INTRODUCTION
Parasites live in an environment of limited resources (their host) which must be allocated efficiently to growth, reproduction and/or survival (Poulin, Reference Poulin1996). This allocation can be affected by host condition; any stress on the host may limit resources available to the parasite (Poulin, Reference Poulin1996, Reference Poulin2007). Selection should favour parasite individuals that adopt the most efficient allocation of limited host resources to different life-history demands (Poulin, Reference Poulin1996).
When hosts are under stress, especially food-deprived, one of two responses by parasites is expected: reduction in resources taken from the host to keep it alive, or, if the parasite cannot reduce its resource consumption and continues to exploit the host at the same rate, increased mortality in hosts under severe stress (Jokela et al. Reference Jokela, Taskinen, Mutikainen and Kopp2005). The latter, when combined with the immunosuppressive effect of stress (Latshaw, Reference Latshaw1991; Pruett et al. Reference Pruett, Ensley and Crittenden1993), can theoretically lead to disease outbreaks in stressed populations (Lloyd, Reference Lloyd, Grenfeld and Dobson1995). In the past, this has been studied by measuring parasite effects on stressed hosts (susceptibility to infection, parasite-induced mortality or reduced development) (Krist et al. Reference Krist, Jokela, Wiehn and Lively2004; Jokela et al. Reference Jokela, Taskinen, Mutikainen and Kopp2005; Saarinen and Taskinen, Reference Saarinen and Taskinen2005; Fellous and Koella, Reference Fellous and Koella2010), and/or parasite reproductive output (Kendall, Reference Kendall1949; Shostak and Dick, Reference Shostak and Dick1986; Keas and Esch, Reference Keas and Esch1997; Ebert et al. Reference Ebert, Zschokke-Rohringer and Carius2000; Sandland and Minchella, Reference Sandland and Minchella2003; Bedhomme et al. Reference Bedhomme, Agnew, Sidobre and Michalakis2004; Seppälä et al. Reference Seppälä, Liljeroos, Karvonen and Jokela2008; Coors and De Meester, Reference Coors and De Meester2011).
In the case of trematodes, which have complex life cycles and multiple life-cycle stages, measuring the reproductive output from the first intermediate host by counting the free-swimming, infective cercariae may not fully capture how the parasite is responding to host stress. Cercariae represent the immediate reproductive output only. This, in relation to numbers of rediae (the within-host stages which produce cercariae), can provide a clearer quantification of the parasite's response in resource allocation between reproduction, growth and survival.
Furthermore, some species of trematodes have a division of labour within their colonies of rediae (Hechinger et al. Reference Hechinger, Wood and Kuris2011; Leung and Poulin, Reference Leung and Poulin2011; Miura, Reference Miura2012). Typically, trematode rediae live within the first intermediate host where they asexually reproduce, forming a clonal colony (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). Cercariae develop within rediae and leave the host to encyst in or on the second intermediate host (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003). In colonies of species with a division of labour, there exist 2 distinct redial morphs: a large, reproducing morph and a small, non-reproducing morph. Morphological and behavioural differences between the 2 morphs indicate that the small, non-reproducing morph appears specialized for defence against hetero- or conspecific colonies trying to establish within the same host, similar to the organization of many social insect colonies (Hechinger et al. Reference Hechinger, Wood and Kuris2011).
Division of labour has been best studied in social insects, where multiple castes have specialized functions. Their response to variations in environment and resource availability has been studied by looking at how the ratios of caste members change. Oster and Wilson (Reference Oster and Wilson1978) predicted that with highly specialized castes, variation in ratios will be linked to overall colony success. Therefore, optimal ratios should vary and be adaptive for any given condition (Oster and Wilson, Reference Oster and Wilson1978). Such adaptation should occur if the system meets 4 assumptions: (1) non-reproducing castes have no other option but to help; (2) there is no constraint on the production of multiple castes; (3) the non-reproductive castes are fully sterile; and (4) the developing individuals have no control over which caste they join (Ratnieks et al. Reference Ratnieks, Foster and Wenseleers2011).
