INTRODUCTION
Approximately 150 years ago, in a lecture to the Royal Academy of Sciences in Vienna, Carl Wedl described a worm from the small intestine of a sheep, which he named Trichosoma papillosum (Wedl, Reference Wedl1856). To my knowledge, this was the first description of a nematode that contemporary taxonomy now places within the genus Strongyloides. Although broader interest in Strongyloides only arose after the human parasite Strongyloides stercoralis was described 20 years later, this event marked the beginning of investigations of this fascinating group of worms that ‘shuttle’ between a parasitic and a free-living mode of existence (for a review of the early studies of Strongyloides and for a historical introduction, see Sandground, Reference Sandground1926; Kreis, Reference Kreis1932; Grove, Reference Grove and Grove1989).
Based on an analysis of sequence data for the small subunit (SSU) of nuclear ribosomal DNA, Blaxter et al. (Reference Blaxter, de Ley, Garey, Liu, Scheldman, Vierstraete, vanFleteren, Mackey, Dorris, Frisse, Vida and Thomas1998) placed the genus Strongyloides within ‘clade IV’ of the Nematoda, and Dorris et al. (Reference Dorris, Viney and Blaxter2002) examined the phylogenetic relationships of Strongyloides species and members of closely related genera. To date, more than 50 species of Strongyloides which parasitize a range of different vertebrates (mostly mammals) have been described (Speare, Reference Speare and Grove1989). A generalized life-cycle of Strongyloides is given in Fig. 1 (reviewed by Schad, Reference Schad and Grove1989; Viney, Reference Viney1999, Reference Viney2006; Viney and Lok, Reference Viney and Lok2007). The adult parthenogenetic females, which parasitize the small intestine of their respective host, produce developing embryos, which are enclosed in an eggshell. Depending on the species, the embryos, early larvae or a mixture of these stages exit the host in the faeces and develop in the external environment. The larvae then undergo either of 2 fundamentally different life-cycles, both of which contain 4 larval stages followed by a reproducing adult stage. Early larvae in the environment may develop into infective third-stage larvae (iL3) and percutaneously invade the host, followed by a migration via the circulatory and respiratory systems to develop further and ultimately establish as adult females in the small intestine (representing the direct or homogonic cycle). Alternatively, such larvae can develop into morphologically distinct, non-infective L3s, which develop into free-living, fourth-stage larvae and finally adult nematodes (representing the indirect or heterogonic cycle). The free-living generation consists of females and males (i.e., dioecious). Usually, the progeny of the free-living adults develop into infective iL3s and must find a host in which to continue the life-cycle. However, under particular culture conditions, up to 9 consecutive free-living generations have been observed for some species.
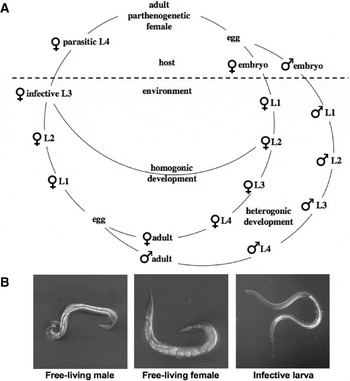
Fig. 1. (A) Generalized life-cycle of Strongyloides. (B) Differential interference contrast pictures of an adult male (left), an adult free-living female (centre) and an infective L3 (iL3) (right) of S. papillosus. The anterior ends point to the left. The total lengths are 0·8 mm for the male, 1 mm for the female and 0·6 mm for the iL3.
While the life-cycle had been investigated by numerous scientists by the mid-1920s, in 1926, in the introduction to a paper that reviewed the research on Strongyloides up to this time and presented a large amount of new data, Sandground (Reference Sandground1926) stated: “ … but these investigations have been chiefly of parasitological interest, and of more recent times no extensive studies on the biological aspects of the life-history have been reported. Yet there remains a number of unsolved problems connected with the life-history of this genus, which to the biologist as well as to the parasitologist is undoubtedly one of the most interesting among the nematodes.” From ecological and evolutionary perspectives, some of the most interesting questions relate to the 2 life-cycles of Strongyloides, particularly the ‘choices’ between clonal and sexual reproduction, between the sexes and between the parasitic and free-living life-cycles.
