
During the administration of the steroids (days 6, 9 and 12 p.c.i.) there were significant effects of IMMUNIZATION, IMMUNOSUPPRESSION, IMMUNIZATION∗IMMUNOSUPPRESSION and DAY (F1,427=91·5, P<0·0001; F1,427=13·1, P=0·0001; F1,427=15·9, P<0·0001; F2,427=17·1, P<0·0001, respectively); there was a significant effect of rat (RAT[IMMUNIZATION∗IMMUNOSUPPRESSION∗DAY] F34,427=4·0, P<0·0001). Thus, during this period of the infection, the worms from the immunized, immunosuppressed rats (Group A) were intermediate in size (mean±S.E. 1765±25·3 μm, for days 6, 9 and 12 p.c.i. combined) between those from naïve rats (Groups C and D) (2033±27·5; 2063±31·6 μm, for Groups C and D, respectively, for days 6, 9 and 12 p.c.i. combined) and from immunized, non-immunosuppressed rats (Group B) (1420±25·7 μm, for days 6, 9 and 12 p.c.i. combined).
A similar pattern remains after the administration of steroids (15 days p.c.i.; there were no data for Group B on day 22 p.c.i) when there were significant effects of IMMUNIZATION and IMMUNOSUPPRESSION (F1,152=17·3, P=0·0001; F1,152=21·7, P<0·0001, respectively); there was a significant effect of rat (RAT[IMMUNIZATION∗IMMUNOSUPPRESSION] F14,152=7·1, P<0·0001). Thus, the worms from the naïve, immunosuppressed rats (Group C) were longer than worms from the immunized, immunosuppressed and the naïve, non-immunosuppressed rats (Groups A and D, respectively) which were, in turn, longer than the immunized, non-immunosuppressed rats (Group B) (Fig. 1).
Thus, overall, these results show that the immunization-dependent reduction in the length of parasitic females (i.e. before steroid administration) was partially reversed by immunosuppression by steroid administration.
Per capita fecundity
The mean per capita fecundities for worms from each treatment group through time are given in Fig. 2. The per capita fecundity was measured both during and after the administration of the steroids. There is a marginally non-significant effect of this PERIOD (F1,60=6·6, P<0·013) and for this reason, and for consistency with the analysis of the worm length data, we then considered the per capita fecundity solely during the period of the administration of steroids. There were significant effects of IMMUNIZATION, IMMUNOSUPPRESSION, DAY, IMMUNOSUPPRESSION∗DAY and IMMUNIZATION∗IMMUNOSUPPRESSION∗DAY (F1,34=49·2, P<0·0001; F1,34=28·1, P<0·0001; F2,34=7·7, P=0·002; F2,34=9·7, P<0·0001; F2,34=5·9, P=0·006, respectively).

Fig. 2. The mean per capita fecundities of parasitic females from rats immunized and immunosuppressed (Group A) (—[bull ]—); immunized only (Group B) (—[squf ]—); naïve (i.e. not immunized) but then immunosuppressed (Group C) (---○---) and naive (i.e. not immunized) only (Group D) (---□---). The period of steroid administration is shown by ↑. Error bars are±1 S.E.
Thus, worms from immunized, non-immunosuppressed rats (Group B) had the lowest per capita fecundity (mean±S.E.=17·3±5·1, for days 6, 9 and 12 p.c.i, combined), but that immunosuppression (Group A) increased the per capita fecundity of the worms (79·1±19·2) to be equivalent to that of the naïve, non-immunosuppressed rats (Group D) (92·2±17·4) and the naïve, immunosuppressed rats (Group C) (201·0±42·2) (Fig. 2). Thus, the immunization-dependent reduction in per capita fecundity was fully reversed upon immunosuppression.
Allometry of parasitic females
The first 3 principal components of the relative positions of the body structures are shown in Table 2. These principal components account for 94% of the variance in the data; principal component 1 accounts for 64% of the variance. The contribution of the 4 relative positions of the body structures to principal component 1 shows this principal component describes parasitic females in which there is increased body size (i.e. greater width and longer oesophagus, shorter intestine) that covaries with reductions in gonad size. Analysis of this principal component of the parasitic females shows that there are significant effects of IMMUNIZATION, IMMUNOSUPPRESSION, IMMUNIZATION∗IMMUNOSUPPRESSION and DAY (F1,188=60·9, P<0·001; F1,188=33·3, P<0·001; F1,188=21·7, P<0·001; F2,188=14·6, P<0·001, respectively). There was also a marginally non-significant effect of IMMUNOSUPPRESSION∗DAY (F2,188=4·6, P=0·017); there was a significant effect of rat (RAT[IMMUNIZATION∗IMMUNOSUPPRESSION∗DAY] F35,188=2·8, P<0·0001). There are no significant effects acting on principal component 2.

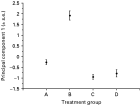
The mean of principal component 1 for each of the treatment groups shows that those in Group B (immunized only) are different from worms in all other treatment groups (Fig. 3). Inspection of the mean relative positions of these body structures also shows the greater relative posterior position of the oesophagus-intestine junction, the shortening of both gonad arms and the increase in width in worms under immune pressure (Group B), fully consistent with the PCA results (Fig. 4)

Fig. 3. The mean of principal component 1 of the relative positions of body structures of worms from the four treatment groups (Groups A–D) at days 6, 9 and 12 p.c.i. Error bars are±1 S.E.
Overall, this shows that the allometry of worms are significantly altered by the host immune response, with a significant reduction in the proportionate size of the gonad occurring in worms under immune pressure (Group B). However, these allometric changes in worms from immunized hosts can be fully reversed by immunosuppression.