In accordance with this theory of optimal caste ratio, it has been shown that caste ratios respond to environmental changes over time: proportionally more workers in response to changes in food resources (McGlynn and Owen, Reference McGlynn and Owen2002), seasonal changes in caste ratios (Passera, Reference Passera1977; Walker and Stamps, Reference Walker and Stamps1986), decreased caste ratio variability after a disturbance (Herbers, Reference Herbers1980) and increased production of non-reproductive caste members (Passera et al. Reference Passera, Roncin, Kaufmann and Keller1996) or reproductive caste members (McGlynn, Reference McGlynn2010) under competitive pressures. However, this response has not been seen in all systems (Calabi and Traniello, Reference Calabi and Traniello1989). Across the social insects, there exists a wide diversity of reproductive strategies resulting in variation in relatedness among colony members, e.g. various types of parthenogenesis (aphids, bees and ants) and polyembryony (parasitoid wasps, bryozoans, trematodes) (Blackman, Reference Blackman1979; Seeley, Reference Seeley1995; Craig et al. Reference Craig, Slobodkin, Wray and Biermann1997; Brusca and Brusca, Reference Brusca and Brusca2003). In cases where colonies are not clonal, partial genetic relatedness make the quantification of colony fitness complicated. Trematode species with divisions of labour are an ideal model system to look at caste ratio theory because all colony members are clonal. Therefore the fitness of the colony is simply a sum of the fitness of all individuals.
A division of labour in Philophthalmus sp. colonies has recently been documented (Leung and Poulin, Reference Leung and Poulin2011). Philophthalmus sp. infects the common New Zealand mudsnail, Zeacumantus subcarinatus, as its first intermediate host. At least 3 other trematode species also infect this mudsnail, the most common being Maritrema novaezealandensis (Martorelli et al. Reference Martorelli, Fredensborg, Mouritsen and Poulin2004, Reference Martorelli, Fredensborg, Leung and Poulin2008). Infections are common (50–80% prevalence by M. novaezealandensis and 3–8% by Philophthalmus sp.). Double species infections are seen in nature, and persist long-term when snails are kept in the laboratory (up to 2 years in some cases) (M. Lloyd, personal observation). Furthermore, the reproductive output of colonies of both M. novaezealandensis and Philophthalmus sp. is reduced when they share the same host individual (Lloyd and Poulin, Reference Lloyd and Poulin2012).
Caste ratio (small rediae to large rediae, i.e. non-reproductives to reproductives) is variable in this system and ranges from 0·24 to 3·27 (Leung and Poulin, Reference Leung and Poulin2011 and the results of this study). According to the theory of optimal caste ratio, this ratio is hypothesized to change under different environmental conditions such as host stress or competition. As the small, non-reproductive rediae appear specialized for defence against co-infecting colonies (Hechinger et al. Reference Hechinger, Wood and Kuris2011), in the case of competitive interactions, it is hypothesized that there will be proportionally more small rediae in a Philophthalmus sp. colony. Since competition between trematodes in their first intermediate host is generally for space and food resources (Sousa, Reference Sousa1992; Poulin, Reference Poulin2001), we predict that colonies in competitive interactions subjected to further stress due to host starvation might intensify a change in caste ratio. Observational studies of Philophthalmus sp. infected snails with and without M. novaezealandensis indicate that the caste ratio is not responding to competition: the number of small rediae (relative to large rediae) in Philophthlamus sp. colonies shows some variation regardless of co-infection by M. novaezealandensis (Leung and Poulin, Reference Leung and Poulin2011). However, a change in caste ratios in response to competition may have been masked by the observational aspect of these studies and the relatively small sample sizes.
These species of trematodes provide a unique and interesting model in which to study resource allocation in parasite infections and caste ratio theory. Resources can be allocated to 3 different life-cycle stages that are specifically used for different functions: large rediae (growth and future reproductive output), small rediae (defence) or cercariae (reproductive output). Numbers of these life-cycle stages are variable (Leung and Poulin, Reference Leung and Poulin2011), and in the case of cercariae, respond to immediate environmental conditions (Lloyd and Poulin, Reference Lloyd and Poulin2012). The aims of this study were to use a long-term experiment to quantify changes in resource allocation and caste ratios in Philophthalmus sp. infections under the combined influence of host stress (induced by starvation) and competition by M. novaezealandensis. Evidence of changing caste ratios could highlight a new way in which parasites can alter the resources taken from the host in response to changes in availability.