Although the understanding of the biology of Strongyloides species has improved substantially since the publication by Sandground (Reference Sandground1926), there are still many controversies and questions. For instance, there are conflicting reports on several aspects of reproductive biology. Some of these contradictory findings are likely to reflect actual biological differences among different species or isolates of a particular species. Frequently, studies were carried out using new isolates of Strongyloides. Given that there are significant limitations in the specific identification of Strongyloides (see Augustine, Reference Augustine1940; Speare, Reference Speare and Grove1989), there are uncertainties regarding the species status of isolates used in some reports. Therefore, it is likely that not all reports about a particular species of Strongyloides actually describe the same species. Other controversies are likely to have resulted from misinterpretations of observations.
The purpose of the present review is to provide an account of key aspects of the life-history and reproductive biology of Strongyloides, with an emphasis on discussing differences in research results among studies, as a foundation for future investigations. With recent progress in the development of genomic and genetic methods for exploring Strongyloides and its sister taxon, Parastrongyloides (e.g., Grant et al. Reference Grant, Skinner, Howes, Grant, Shuttleworth, Heath and Shoemaker2006a, Reference Grant, Stasiuk, Newton-Howes, Ralston, Bisset, Heath and Shoemakerb; Guiliano and Blaxter, Reference Guiliano and Blaxter2006; Li et al. Reference Li, Massey, Nolan, Schad, Kraus, Sundaram and Lok2006; Thompson et al. Reference Thompson, Barker, Hughes, Wilkes, Coghill and Viney2006; Viney, Reference Viney2006; Lok, Reference Lok2007), it should be possible to address some of these controversies.
THE PARASITIC GENERATION AND ITS PROGENY
The mode of reproduction in the parasitic female: parthenogenesis, self-fertilization or sex?
Adult females of Strongyloides parasitizing a host are generally considered to reproduce parthenogenetically. This statement is based on a large body of evidence. (i) With the exception of 2 reports from the early 1930s, which were subsequently rejected by some authors, no parasitic males have been described for any species of Strongyloides. (ii) Multiple authors sought, but failed, to find sperm in the reproductive tracts of parasitic females (Chitwood and Graham, Reference Chitwood and Graham1940 for S. ratti and a species of Strongyloides derived from a grey squirrel; Nigon and Roman, Reference Nigon and Roman1952; Bolla and Roberts, Reference Bolla and Roberts1968 for S. ratti; Chang and Graham, Reference Chang and Graham1957 for S. papillosus). (iii) Infection of rats with single iL3s of S. ratti allowed the establishment of adult worms that produced homogonically developing larvae and heterogonic individuals of both sexes. Therefore male worms were not required for reproduction (Graham, Reference Graham1936, Reference Graham1938; Viney et al. Reference Viney, Matthews and Walliker1992; Viney, Reference Viney1994). (iv) Cytological observations of the maturing oocytes in S. ratti (see Chitwood and Graham, Reference Chitwood and Graham1940; Nigon and Roman, Reference Nigon and Roman1952), S. ransomi (see Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977) and S. papillosus (see Zaffagnini, Reference Zaffagnini1973; Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977) led to the conclusion that no meiosis occurred during oogenesis in the parasitic female and that the reproduction was by mitotic parthenogenesis. These authors did indicate, however, that some of the processes during oocyte maturation were very difficult to observe microscopically. (v) In S. ratti, inheritance has been studied genetically. Using worms from rats infected with a single female S. ratti, Viney (Reference Viney1994) showed that all individuals of the progeny were genetically identical, as they were to the original female worm, demonstrating that reproduction in these parasitic females was functionally mitotic.
This evidence indicates that parthenogenesis can occur and is probably the most common mode of reproduction in the parasitic females of Strongyloides. However, the only instance for which clonal reproduction has been fully demonstrated is for S. ratti in the case of single worm infections, where it is certain that no males were present. It cannot yet be excluded that, under particular circumstances, one or more other modes of reproduction could occur for some members of Strongyloides. Indeed, alternative modes have been proposed, such as self-fertilizing hermaphroditism and dioecious sex. Sandground (Reference Sandground1926) described structures, which he considered to represent sperm in the reproductive tract of parasitic S. ratti females, but failed to find parasitic males. Therefore, he concluded that S. ratti is a self-fertilizing hermaphrodite. This conclusion was questioned by other authors (Chitwood and Graham, Reference Chitwood and Graham1940; Nigon and Roman, Reference Nigon and Roman1952), who argued that Sandground had probably misidentified somatic cells as sperm. The occurrence of parasitic males and dioecious sexual reproduction was first described by Kreis (Reference Kreis1932) and later confirmed by Faust (Reference Faust1933). The latter author infected 40 dogs with Strongyloides isolated from faeces from chimpanzees, rhesus and capucin monkeys, dogs or humans and subsequently found parasitic males in two thirds of them. Unfortunately, he did not provide details regarding a possible relationship between the presence or absence of males and the original source of Strongyloides used for infection However, he did notice a strong ‘correlation’ between the presence of males and a preference for a heterogonic development in the progeny. Faust (Reference Faust1933) also provided a possible explanation as to why earlier authors might not have detected parasitic males. He pointed out that the male worms occurred mainly during the pre-patent period of infection and were predominantly in the lung rather than in the intestine. He speculated that mating took place early and that the males were eliminated relatively rapidly, such that they were not found later in the infection (when most other authors undertook examinations).
