Published online by Cambridge University Press: 14 September 2004
Reservosomes are large membrane-bound organelles found at the posterior end of epimastigote forms of Trypanosoma cruzi, but absent in amastigotes and trypomastigotes. We have transferred bloodstream trypomastigotes to LIT medium supplemented with gold-labelled transferrin in order to analyse, at the ultrastructural level, the occurrence of reservosomes and endocytosis during the trypomastigote to epimastigote differentiation. After 24 h, the trypomastigotes differentiated into amastigotes, which adhered to each other forming large clusters. Electron-dense vesicles were detected close to the Golgi complex in cells with intermediary characteristics between amastigotes and epimastigotes, but typical reservosomes at the posterior cell tip were still absent. Transferrin–gold complexes were observed only bound to the surface of clustered cells. After 72 h, epimastigotes were observed being released from the clusters and free-swimming epimastigotes appeared, containing electron-dense vesicles at their posterior region. Typical reservosomes, labelled with transferrin–gold, were observed only in free-swimming epimastigotes. When fully differentiated epimastigotes were incubated with transferrin–gold complexes and then processed for the immunocytochemical detection of cysteine proteinase, all reservosomes were positive for the enzyme, but co-localization of both markers did not occur in all organelles. Our data demonstrate that in T. cruzi epimastigotes endocytosis is strongly related to reservosome biogenesis during the trypomastigote to epimastigote differentiation process.
Reservosomes are large membrane-bound organelles found at the posterior region of epimastigote forms of Trypanosoma cruzi, the aetiological agent of Chagas' disease (Soares, 1999; De Souza et al. 2000; De Souza, 2002). These structures were initially described as multivesicular bodies (De Souza et al. 1978), but cytochemical analysis revealed that reservosomes present, in fact, an electron-dense proteic matrix with lipid droplets (Soares & De Souza, 1988). Studies using gold-labelled proteins have demonstrated that nutrients taken up through the cytostome and flagellar pocket are delivered to the reservosomes (Soares & De Souza, 1991; Soares, Souto-Padrón & de Souza, 1992; Porto-Carreiro et al. 2000), thus showing that these organelles are compartments of the endocytic pathway.
In culture epimastigote forms, the reservosomes occupy about 6% of the total cell volume (Soares & De Souza, 1988), but these structures gradually vanish in old cultures and during the in vitro differentiation from epimastigotes to metacyclic trypomastigotes (Soares et al. 1989; Figueiredo, Rosa & Soares, 2000). It has been suggested that proteins accumulated in the reservosomes are used as an energy source for this differentiation process (Soares et al. 1992; Figueiredo, Steindel & Soares, 1994). Indeed, biochemical studies revealed that amino acid metabolism is favoured during metacyclogenesis (Urbina, 1994). It has been proposed that a nutritional stress triggers the differentiation process, leading to the utilization of reservosome contents and the complete disappearance of these organelles in amastigote and trypomastigote forms (Soares et al. 1989; Figueiredo et al. 1994). Recent data demonstrated this close relationship between nutritional stress and metacyclogenesis in T. cruzi: addition of transferrin to the TAU3AAG medium developed by Contreras et al. (1985) reverted the metacyclogenesis and promoted epimastigote growth (Figueiredo et al. 2000). Intense transferrin uptake was observed in differentiating epimastigotes, but no transferrin labelling could be observed in trypomastigote forms.
Although reservosomes seem to play a major role in the life-cycle of T. cruzi, the biochemical and functional characteristics of these organelles, as well as their biogenesis in epimastigotes, are only partially known. Immunocytochemical data showed that reservosomes present an acidic pH (about 6·0), contain cysteine proteinase (cruzipain) and may correspond to pre-lysosomal compartments, where ingested proteins are accumulated (Soares et al. 1992; De Souza et al. 2000). However, molecular markers of the endocytic pathway are still lacking to more precisely define this organelle. It has been recently suggested that a homologue of Rab11 – a GTPase involved in receptor recycling to the plasma membrane (Sheff et al. 1999) – may be localized in the reservosomes (Mendonça et al. 2000). The recent isolation and biochemical characterization of a purified reservosome fraction (Cunha-e-Silva et al. 2002) may allow the future identification of molecular markers for this organelle.