Fig. 4. A diagrammatic representation of the mean relative positions of body structures of worms from the four treatment groups (Groups A–D) at days 6, 9 and 12 p.c.i., where O – oesophagus-intestine junction; A – anterior extremity of anterior gonad arm, V – vulva and P – posterior extremity of posterior gonad arm. Body width (W) at the vulva is shown as separate horizontal bars (right); width is shown at 10-times the scale of the length proportions.
DISCUSSION
The purpose of this study was to determine if the putative stresses imposed on developing larvae and young adults by hosts immunized against S. ratti, imposes a life-long constraint on the size and per capita fecundity of adult worms. This was investigated by comparing the size and fecundity of parasitic females that had developed in rats immunized with S. ratti that were subsequently immunosuppressed, with parasitic females from naïve rats. Overall, the results showed that the immunization regime resulted in shorter and less fecund parasitic females, compared to non-immunized rats. This reduction in size was comparable to that observed with a range of immune pressures used in directly analogous studies (Wilkes et al. 2004). The size reduction observed here was partially reversed and the per capita fecundity reduction was fully reversed, upon host immunosuppression. Thus, it can be concluded that this immunization regime imposes a life-long constraint on the size of parasitic females of S. ratti, but not upon their per capita fecundity. A previous study in which parasitic females from a secondary infection were transplanted to naïve rats, showed a partial recovery of the size and per capita fecundity of these parasitic females (Moqbel et al. 1980). In this work (Moqbel et al. 1980), the secondary infections appear to have been subject to a more severe immunization effect (as judged by the size of the parasitic females) than in the study presented here. This may suggest that the ability of parasitic females of S. ratti to recover from the deleterious effects of the immune response may be inversely related to the severity of the effects of the immune response.
The fecundity of parasitic nematodes is central to their transmission and thus is likely to be a major component of their fitness. In this respect, the absence of a life-long constraint on the fecundity of S. ratti is notable. In natural infections it can be envisaged that the immune status of hosts may vary due to a number of factors (e.g. other infections, nutrient limitation, reproductive status, etc.) during the life (including migratory and pre-patent periods) of a parasitic nematode. Therefore, the ability of a parasitic nematode to ‘recover’ from the deleterious effects of the host immune response is likely to be advantageous.
Cross-species comparisons of life-history traits of parasitic nematodes has shown an association between the per capita fecundity and adult female body size, such that bigger nematodes have a greater per capita fecundity (Skorping et al. 1991). For Teladorsagia circumcincta infections it has been shown that the immune response of young sheep reduces the faecal egg output, by reducing the size, and hence per capita fecundity, of worms rather than by reducing the number of worms (Stear et al. 1997). The control of body size of the model free-living nematode Caenorhabditis elegans and of other rhabditid nematodes has been investigated. This has shown that the principal control of body size is via the size, rather than the number, of somatic cells (Suzuki et al. 1999; Flemming et al. 2000), athough there are also effects of collagen genes, which presumably affect the cuticle (Nyström et al. 2002). These studies with C. elegans do not suggest the basis of a causal relationship between body size and fecundity. It is possible that there are pleiotropic effects of the control of cell size on fecundity, though this has not been demonstrated (Knight et al. 2001; Leroi, 2001). Alternatively, fecundity could, simply, be constrained by an individual's body size, for example due to the physical limitation of the size of the gonad etc. Assuming that size is similarly determined for S. ratti (order Rhabditida) this would suggest that the immunization regime used here in some way constrains the growth of cells of developing larvae. For free-living nematodes in which the control of body size has been investigated, it is not known how alterations in the growth of juveniles affect adult body size. The observations on S. ratti presented here, that the immunization-dependent constraint on size is reversed by immunosuppression, show that parasitic females of S. ratti can increase in size during adulthood. Substantial adult growth of rhabditid nematodes has also been observed (Flemming et al. 2000).
In addition to the observations on the overall size of parasitic females of S. ratti in response to different immunization and immunosuppression regimes, the allometry of these parasitic females was also determined. These observations showed that parasitic females under immune pressure (Group B) are not only small, but also that proportionately less of their body is gonad; these worms also have the lowest per capita fecundity. These results suggest that in seeking to understand the relationship between body size and fecundity of nematodes (including free-living species), the allometric changes also need to be considered. These observations with S. ratti suggest that the physical size of the gonad per se determines or limits the fecundity of nematodes.
The means by which the host immune response brings about these effects on the size and per capita fecundity of S. ratti has been previously considered. This showed that as a primary infection continued there was evidence of damage to the intestine, and other tissues, of the parasitic females and that the parasitic females had oral plugs that contained host antibody (Moqbel and McLaren, 1980). Overall, this suggests that the metabolic activity of the parasitic females is detrimentally altered (directly or indirectly) by the host immune response. The apparent consequences of this (reduced body size, gonad size, per capita fecundity) suggest that maintenance of these features is an active process. Subcurative doses of anthelmintic drugs can bring about changes in the per capita fecundity of S. ratti analogously to the effect of the host immune response, which has also been hypothesized to occur by the drugs bringing about energetic constraints (Crook and Viney, 2005).
Substantial programmes of gene discovery are underway for a range of parasitic nematodes, including S. ratti and S. stercoralis (Parkinson et al. 2003; Mitreva et al. 2004; Thompson et al. 2005). These resources are now being used particularly to construct DNA microarrays which are being used to determine changes in gene expression that occur as S. ratti is subject to the host immune response (Viney, 2002). The observations presented here of how parasitic females of S. ratti are affected by changes in its immune environment will allow precise manipulation of the status of these parasites with respect to the host immune response.
We would like to thank W. Nickell, K Faniodoukas, M. Wilson, V. Panizzon and S. Iles-Ryan for technical help, M. Gardner for statistical advice and F. Thompson for comments on this work. This work was supported by the MRC.