Subsequently, Basir (Reference Basir1950) explicitly questioned whether Kreis (Reference Kreis1932) and Faust (Reference Faust1933) had correctly identified parasitic males of Strongyloides, given that the males described were rhabtidiform, like males from the free-living generation rather than filariform (such as the parasitic males of the dioecious species of Parastrongyloides). Also, in an extensive study, Premvati (Reference Premvati1958a) did not find any evidence of parasitic males in the small intestines from approximately 1500 rhesus monkeys with heavy infections of S. fuelleborni. Therefore, most authors have disregarded the reports by Kreis (Reference Kreis1932) and Faust (Reference Faust1933), and the absence of parasitic males has become a widely accepted belief in the field (cf. Schad, Reference Schad and Grove1989). In contrast, Schad (Reference Schad and Grove1989) considered that it was premature to ‘discard’ the studies by Kreis (Reference Kreis1932) and Faust (Reference Faust1933). He pointed out clearly that the occurrence of parasitic males could be a strain-specific characteristic and that this issue required further investigation, also because both of these authors had been leading experts in the field and that their studies were extensive, with large sample sizes.
‘Choices’ between alternative developmental routes in the progeny of the parasitic generation
If one accepts that usually no parasitic males arise, the newly formed Strongyloides embryo has the following developmental options: it can either develop homogonically into an iL3 or heterogonically into a free-living male or a free-living female. Another option for S. stercoralis and S. felis is that iL3s are autoinfective. Under particular circumstances, larvae of these species can develop ‘precociously’ into iL3s before leaving the host and can then re-infect the same host, thereby establishing a self-sustaining ‘autoinfective cycle’ (reviewed by Schad, Reference Schad and Grove1989; Speare, Reference Speare and Grove1989; Keiser and Nutman, Reference Keiser and Nutman2004). Although these larvae are morphologically very similar to iL3s, which developed in the external environment, they have been described as being smaller in size (Speare, Reference Speare and Grove1989). The autoinfective cycle is of significant medical importance, since it leads to the most severe clinical expression of human strongyloidiasis (Keiser and Nutman, Reference Keiser and Nutman2004). However, autoinfection appears to be exclusive to S. stercoralis and S. felis (see Speare, Reference Speare and Grove1989) and is not considered further.
The early ‘developmental switches’ are of great ecological importance. Making the ‘right choice’ of sex and life-cycle under particular circumstances seems to be very important, as it is likely to have a very profound effect on the expected reproductive success of an individual of Strongyloides (see Viney, Reference Viney, Lewis, Campbell and Sukhedo2002). The proportion of males produced and the ratio of females developing homogonically into iL3s versus those that develop into free-living adults are both influenced by the genetic background of the respective isolate of Strongyloides (demonstrated by Sandground, Reference Sandground1926; Graham, Reference Graham1939b, Reference Graham1940; Viney et al. Reference Viney, Matthews and Walliker1992; Viney, Reference Viney1996 for S. ratti, and by Sandground, Reference Sandground1926 for S. stercoralis and S. papillosus). Also, host factors, such as the immune status of the host, affect the sex ratio and the homogonic versus heterogonic switch. Increased host immunity against Strongyloides has been shown to lead to a higher proportion of males and to predispose females to undergo heterogonic development (e.g., Moncol and Triantaphyllou, Reference Moncol and Triantaphyllou1978 for S. ransomi, and Gemmill et al. Reference Gemmill, Viney and Read1997; Harvey et al. Reference Harvey, Gemmill, Read and Viney2000 for S. ratti). Crook and Viney (Reference Crook and Viney2005) showed for S. ratti that some ‘non-immunological stressors’ decreased the proportion of heterogonically developing individuals of both sexes, indicating that an increase in the proportion of free-living individuals was not a general response to stress. Matoff (Reference Matoff1936) and Graham (Reference Graham1939a), studying S. papillosus and S. ratti, respectively, observed a seasonal variation in the life-cycle ‘choice’, such that both developmental cycles occurred in summer, whereas in winter the worms developed almost exclusively via the homogonic cycle. In addition, experimental transfer to a different host species was shown to alter the sex ratio and the ratio of homogonically to heterogonically developing females (Sandground, Reference Sandground1926 for S. stercoralis and S. fuelleborni; Brumpt, Reference Brumpt1921; Sandground, Reference Sandground1926; Matoff, Reference Matoff1936; Basir, Reference