In this study we have transferred Trypanosoma cruzi bloodstream trypomastigote forms to LIT medium supplemented with gold-labelled transferrin in order to analyse, at the ultrastructural level, the occurrence of reservosomes and the uptake of nutrients during the differentiation of trypomastigotes to epimastigotes.
The Y strain of Trypanosoma cruzi was used in all experiments. Bloodstream trypomastigotes were obtained from Swiss albino mice at the peak of parasitaemia, as previously described (Meirelles, Souto-Padrón & De Souza, 1984). Epimastigotes were obtained from axenic cultures grown at 28 °C in LIT (Liver Infusion-Tryptose) medium (Camargo, 1964) supplemented with 10% fetal calf serum.
In order to follow the biogenesis of reservosomes during the differentiation process, bloodstream trypomastigotes were transferred (final concentration 4×106 cells/ml) to LIT medium supplemented with 10% fetal calf serum, at 28 °C. To follow the endocytosis of proteins, the parasites were grown in LIT medium supplemented with 5% (v/v) sterile holo-transferrin (Sigma Co, USA), coupled to 10 nm colloidal gold particles as described by Slot & Geuze (1985). The parasites were then cultivated at 28 °C in culture flasks, in 10 ml aliquots. The differentiation process and the uptake of transferrin–gold complexes were followed during 4 days, by examining the parasites at 24-h intervals by light microscopy (phase contrast) and by transmission electron microscopy.
Differentiating parasites in LIT medium were collected daily by centrifugation for 5 min at 1500 g, fixed for 2 h with 2·5% glutaraldehyde/4% paraformaldehyde in 0·1 M cacodylate buffer (pH 7·2), washed in the same buffer and post-fixed for 1 h in 1% OsO4/0·8% potassium ferricyanide/5 mm CaCl2 in 0·1 M cacodylate buffer (pH 7·2). The cells were then rinsed in cacodylate buffer, dehydrated in graded acetone and embedded in Poly/Bed 812 resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed in a Zeiss EM109B transmission electron microscope.
For the co-localization of transferrin–gold complexes and the cysteine proteinase enzyme, 5-day-old culture epimastigotes were collected by centrifugation, washed in phosphate-buffered saline (PBS), pH 7·2, and then incubated for 30 min at 28 °C in PBS containing transferrin–gold (15 nm) complexes (1[ratio ]5 dilution), to label the reservosomes. The cells were then fixed for 2 h with 0·1% glutaraldehyde/4% paraformaldehyde diluted in 0·1 M phosphate buffer, pH 7·2, washed in buffer, dehydrated in methanol and embedded in Lowicryl K4M.
Ultra-thin sections were collected on nickel grids, incubated for 30 min in PBS, pH 8·0, containing 5% non-fatty milk/0·01% Tween 20 (PMT), and then for 1 h in this medium containing a 1[ratio ]20 dilution of a rabbit anti-T. cruzi cysteine proteinase antibody. After washing in PBS/1% bovine serum albumin/0·01% Tween 20, the grids were incubated for 1 h with gold (5 nm)-labelled goat anti-rabbit IgG diluted 1[ratio ]20 in PMT. The grids were then washed in PBS, pH 7·2, rinsed in distilled water, stained with uranyl acetate and lead citrate, and observed by transmission electron microscopy.
Transfer of T. cruzi bloodstream trypomastigotes to LIT medium resulted in gradual morphological changes, leading to differentiation into epimastigote forms after 96 h. To analyse the uptake of nutrients by differentiating cells, transferrin–gold complexes were added to the LIT medium and aliquots of the culture were collected and processed daily for transmission electron microscopy.
After the first 24 h most trypomastigotes differentiated into isolated amastigote forms, which displayed intense cell division activity. At the ultrastructural level, these round cells presented typical amastigote morphology, with rod-shaped kinetoplast, short flagellum and a thick surface coat (Fig. 1A). After 48 h, free amastigotes strongly adhered to each other, forming large clusters. At this time, differentiating epimastigotes could be observed at the border of the clusters. At the ultrastructural level, the adhered cells usually showed tight interdigitating cell bodies (Fig. 1B). Transferrin–gold particles could be detected bound only to the plasma membrane of the clustered amastigotes/epimastigotes (Fig. 1C). These cells presented a large number of glycosomes dispersed throughout the cytoplasm. No transferrin– gold labelling could be detected in free amastigotes and trypomastigotes at any time of cultivation.