MATERIALS AND METHODS
Study system
Zeacumantus subcarinatus is a common mudsnail inhabiting the intertidal zone of New Zealand mudflats. Macro-algae make up 80–90% of the snail's diet, specifically the sea lettuce, Ulva lactuca (McClatchie, Reference McClatchie1979). The snail is the first intermediate host to several trematode parthenitae (sporocysts or rediae, depending on the species), the 2 most common of which were used in this study: M. novaezealandensis (family Microphallidae) and Philophthalmus sp. (family Philophthalmidae) (Martorelli et al. Reference Martorelli, Fredensborg, Mouritsen and Poulin2004, Reference Martorelli, Fredensborg, Leung and Poulin2008). As is the case with most trematode species, Philophthalmus sp. and M. novaezealandensis asexually reproduce within the snail, filling it with sporocysts (in the case of M. novaezealandensis) or rediae (in the case of Philophthalmus sp.). Free-swimming cercariae develop in either the sporocysts or rediae and are released into the water where they look to encyst in or on a second intermediate host (West, Reference West1961; Martorelli et al. Reference Martorelli, Fredensborg, Mouritsen and Poulin2004). Philophthalmus sp. is somewhat unusual, in that the cercariae do not encyst inside the tissue of a second intermediate host, but on the outer shells of gastropods (Neal and Poulin, Reference Neal and Poulin2012). This makes the system ideal for laboratory studies; encysted metacercariae form on any hard substrate (glass, plastic, etc.), where they accumulate and are easily counted (Lei and Poulin, Reference Lei and Poulin2011). Sexual reproduction occurs after the encysted metacercariae are ingested by the adult host (a sea bird), and eggs are subsequently released back into the marine environment to re-start the cycle (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003).
Snails were collected from Lower Portobello Bay, Otago Harbour, South Island, New Zealand (45°52′S, 170°42′E) between 28 February and 5 March 2012. Individual snails were placed in 5 mL wells of a 12-well culture plate with 4 mL of filtered seawater and incubated overnight at 27 °C under bright light to encourage emergence of cercariae. Snails identified as being infected by either Philophthalmus sp. or both M. novaezealandensis and Philophthalmus sp. were retained; those infected by M. novaezealandensis only and uninfected snails were not used in this study. All snails were kept in well-aerated plastic containers (17 × 17 cm) with U. lactuca for up to 2 weeks prior to the start of the experiment.
Experimental design
On day 0 of the experiment, snails were incubated overnight again to confirm their infection status and to release any prior accumulation of mature cercariae. On day 1 of the experiment, the maximum length of each snail was measured with callipers (to 0·01 mm) and snails within each infection type (Philophthalmus sp. only, or both M. novaezealandensis and Philophthalmus sp.) were separated into size classes to be assigned evenly across food treatments. This ensured that the average snail length (which indirectly corresponds to snail age) was equal across food treatments. Individual snails were placed into plastic cups (4·5 cm diameter × 5 cm height) filled with 65 mL of filtered seawater. Cups were numbered to allow for repeated counts of individual snails over the 10 weeks of the experiment.
Food stress was induced by controlling the amount of U. lactuca added to each plastic cup. The 3 treatments consisted of either a 5 cm2 piece of U. lactuca (referred to as the well-fed treatment), a 1 cm2 piece of U. lactuca (referred to as the intermediate food treatment) or no U. lactuca (referred to as the starved treatment). Food was replaced every 7 days throughout the experiment. At the time that food was replaced each week, some portion of all 5 cm2 pieces remained and the majority of the 1 cm2 portions were consumed, indicating that 1 cm2 was just sufficient to meet the snail's diet. Sixteen Philophthalmus sp. infected snails were used per food treatment. Since snails infected with both M. novaezealandensis and Philophthalmus sp. are more rare, there were 7 or 8 snails per food treatment.
To measure cercarial output of individual colonies within snails, a standard glass microscope slide was placed in each cup. Once weekly, encysted metacercariae on the glass slides were counted under a dissecting microscope. This method has been used in the past to measure relative, if not absolute, cercarial output from Philophthalmus sp. infected snails kept in laboratory conditions (Lei and Poulin, Reference Lei and Poulin2011). Since the plastic cups were small, encysted metacercariae on the bottom of the cups were also easily counted. The slides were replaced, water was changed and plastic cups were cleaned once weekly. Cercarial counts were completed just prior to cleaning to ensure that no cercariae were lost during the cleaning process.
Caste ratio
After 10 weeks of exposure to the above food treatment, snails were dissected and small and large rediae in each snail were counted. The snail visceral mass was dissected out of the snail shell and teased apart to release Philophthalmus sp. rediae and M. novaezealandensis sporocysts. Parasite parthenitae were dyed with neutral red, and pressed between 2 glass slides. Philophthalmus sp. small and large rediae were counted separately. The caste ratio was calculated as the number of small rediae divided by the number of large ones.