Basir1950; Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977 for S. papillosus; Crook and Viney, Reference Crook and Viney2005 for S. ratti). Interestingly, the effect was not the same among different experiments involving different species of Strongyloides and various hosts. For example, when S. papillosus from sheep was raised in rabbits, the proportion of males and free-living females increased (Brumpt, Reference Brumpt1921; Sandground, Reference Sandground1926; Matoff, Reference Matoff1936; Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977). This reflects the reaction of Strongyloides to increasing host immunity and may indicate that the transfer of S. papillosus to the atypical host acted as an immunological stressor. By contrast, Crook and Viney (Reference Crook and Viney2005) demonstrated that transferring S. ratti into the atypical murine host acted as a non-immunological stressor and resulted in a decrease in the proportion of free-living adults of both sexes.
During an infection, the increase in the proportion of males parallels the increase in the proportion of females that undergo heterogonic development. Nevertheless, sex determination and the switch between homogonic and heterogonic development in females are not intimately linked, rather they represent 2 independent life-cycle switches. Somewhat counter-intuitively, sex determination appears to precede the homogonic versus heterogonic switch. This hypothesis is supported by the following observations. (i) Several authors have shown for multiple species of Strongyloides that the proportion of homogonically to heterogonically developing females, but not the number of males, can be altered by environmental factors, even after the embryos or early larvae have left the host (Table 1). (ii) The treatment of S. ratti infection with a sublethal dose of the anthelmintic compound thiabendazole leads to an increase in the proportion of males but not to a change in the ratio of homogonically to heterogonically developing females (Crook and Viney, Reference Crook and Viney2005). (iii) S. papillosus males are extremely rare (Brumpt, Reference Brumpt1921; Sandground, Reference Sandground1926; Basir, Reference Basir1950) or much less abundant than females (Matoff, Reference Matoff1936) when raised in sheep, whereas a much higher proportion of males (sometimes even exceeding that of females) occurs when the same isolates are raised in rabbits (Brumpt, Reference Brumpt1921; Sandground, Reference Sandground1926; Matoff, Reference Matoff1936; Basir, Reference Basir1950).
Table 1. Examples of non-host environmental factors described to influence the ‘choice’ between homogonic and heterogonic development

a This author speculated that also individuals that would have developed into males redirected their development to iL3.
b Loke's solution contains several salts and dextrose.
Sex determination in the progeny of the parasitic female
The mechanism by which parthenogenetic Strongyloides females produce progeny of both sexes is interesting but unclear. Although sex is determined, at least in part, environmentally (either directly by host factors, particularly the immune response, or by the mother in response to these factors), some species of Strongyloides have a sex chromosome (Fig. 2). Cytological analyses of S. ratti (see Nigon and Roman, Reference Nigon and Roman1952; Bolla and Roberts, Reference Bolla and Roberts1968) and S. stercoralis (see Hammond and Robinson, Reference Hammond and Robinson1994) indicate that females have 6 chromosomes (2 pairs of autosomes and 1 pair of X chromosomes) whereas males possess only 5 (XX/XO sex determination; Fig. 2A). For S. ratti, the XX/XO sex determination has also been confirmed genetically (Harvey and Viney, Reference Harvey and Viney2001). XX/XO sex determination occurs also in numerous other nematodes representing all 5 major clades (cf. Blaxter et al. Reference Blaxter, de Ley, Garey, Liu, Scheldman, Vierstraete, vanFleteren, Mackey, Dorris, Frisse, Vida and Thomas1998) and is best characterized in the free-living nematode Caenorhabditis elegans, in which sex is determined genetically by the ratio of X-chromosomes to autosomes (reviewed by Boag et al. Reference Boag, Newton and Gasser2001; Pires-daSilva, Reference Pires-daSilva2007). It is interesting to speculate that the X chromosome is an evolutionary relict of an ancestor of Strongyloides, which had a genetic sex determining system. However, at least 2 species of Strongyloides have been found to lack an X chromosome. Based on cytological evidence, Triantaphyllou and Moncol (Reference Triantaphyllou and Moncol1977) concluded that free-living females and males of S. ransomi and S. papillosus do not differ in chromosome number. Interestingly, both of these species have 2 pairs of chromosomes (as opposed to the 3 pairs in S. ratti and S. stercoralis) and 1 pair is considerably larger than the other (referred to henceforth as 2 large (2L) and 2 medium (2M) chromosomes; Fig. 2B). Triantaphyllou and Moncol (Reference