Fig. 1. (A) Free amastigote from the supernatant, after 24 h of differentiation at 28 °C in LIT medium. Note the oval body and the rod-shaped kinetoplast (K). No transferrin–gold labelling is detected either at the plasma membrane or inside intracellular compartments. (B) Detail of adhered amastigotes showing interdigitating plasma membranes (arrowheads). Note the surface coat. Gold labelling is found at the plasma membrane (arrow), but is absent in the interdigitating regions. (C) Ultrastructural aspect of an amastigote cluster (C) after 48 h of differentiation in LIT medium, showing the strong adhesion between the cells. Transferrin–gold labelling is detected dispersed at the plasma membranes (arrowheads). Note an elongated cell (I) with an intermediary morphology between the amastigote and epimastigote forms.
The clustered epimastigotes presented several electron-dense vesicles close to the Golgi complex (Fig. 2A). These dense organelles morphologically differed from typical reservosomes of epimastigotes maintained for longer periods in LIT medium, which have an electron-dense matrix and well-delimited electron lucent lipid inclusions. With 72 h, typical epimastigotes were observed pinching off from the cluster borders and free-swimming epimastigotes were already observed in the supernatant. Only after 96 h were typical reservosomes detected in free-swimming epimastigotes, although electron-dense vesicles were still present close to the Golgi apparatus. These cells presented intracellular labelling with transferrin–gold complexes (Fig. 2B). The markers were present inside the cytostome, small cytoplasmic carrier vesicles and the reservosomes (Fig. 2B).

Fig. 2. (A) Large magnification of a differentiating epimastigote form after 48 h of cultivation, showing an electron-dense vesicle (*) close to the Golgi (G) complex. This cell presents a rod-shaped kinetoplast (K) typical of an epimastigote form, but no reservosomes and endocytic activity for transferrin. (B) Differentiated epimastigote after 96 h of cultivation at 28 °C in LIT medium, showing transferrin labelling inside typical reservosomes (R) at the posterior end, as well as inside endocytic vesicles (arrow). (C) Detail of reservosomes (R) in a 5-day-old, fully differentiated epimastigote form, presenting labelling only with cysteine proteinase (small gold particles), or double labelling with transferrin (large gold particles) and cysteine proteinase (small particles).
When 5-day-old, fully differentiated, epimastigotes were allowed to endocytose transferrin–gold (15 nm) complexes and were then processed for the immunocytochemical detection of cysteine proteinase (using 5 nm gold particles), all reservosomes at the cell posterior end were positive for the enzyme, but not all reservosomes presented double labelling with transferrin–gold and cysteine proteinase (Fig. 2C). Small carrier vesicles, loaded with gold-labelled transferrin, but with no labelling for cysteine proteinase, could be observed close to the reservosomes (data not shown).
In Trypanosoma cruzi epimastigotes, proteins are ingested through the cytostome and the flagellar pocket and are carried in endocytic vesicles to the reservosomes, thus showing that these organelles are compartments of the endocytic pathway (Soares & De Souza, 1991: Soares, 1999; De Souza et al. 2000; Porto-Carreiro et al. 2000). Furthermore, reservosomes may play a major role in the epimastigote to trypomastigote differentiation process, as they disappear during this process and are absent in amastigote and trypomastigote forms (Soares & De Souza, 1989). Although the reverse differentiation from trypomastigotes to epimastigotes in T. cruzi is long known (Chagas, 1909), little information is available on ultrastructural aspects of this process, as well as on the fine structure of the ingestion of nutrients and biogenesis of reservosomes in these differentiating parasites.