Statistical analysis
Differences in numbers of Philophthalmus sp. encysted metacercariae from single and double infected snails in different treatments were tested using a Linear Mixed Model (LMM) performed in R version 2.14.0 (R Development Core Team, 2011) using the package LME4 (Bates et al. Reference Bates, Maechler and Bolker2011). Fixed effects included food treatment, infection type (either Philophthalmus sp. only or Philophthalmus sp. and M. novaezealandensis), the interaction between food treatment and infection type, and host size (as measured at the start of the experiment). Host size was included as a factor because the average size between snails of different infection types was slightly different (single infection 17·47 mm ± 2·90 mm, double infection 14·91 mm ± 1·90 mm). To account for temporal pseudo-replication, snail identity was included as a random effect. The number of encysted metacercariae was log(x + 0·5) transformed to meet assumptions of normality.
The small-to-large rediae caste ratio, total number of rediae, and number of large and small rediae per colony (i.e. per snail) were all compared between treatments using separate Linear Models (LMs). When comparing caste ratios between treatment groups, main factors included food treatment, infection type (either Philophthalmus sp. only or Philophthalmus sp. and M. novaezealandensis), the interaction between food and infection, and total number of rediae. Host size was considered as a factor, but was not significant, and removing it increased the fit of the model (according to Akaike Information Criterion (AIC) values). Differences in total number of rediae between treatments were also analysed using a LM. In this case, the main factors were infection, food treatment, the interaction between food and infection, host size and caste ratio.
Additional LMs were performed to verify whether the treatment levels affected only one caste independently of the other. When analysing numbers of small rediae, factors included food treatment, infection type (either Philophthalmus sp. only or Philophthalmus sp. and M. novaezealandensis), the interaction between the two, host size and the number of large