Triantaphyllou and Moncol1977) speculated that the 2L2M configuration is the result of a chromosome fusion event, in which the X chromosome combines with one of the autosomes. This hypothesis requires testing. While agreeing that females are 2L2M, Albertson et al. (Reference Albertson, Nwaorgu and Sulston1979) described a chromosomal difference between the males and females of S. papillosus, which relates to a sex-specific chromatin diminution event. According to these authors, a portion of one of the homologous large chromosomes in eggs developing to males is eliminated during the single, mitotic, maturation division. Consequentially, the males possess, in addition to their 2M chromosomes, only a single large chromosome, another medium-sized and 1 tiny chromosome. The latter 2 chromosomes are the remnants of the second large chromosome on either side of the region eliminated (1 Large, 3 Medium, 1 Small, 1L3M1S; Fig. 2C). This process creates a ‘hemizygous region’ in males comparable to the X chromosome in the species with XX/XO sex determination. Currently, it is not known which genes are located in this region. However, if the hypothesis that the long chromosome is the result of a fusion event, one would expect that the region eliminated is homologous to the X chromosome in S. ratti and S. stercoralis. However, although Albertson et al. (Reference Albertson, Nwaorgu and Sulston1979) presented quite convincing evidence that embryos with a 1L3M1S chromosomal configuration become males, these authors did not directly observe that adult males actually have the 1L3M1S karyotype. Triantaphyllou and Moncol (Reference Triantaphyllou and Moncol1977), who studied chromosomes in the adult male germline (the only tissue in the adult in which cell divisions take place, and thus metaphase chromosomes are available), did not observe diminuted chromosomes. These authors, in turn, did not analyse embryos or any type of somatic cells. There are at least 2 possible explanations for this discrepancy: (a) the different isolates of S. papillosus studied differed in this trait and perhaps were different species or (b) this could be an indication that the eliminated portion is reconstructed (by an unknown mechanism or process) in the germline to ensure that all offspring receive an intact chromosome and are thus karyotypically female.
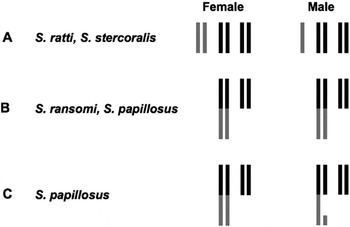
Fig. 2. Schematic representation of the various karyotypes in females and males proposed for different species of Strongyloides. (A) XX/XO sex determination. Autosomes (two pairs) are in black, X chromosomes are in grey. (B) No karyotypic difference between the sexes. (C) Sex specific chromatin diminution in males. In (B) and (C) the regions that were proposed to be derived from an X chromosome are in grey.
Another interesting question is whether a particular adult parasitic female produces progeny of both sexes simultaneously. Cytological observations (Albertson et al. Reference Albertson, Nwaorgu and Sulston1979) suggest that an S. papillosus female produces progeny of only one sex at any given time. On the other hand, Graham (Reference Graham1938) and Viney (Reference Viney1994) both observed similarly aged individuals of both sexes among the worms isolated from faecal samples from rats infected with a single worm. There are 2 possible explanations for this apparent contradiction: (a) individual females switch back and forth (relatively rapidly) between producing male and female progeny or (b) S. ratti but not S. papillosus can produce progeny of both sexes simultaneously.
The ‘choice’ between homogonic and heterogonic development in females
Some early literature contains discussions regarding possible genetic differences between homogonically and heterogonically developing females (e.g., Sandground, Reference Sandground1926; Faust, Reference Faust1933). Chang and Graham (Reference Chang and Graham1957), in a conference abstract which does not contain any detailed description of data, proposed that parasitic and free-living S. papillosus females differ in ploidy, such that free-living females are diploid and parasitic females triploid. According to these authors, parasitic females produce 2 different types of eggs, namely triploid ones that develop homogonically and diploid ones that develop into free-living worms. Triploidy is restored in the progeny of the free-living generation by the fertilization of diploid eggs with a haploid sperm. Although Chang and Graham (Reference Chang and Graham1957) did not publish their findings in an original article, their conference abstract has been cited relatively frequently in peer-reviewed articles and some textbooks. Bolla and Roberts (Reference Bolla and Roberts1968) proposed that also parasitic and free-living S. ratti might differ in ploidy. However, these authors considered the parasitic female to be haploid, although they concluded that “ … the difficulties inherent in the study of this material … do not allow us to draw definite conclusions”.