Our results show that the first event during the trypomastigote to epimastigote differentiation process in T. cruzi is the transformation of trypomastigotes into amastigotes, followed by strong adhesion of the latter cells. Previous studies have demonstrated that adhesion is an important step in the metacyclogenesis (epimastigote to trypomastigote differentiation) in vitro (Bonaldo et al. 1988; Figueiredo et al. 2000). Our results show that adhesion is also required for the transformation, in vitro, from the amastigote to the epimastigote stage. The presence of a coat at the interdigiting membranes of clustered amastigotes suggests that surface glycoproteins are involved in the adhesion process. Since parasite–parasite interdigitations are also observed in the insect vector (Kollien, Schmidt & Schaub, 1998), our data suggest that the trypomastigote to epimastigote differentiation system in LIT medium may reproduce, at least in part, the in vivo differentiation process that occurs in the digestive tract of the triatomines. Accordingly, we have identified reservosome-like structures within epimastigotes present in the digestive tract of the insect vector (unpublished observations).
It is well known that trypanosomatids require iron for growth and division (Schell, Borowy & Overath, 1991; Landfear & Ignatushchenko, 2001; Morgan et al. 2002). It is also known that transferrin binding is limited to the flagellar pocket in T. brucei bloodstream forms (Steverding, 2000) and in Leishmania promastigotes (Wilson et al. 2002; Britigan et al. 1998; Wilson et al. 1994). One the other hand, in T. cruzi epimastigotes the cytostome seems to be the major site for transferrin uptake (Figueiredo et al. 2000; Porto-Carneiro et al. 2000). In T. cruzi epimastigotes, transferrin uptake occurs by receptor-mediated endocytosis (Soares & De Souza, 1991; Soares et al. 1992; Porto-Carreiro et al. 2000), but little is known about the transferrin uptake in amastigotes and trypomastigotes. A previous biochemical study has shown the presence of a putative transferrin receptor in amastigotes, but not in trypomastigote forms (Lima & Villalta, 1990). Accordingly, we could find transferrin–gold labelling at the surface of differentiating amastigotes/epimastigotes, as well as inside intracellular compartments of typical epimastigote forms. Our results suggest that transferrin receptor expression precedes the acquisition of endocytic activity by epimastigote forms.
The absence of transferrin uptake in trypomastigotes (Corrêa, Andrade & Soares, 2002; this study) suggests that other mechanisms are implicated in the iron acquisition by this stage. As, differently from the African trypanosomes, T. cruzi bloodstream trypomastigotes derive from amastigotes, are non-proliferative and have a relatively short extracellular life, it is also possible that they do not rely exclusively on iron uptake by transferrin receptors to survive. On the other hand, metacyclic trypomastigotes – those found in the rectum of the insect vector (triatomine bugs) – derive from epimastigotes and may rely on iron that was stored in reservosomes during the epimastigote stage at the insect midgut. The importance of transferrin receptors for T. cruzi intracellular amastigote forms is still unclear. Thus, it seems that the different expression of transferrin receptors in T. cruzi reflects adaptations to the different environments where the parasites live.
Our results demonstrated that the appearance of electron-dense vesicles at the posterior end of differentiating epimastigotes preceded the capacity of these cells to ingest gold-labelled transferrin from the environment. It is known that reservosomes contain cysteine proteinase, the main T. cruzi proteinase (Soares et al. 1992; Souto-Padrón et al. 1990). Our data suggest that reservosomes are first formed from homotypic fusion of Golgi-derived vesicles loaded with cysteine proteinase (and possibly other lysosomal enzymes), to which endocytic vesicles are then able to fuse. Some data support this idea. First, treatment of T. cruzi epimastigotes with cysteine proteinase inhibitors arrested the transport of the enzyme to the reservosomes at the Golgi complex, thus demonstrating a functional relationship between these two organelles (Engel et al. 1998). Second, our data using transferrin as endocytic tracer and an anti-cysteine proteinase antibody clearly demonstrated reservosomes with different labelling patterns: some presented only cysteine proteinase labelling, while others were positive for both cysteine proteinase and transferrin. These data show that endocytic vesicles fuse with pre-existing compartments enriched with cysteine proteinase. An early endosomal compartment in T. cruzi epimastigotes, as already proposed (Porto-Carreiro et al. 2000), has not been found in our observations.