rediae. The number of small rediae was log(x + 0·5) transformed to meet assumptions of normality. Similarly, when analysing numbers of large rediae, factors included food treatment, infection type, the interaction between the two, host size and number of small rediae. The number of large rediae was log(x + 0·5) transformed to meet assumptions of normality.
RESULTS
Over the 10 weeks of the experiment, 8 Philophthalmus sp. infected snails died, 1 from the well-fed treatment, 3 from the intermediate food treatment and 4 from the starved treatment. One double infected snail from the intermediate food treatment group died.
Cercarial output
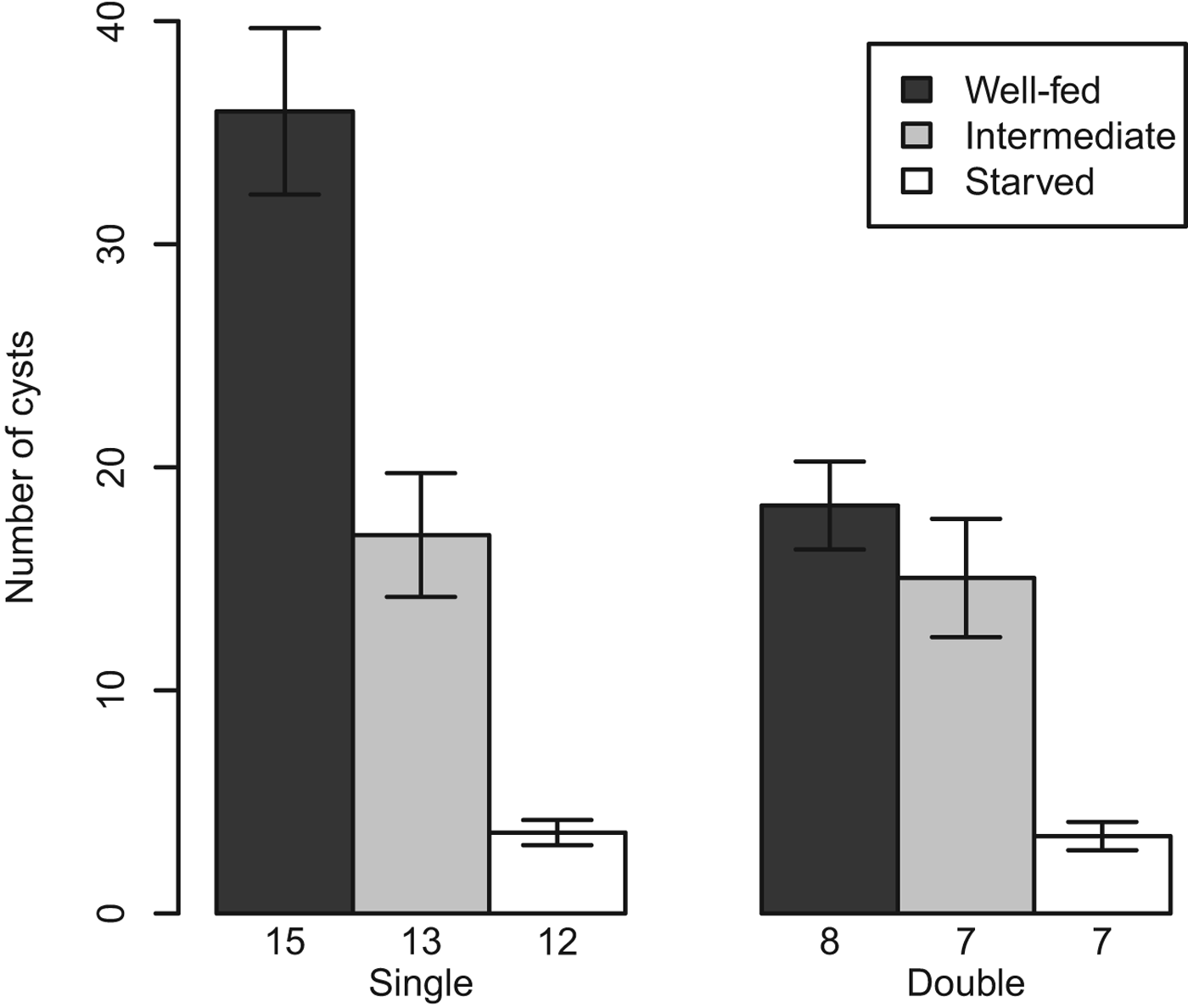
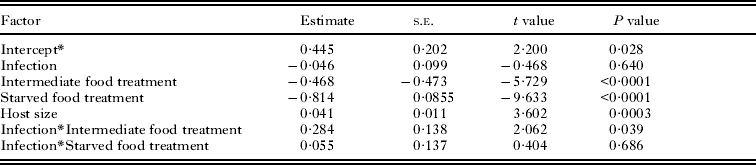
The numbers of cercariae (counted as encysted metacercariae) that emerged from well-fed snails of both infection types were significantly higher than those of snails in the intermediate and starved food treatments (P < 0·0001). Also significant was the interaction between infection type and the intermediate food treatment (P = 0·039), but not the effect of infection itself (Fig. 1, Table 1). Well-fed snails with only Philophthalmus sp. infections released approximately twice as many cercariae as well-fed snails with both Philophthalmus sp. and M. novaezealandensis. However, this difference between infection types was only seen in the well-fed treatment. In the intermediate food treatment, the numbers of cercariae that emerged from single infected snails were only slightly higher than those of double infected snails; and in the starved treatment, the numbers of cercariae emerged from single and double infected snails were similar (Fig. 1, Table 1).

Fig. 1. Average (± s.e.) total number of Philophthalmus sp. encysted metacercariae released over the ten week period from colonies without or with competition (i.e. single or double infections) by Maritrema novaezealandensis in three food treatments. Sample size is indicated at the bottom of each bar.
Table 1. Factors affecting number of encysted metacercariae from Philophthalmus sp. colonies with or without competition in three food treatments

* The effect of the well-fed treatment is included in the intercept.