Most findings published to date are inconsistent with a genetic difference between the parasitic and the free-living females; these are listed as follows. (i) Based on cytological observations, multiple authors concluded that all females (parasitic and free-living) have the same number of chromosomes (Hammond and Robinson, Reference Hammond and Robinson1994 for S. stercoralis; Nigon and Roman, Reference Nigon and Roman1952 for S. ratti; Zaffagnini, Reference Zaffagnini1973; Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977; Albertson et al. Reference Albertson, Nwaorgu and Sulston1979 for S. papillosus). (ii) Molecular genetic studies of S. ratti (see Viney, Reference Viney1994) and S. papillosus (see Eberhardt et al. Reference Eberhardt, Mayer and Streit2007) support the hypothesis that both generations are diploid. (iii) Female larvae can switch between the homogonic and heterogonic development in early larval stages (see Table 1). It is highly unlikely that they can change genetically at that time of development.
A number of studies of various species of Strongyloides have shown that, in addition to the host-related factors, external environmental factors influence the ‘choice’ between the homogonic and heterogonic cycles after the female larvae have left the host animal. Examples of such (external) environmental factors with the corresponding references are listed in Table 1. Some authors have hypothesized that, in general, adverse conditions within the host and favourable culture conditions outside of the host promote heterogonic development, whereas favourable conditions within the host and deteriorating conditions in the external environment lead to increased homogonic development (Moncol and Triantaphyllou, Reference Moncol and Triantaphyllou1978; Schad, Reference Schad and Grove1989; Yamada et al. Reference Yamada, Matsuda, Nakazawa and Arizono1991). Several laboratories have started to investigate the genetic and molecular basis of the homogonic/heterogonic switch (e.g., Crook et al. Reference Crook, Thompson, Grant and Viney2005; Massey et al. Reference Massey, Castelletto, Bhopale, Schad and Lok2005, Reference Massey, Bhopale, Li, Castelletto and Lok2006; Viney et al. Reference Viney, Thompson and Crook2005; Thompson et al. Reference Thompson, Barker, Hughes, Wilkes, Coghill and Viney2006; Viney, Reference Viney2006).
THE FREE-LIVING GENERATION AND ITS PROGENY
The mode of reproduction in the free-living generation: parthenogenesis, pseudogamy or sex?
There is agreement that the free-living generation consists of males and females and most authors agree that both sexes are required for successful reproduction [systematically tested for S. simiae by Beach (Reference Beach1936), for S. fuelleborni by Premvati (Reference Premvati1958b), for S. ransomi by Triantaphyllou and Moncol (Reference Triantaphyllou and Moncol1977), for S. papillosus by Triantaphyllou and Moncol (Reference Triantaphyllou and Moncol1977) ; Eberhardt et al. (Reference Eberhardt, Mayer and Streit2007) and for S. stercoralis by the author (unpublished observations)]. However, there are at least 2 reports suggesting that male-independent parthenogenetic reproduction can also occur. (i) Sandground (Reference Sandground1926) failed to isolate males and observe sperm in the reproductive tract of females from some coprocultures of S. ratti and S. stercoralis (but not S. papillosus) which did produce progeny. From this he concluded that parthenogenesis did occur when males were absent. However, the author explicitly stated that these were casual observations, which he did not follow up on systematically. (ii) Zaffagnini (Reference Zaffagnini1973) did not observe sperm in the reproductive tract of free-living females and he could not find males in some coprocultures containing S. papillosus. Based on these observations, he proposed that reproduction in S. papillosus was clonal and independent of sperm. Nevertheless, to date, there is no well-controlled study of any species of Strongyloides showing that known virgin free-living females ever produce live progeny. However, there are major disagreements regarding the role of Strongyloides males. Most authors who investigated the cytological processes during mating, fertilization and the early cleavage divisions of different species of Strongyloides concluded that members of this genus reproduce (at least most of the time) by sperm-dependent parthenogenesis (pseudogamy) (Nigon and Roman, Reference Nigon and Roman1952; Bolla and Roberts, Reference Bolla and Roberts1968 for S. ratti; Triantaphyllou and Moncol, Reference Triantaphyllou and Moncol1977 for S. papillosus; Hammond and Robinson, Reference Hammond and Robinson1994 for S. stercoralis). Also Chang and Graham (Reference Chang and Graham1957) agreed that no reduction division takes place in S. papillosus but considered that the diploid egg is fertilized by sperm, resulting in a triploid embryo destined to become an iL3.