Our data indicate that endocytosis of nutrients, biogenesis of reservosomes and cell differentiation can be correlated in epimastigote forms of T. cruzi. Biogenesis of reservosomes begins early during the trypo-/ama- to epimastigote differentiation, with the formation of electron-dense vesicles from the Golgi complex containing cysteine proteinases and other hydrolases. These organelles move from the Golgi complex region (close to the flagellar pocket) to the posterior end of the parasites. Endocytic vesicles with ingested molecules (which arose from the cytostome and the flagellar pocket) will eventually fuse with these electron-dense vesicles, forming the reservosomes of T. cruzi epimastigotes (Fig. 3). As long as nutrients are available, large reservosomes are formed by continuous fusions, so that the number of these structures per cell remains low, about 4–5 per cell. However, when exogenous nutrients are absent, endocytosis is hindered and no new reservosomes are formed. Existing reservosomes will be consumed as an energy source. With the absence of endogenous nutrients, and the resulting nutritional stress, the epimastigotes transform into trypomastigotes.
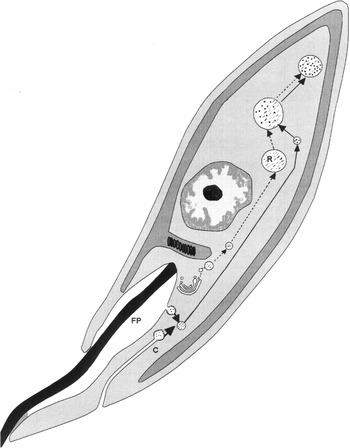
Fig. 3. Hypothetical model for the biogenesis of reservosomes (traced line) and the endocytic pathway (thick line) in epimastigote forms of Trypanosoma cruzi. electron-dense vesicles containing cysteine proteinase and other lysosomal enzymes (/) are formed at the Golgi complex and fuse at the posterior end of the parasites. Exogenous molecules (black dots) are taken up in small uncoated endocytic vesicles formed at the cytostome (C) and the flagellar pocket (FP). These endocytic vesicles will fuse with the Golgi-derived electron-dense vesicles at the posterior end of the cells, to form the typical reservosomes (R).
The authors would like to thank Mr Raimundo N. C. Pimentel and Mrs Verônica G. Mendes for their technical assistance. This work was supported by CPqAM/ FIOCRUZ, IOC/FIOCRUZ, FACEPE, PADCT/CNPq and PAPES-III/FIOCRUZ.

Fig. 1. (A) Free amastigote from the supernatant, after 24 h of differentiation at 28 °C in LIT medium. Note the oval body and the rod-shaped kinetoplast (K). No transferrin–gold labelling is detected either at the plasma membrane or inside intracellular compartments. (B) Detail of adhered amastigotes showing interdigitating plasma membranes (arrowheads). Note the surface coat. Gold labelling is found at the plasma membrane (arrow), but is absent in the interdigitating regions. (C) Ultrastructural aspect of an amastigote cluster (C) after 48 h of differentiation in LIT medium, showing the strong adhesion between the cells. Transferrin–gold labelling is detected dispersed at the plasma membranes (arrowheads). Note an elongated cell (I) with an intermediary morphology between the amastigote and epimastigote forms.

Fig. 2. (A) Large magnification of a differentiating epimastigote form after 48 h of cultivation, showing an electron-dense vesicle (*) close to the Golgi (G) complex. This cell presents a rod-shaped kinetoplast (K) typical of an epimastigote form, but no reservosomes and endocytic activity for transferrin. (B) Differentiated epimastigote after 96 h of cultivation at 28 °C in LIT medium, showing transferrin labelling inside typical reservosomes (R) at the posterior end, as well as inside endocytic vesicles (arrow). (C) Detail of reservosomes (R) in a 5-day-old, fully differentiated epimastigote form, presenting labelling only with cysteine proteinase (small gold particles), or double labelling with transferrin (large gold particles) and cysteine proteinase (small particles).

Fig. 3. Hypothetical model for the biogenesis of reservosomes (traced line) and the endocytic pathway (thick line) in epimastigote forms of Trypanosoma cruzi. electron-dense vesicles containing cysteine proteinase and other lysosomal enzymes (/) are formed at the Golgi complex and fuse at the posterior end of the parasites. Exogenous molecules (black dots) are taken up in small uncoated endocytic vesicles formed at the cytostome (C) and the flagellar pocket (FP). These endocytic vesicles will fuse with the Golgi-derived electron-dense vesicles at the posterior end of the cells, to form the typical reservosomes (R).