Caste ratio and rediae
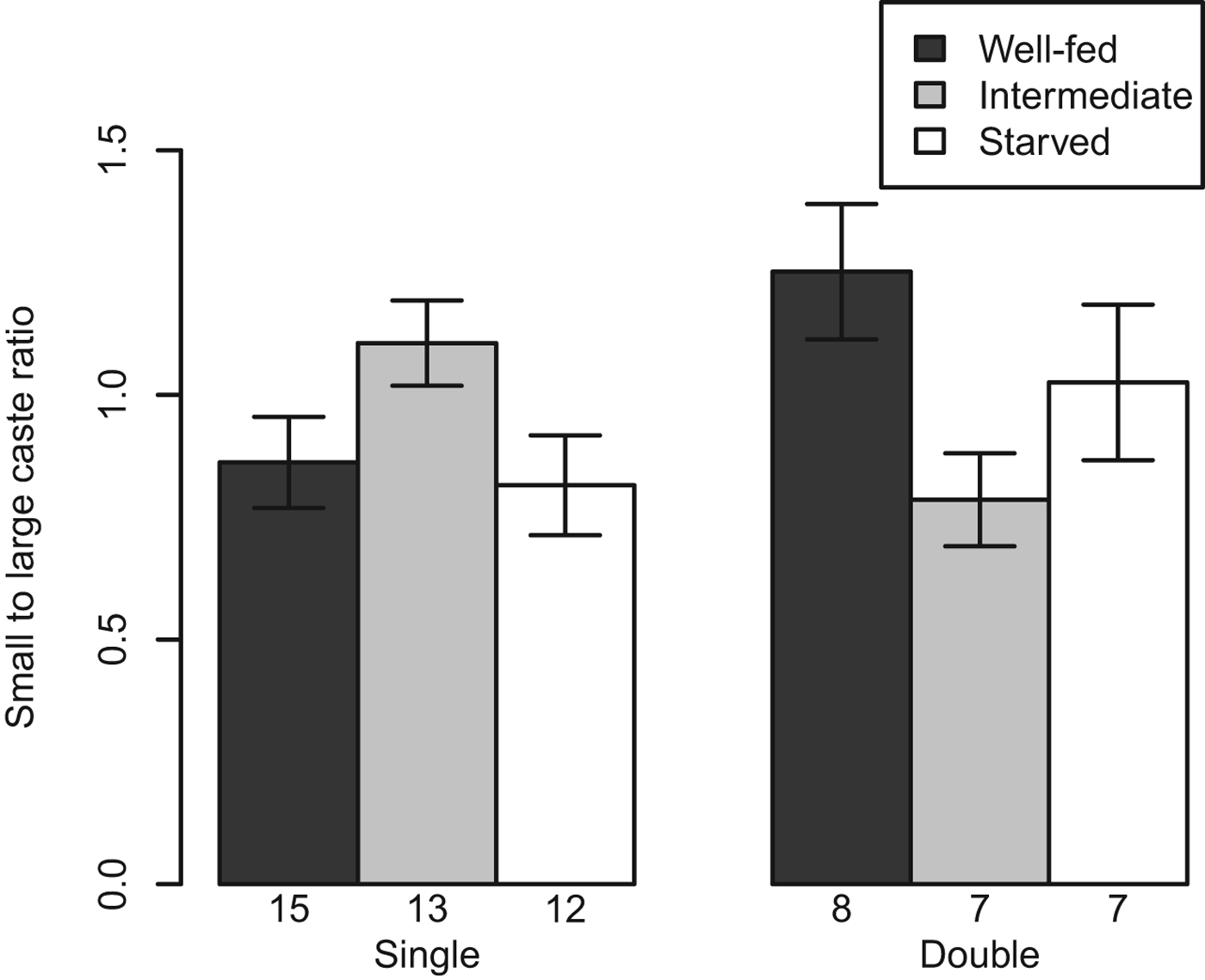
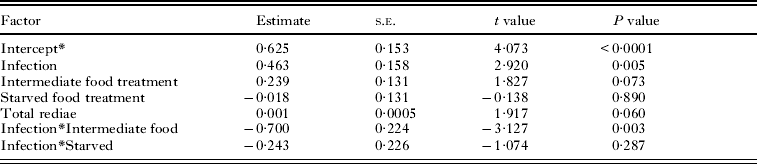
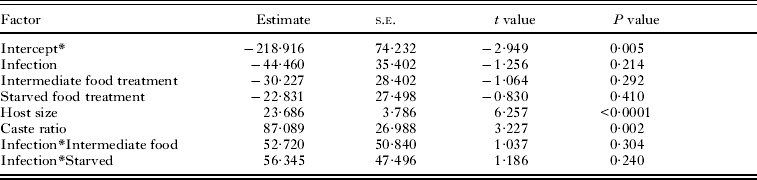
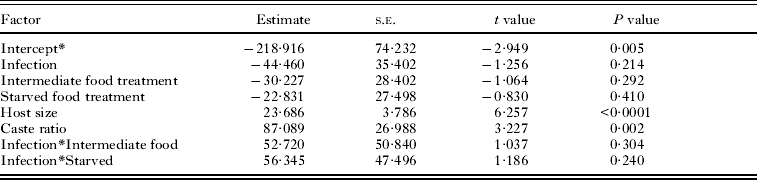
At the end of the experiment, 43 single infected snails and 22 double infected snails were dissected and their rediae counted. Snails were not included if they died before they could be dissected and the rediae inside had started to decompose. The caste ratio of small-to-large rediae ranged from 0·25 to 1·75 and was significantly higher in snails with both Philophthalmus sp. and M. novaezealandensis than in snails with only Philophthalmus sp. (P = 0·004). In addition, there was a significant interaction between the intermediate food treatment and infection type, although no effect of food treatment was detected (Fig. 2, Table 2). The double infected snails in the intermediate food treatment group hosted colonies with a lower ratio of small-to-large rediae than either the well-fed treatment or the starved treatment group. The total number of rediae per colony ranged from 66 to 510. The only significant factors affecting total number of rediae were host size and the caste ratio (Table 3).