In contrast to these cytological studies are genetic studies. Graham (Reference Graham1939b) stated that, in consecutive passages of homogonically developing S. ratti “… a remarkable constancy of characteristics has been noted. On the other hand it is definitively known that new and distinctive lines of S. ratti do arise from the indirect filariform larvae of the bisexual free-living generation.” Similar observations were made by Sandground (Reference Sandground1926) for S. ratti, S. papillosus and S. stercoralis. This statement could suggest that, in contrast to the parasitic generation, the free-living generation reproduces sexually. More recently, some papers investigating the inheritance of molecular genetic markers in S. ratti (see Viney et al. Reference Viney, Matthews and Walliker1993; Viney, Reference Viney1994) and in S. papillosus (see Eberhardt et al. Reference Eberhardt, Mayer and Streit2007) showed that males can contribute genetic material to the next generation (and, in the cited studies, they did so most, if not all of the time). Also, a population genetic study of S. ratti (see Fisher and Viney, Reference Fisher and Viney1998) revealed that there was extensive gene flow between different sampling sites and that the frequencies of genetic markers were in a Hardy Weinberg equilibrium, suggesting that sexual reproduction occurs in natural populations, although maybe only sporadically. Why the cytological and the genetic studies have yielded contradictory results remains to be elucidated.
Alternative developmental routes in the progeny of the free-living generation
The two most comprehensively studied species, S. ratti (see Viney, Reference Viney1999) and S. stercoralis (see Yamada et al. Reference Yamada, Matsuda, Nakazawa and Arizono1991), appear to be incapable of undergoing multiple, consecutive free-living generations; all viable progeny of the free-living generation develop into female iL3s. It is not known how this occurs. In particular, it is not known whether nullo-X sperm or zygotes with only 1 X chromosome arise. However, Yamada et al. (Reference Yamada, Matsuda, Nakazawa and Arizono1991) found that, under particular culture conditions, up to 9 consecutive free-living generations of S. planiceps are possible, although with a gradual decrease in fertility over time. Beach (Reference Beach1936) showed 3 consecutive free-living generations in S. simiae and speculated that, under optimum culture conditions, this species might be able to propagate indefinitely as a free-living form. Hansen et al. (Reference Hansen, Buecher and Cryan1969) noted the occurrence of free-living females but not males in the progeny of the free-living generation of S. fuelleborni. Augustine (Reference Augustine1940) found consecutive free-living generations in S. simiae, S. fuelleborni and a Strongyloides isolate from the primate Cebus apella, but not in an isolate from a dog. In this latter report, fertile males were found in all 3 species but in numbers that were insufficient to maintain in culture. Males from the progeny of parasitic females were used to obtain consecutive generations of free-living females in this case. Beg (Reference Beg1968), also studying S. fuelleborni, described large numbers of second generation free-living adults of both sexes without providing separate numbers for males and females. In contrast, a large experiment by Premvati (Reference Premvati1958b) revealed no second-generation free-living forms of S. fuelleborni, even under culture conditions considered optimal for heterogonic development in progeny of the parasitic generation. It is striking that whenever consecutive free-living generations were observed (for any species of Strongyloides), second generation free-living worms were rare and appeared only under optimal culture conditions and at low population densities. It remains to be determined whether consecutive free-living generations is a ‘normal’ means of propagation for a range of Strongyloides species in nature or whether they represent an anomaly occurring exclusively under particular laboratory conditions. Interestingly, Parastrongyloides trichosuri (a relative of Strongyloides spp.) can be maintained for a very long time (>50 generations) if not indefinitely in a free-living cycle, provided the worms are kept in culture at a very low population density (Grant et al. Reference Grant, Stasiuk, Newton-Howes, Ralston, Bisset, Heath and Shoemaker2006b).
CONCLUDING REMARKS
The complex life-cycles of Strongyloides and the availability of numerous species within this genus provide a unique opportunity to study the basic principles of their life-history switches, ecology and evolution. The basis for conducting fundamental comparative investigations is being able to unequivocally identify the species of Strongyloides under study. Molecular methods employing species-specific genetic markers should assist in overcoming the substantial limitations in the specific identification of Strongyloides due to a lack of morphological characters for delineation (Augustine, Reference Augustine1940). Also, investigating the different modes of reproduction and of sex determination within and among species and under different environmental conditions would be of major fundamental relevance. Comparative studies with Parastrongyloides trichosuri, a relatively closely related species, which reproduces sexually in the parasitic and the free-living generation (Grant et al. Reference Grant, Stasiuk, Newton-Howes, Ralston, Bisset, Heath and Shoemaker2006b), may assist in elucidating various aspects of Strongyloides life-cycles. There are several other nematodes in which multiple modes of reproduction and chromosomal configuration occur. For instance, within the free-living nematode genera Caenorhabditis and Pristionchus (see Kiontke et al. Reference Kiontke, Gavin, Raynes, Roehrig, Piano and Fitch2004; Herrmann et al. Reference Herrmann, Mayer and Sommer2006a, Reference Herrmann, Mayer and Sommerb; Mayer et al. Reference Mayer, Herrmann and Sommer2007), facultative self-fertilizing hermaphroditic species exist as well as gonochoristic species. In both cases, it is likely that hermaphroditism has arisen several times independently during evolution. While in these 2 genera reproduction is always sexual (either by self- or by cross-fertilization), sexual and clonal species coexist in Meloidogyne (root knot nematodes) (Castagnone-Sereno, Reference Castagnone-Sereno2006). These examples illustrate that the modes of reproduction and sex determination in nematodes are very diverse and could relate directly to their evolutionary history. In Caenorhabditis elegans, Hodgkin (Reference Hodgkin2002) demonstrated elegantly that very few or even single-point mutations can lead to stable strains with very different, genetic or environmental sex determining systems and hermaphroditic or gonochoristic modes of reproduction.
Many of the controversies and uncertainties described in the present article are due to limitations in cytological, genetic and taxonomic methods that authors faced at the time of their studies. Recently, there has been tremendous progress in the development of molecular, genetic and genomic tools for Strongyloides. Expressed sequence tag (EST) sequencing projects for S. ratti and S. stercoralis have identified a large number of genes in these 2 species (Viney, Reference Viney2006) and the genomes of S. ratti and S. stercoralis have been selected for whole genome sequencing (http://www.sanger.ac.uk/Projects/Helminths/). Transgenic techniques are emerging for S. stercoralis (see Li et al. Reference Li, Massey, Nolan, Schad, Kraus, Sundaram and Lok2006; Junio et al. Reference Junio, Li, Massey, Nolan, Todd Lamitina, Sundaram and Lok2007) and P. trichosuri (see Grant et al. Reference Grant, Skinner, Howes, Grant, Shuttleworth, Heath and Shoemaker2006a). The availability of these and other functional genomic tools provides new avenues for detailed investigations into species of Strongyloides and will allow several of the issues raised in this article to be addressed. For example, the genomic sequences of reference strains of S. ratti and S. stercoralis will pave the way for the definition of a large set of molecular genetic markers, as has been carried out for C. elegans (see Wicks et al. Reference Wicks, Yeh, Gish, Waterston and Plasterk2001), for the genetic delineation between clonal and sexual reproduction and for the mapping of quantitative trait loci (QTLs) or classical mutations. Transgenic techniques will not only be useful for the analysis of gene expression (Li et al. Reference Li, Massey, Nolan, Schad, Kraus, Sundaram and Lok2006; Junio et al. Reference Junio, Li, Massey, Nolan, Todd Lamitina, Sundaram and Lok2007) but should assist in addressing the cytological issues raised herein. In C. elegans, transgene-encoded components of chromatin or the cytoskeleton, tagged with, for example, the green fluorescent protein (GFP), have enabled the analysis of the cytological processes during fertilization and early development in live embryos at high resolution (e.g., Hannak et al. Reference Hannack, Kirkham, Hymann and Oegema2001). These examples indicate how modern genomic and genetic techniques could assist in gaining detailed insights into the fundamental aspects of developmental and reproductive biology of Strongyloides.
I thank Drs Mark E. Viney, Ralf J. Sommer, Matthias Herrmann and Robbie Rae and Alexander Eberhardt and Benjamin Schlager for the critical reading of the draft manuscript and Professor Robin. B. Gasser for editorial support in finalizing the manuscript. The research in my laboratory was supported by the Max Planck Society.