Fig. 2. Average (± s.e.) caste ratio of small-to-large rediae in Philophthalmus sp. colonies without or with competition (i.e. single or double infections) by Maritrema novaezealandensis in three food treatments after 10 weeks. Sample size is indicated at the bottom of each bar.
Table 2. Factors affecting caste ratio of small : large rediae
* The effect of the well-fed treatment is included in the intercept.
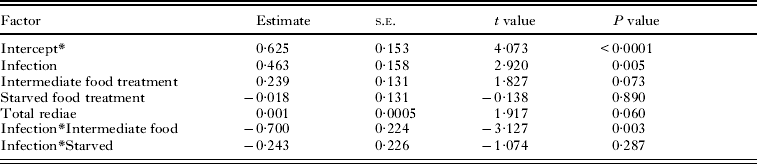
Table 3. Factors affecting the total number of rediae
* The effect of the well-fed treatment is included in the intercept.
The number of small rediae per colony ranged from 20 to 265; the significant factors affecting the number of small rediae were the number of large rediae and the interaction between infection type and the intermediate food treatment (Supplementary Table 1, in Online version only). In a close parallel, the number of large rediae per colony ranged from 35 to 235; the significant factors affecting the number of large rediae were host size, the number of small rediae, and the interaction between infection type and the intermediate food treatment (Supplementary Table 2, in Online version only).
DISCUSSION
Caste ratio
This study presents the first evidence that caste ratios in trematodes with a division of labour are influenced by their environment. Philophthalmus sp. colonies which competed with M. novaezealandensis had a higher caste ratio of non-reproducing rediae to reproducing rediae than Philophthalmus sp. colonies without a competitor. The caste ratio of colonies in ideal conditions (those in well-fed snails) increased from a mean of 0·86 small rediae per large rediae in single infected snails to a mean of 1·25 small rediae per large rediae in double infected snails (Fig. 2). In previous observational studies, the caste ratio of small, non-reproducing to large, reproducing rediae in double infected snails was not higher than that in single infected snails (Leung and Poulin, Reference Leung and Poulin2011). However, these studies used field-collected snails and/or snails maintained in the laboratory for only a short period of time, thus ignoring long-lasting effects of what resources had been available to the snail host in nature. If non-reproducing rediae are specialized for defence, as was previously suggested (Hechinger et al. Reference Hechinger, Wood and Kuris2011), one would expect the caste ratio in Philophthalmus sp. colonies to respond to co-infection by M. novaezealandensis with an increase in the relative numbers of non-reproductive rediae. Similar to our results, caste ratios in social insects did not vary due to ecological factors (one being competition) in a field observational study (Calabi and Traniello, Reference Calabi and Traniello1989), but caste ratios did respond to competition by increasing soldier production in a 7-week laboratory experiment (Passera et al. Reference Passera, Roncin, Kaufmann and Keller1996). This may indicate that there are many confounding environmental factors that determine caste ratio which can best be standardized by using experimental treatments either in the field or in the laboratory.
These results could also be interpreted within the traditional understanding of redial generations occurring in most trematode species, in which young, immature rediae with higher mobility grow into larger rediae capable of producing cercariae (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003; Rondelaud et al. Reference Rondelaud, Belfaiza, Vignoles, Moncef and Dreyfuss2009). In this case, the division of labour would be sequential and follow the ontogeny of rediae, as opposed to involving distinct morphs. If this were the case, the changes in caste ratios we observed here among treatments would represent demographic processes, i.e. adjustments to the developmental schedule of growing rediae, and not the differential production of distinct morphs. These could also be interpreted as adaptive responses as we explain above and below, following the same rationale driven by what is the optimal strategy for the whole colony. However, given that the small rediae of our study species, when left in a suitable culture medium for 2 months, never develop, even partially, toward becoming large reproductive rediae (M. Lloyd, personal observation), we believe that distinct castes do exist in this species as in other trematodes (Hechinger et al. Reference Hechinger, Wood and Kuris2011; Miura, Reference Miura2012).
Analyses of caste ratios of colonies in snails within different food treatments indicate that the ratios may be altered in response to available food treatments, although the mechanism for this is not clear. In well-fed snails, the caste ratio of Philophthalmus sp. colonies in double infected snails was higher than single infected snails. In contrast, the caste ratio of Philophthalmus sp. colonies in snails in the intermediate food group decreased when co-infected with M. novaezealandensis (Fig. 2). This interaction effect between infection type and the intermediate food treatment was seen in the LMs for caste ratio, large rediae and small rediae (Table 2, Supplementary Table S1, Supplementary Table S2, in Online version only). In social insects, there is a temporal constraint on caste ratio responses to changes in environmental condition (Herbers, Reference Herbers1980; Walker and Stamps, Reference Walker and Stamps1986). Perhaps the caste ratio in the trematode system responds over a long time period and had not had enough time to fully respond to the food stress treatment over 10 weeks. For instance, caste ratios of social ants respond to changes in food availability over 4 months (McGlynn and Owen, Reference McGlynn and Owen2002). Another explanation for this interaction effect may be that only colonies in unstressed hosts are able to increase production of small per large rediae: the caste ratio of colonies in well-fed hosts increased in response to competition while the caste ratio of colonies in stressed hosts either decreased or stayed the same in response to competition (Fig. 2).
Previous studies show that non-reproducing rediae provide some benefit to Philophthalmus sp. colonies resulting in a higher reproductive output (Lloyd and Poulin, Reference Lloyd and Poulin2012). While the mechanisms of this benefit remain unclear, the present experiment shows that colonies may be able to alter caste ratios in response to changes in environmental conditions, further indicating that small rediae play an important functional role.
Cercarial output strategy
Results also show Philophthalmus sp. colonies alter the way in which they use resources taken from the host in response to both starvation and competition. Colonies in well-fed snails had a higher reproductive output (up to 2-fold difference) than colonies in snails in the intermediate or starved food treatments (Fig. 1). When faced with a competitor, Philophthalmus sp. colonies had a lower reproductive output, but this difference was seen only between snails in the well-fed treatment group. Compared with the least stressed colonies (i.e. those in well-fed, single infection snails), those in the intermediate food treatment group showed a 2-fold decrease in reproductive output regardless of infection type. Colonies in snails in the starved treatment group showed a dramatic reduction in reproductive output regardless of infection type. The generally negative impact of food availability for the host on parasite reproductive output is not surprising as this trend has been recorded multiple times (Kendall, Reference Kendall1949; Keas and Esch, Reference Keas and Esch1997; Seppälä et al. Reference Seppälä, Liljeroos, Karvonen and Jokela2008). The interaction between food treatment and infection type provides more information on the effect of both: the fact that competition was only seen between the well-fed treatments groups indicates that the effects of host nutrition might outweigh those of competition, and thus that competition has resource-dependent effects on trematode colonies.
The total number of small and large rediae per colony did not respond to the infection type of its host. This provides evidence that Philophthalmus sp. colonies do not change their investment into growth or future reproductive output (large rediae) in response to competition. The same result has been seen in Philophthlamus sp. colonies in response to co-infection by M. novaezealandensis (Keeney et al. Reference Keeney, Boessenkool, King, Leung and Poulin2008), as well as in another similar trematode species pair (Hendrickson and Curtis, Reference Hendrickson and Curtis2002). The difference in the numbers of cercariae produced by Philophthalmus sp. colonies in unstressed hosts (well-fed, single infections) and stressed hosts (starved, double infections) indicates that rediae appear able to increase their per capita cercarial output when not under stress.
Taken together, the results from this study indicate that Philophthalmus sp. colony organization may be altered in response to environmental conditions. In addition, there was no difference in host survival between any treatment groups; only 8 out of 71 snails died over the course of the experiment. This provides further support for the hypothesis put forth by Jokela et al. (Reference Jokela, Taskinen, Mutikainen and Kopp2005) that parasites can alter the amount of resources taken from a host under stress to avoid over-exploitation and parasite-induced mortality.
ACKNOWLEDGEMENTS
We thank Shannon Smith for field and laboratory assistance and the Otago Parasite Laboratory for comments on an earlier version. This project was funded by the Department of Zoology, University of Otago.