INTRODUCTION
Empirical studies have identified that individual hosts vary greatly in terms of both the rate at which they produce infectious particles and the rate at which infectious individuals come into contact with susceptible individuals. In terms of potential onward transmission, individuals can be classified as the ‘insignificant many’ or the ‘vital few’ (Woolhouse et al. Reference Woolhouse, Dye, Etard, Smith, Charlwood, Garnett, Hagan, Hii, Ndhlovu, Quinnell, Watts, Chandiwana and Anderson1997; Perkins et al. Reference Perkins, Cattadori, Tagliapietra, Rizzoli and Hudson2003; Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004). Amongst macroparasite infections, the majority of a helminth population is usually concentrated in a minority of the host population, and is invariably best described by the negative binomial distribution in parasite intensity, because the variance in the intensity of infection between populations rises faster than the mean (e.g. Shaw and Dobson, Reference Shaw and Dobson1995; Wilson et al. Reference Wilson, Bjornstad, Dobson, Merler, Poglayen, Randolf, Read, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2001). There are 3 ways the vital few individuals may be responsible for higher than average number of transmission events: (1) these individuals have higher than average contact rates with susceptible hosts, irrespective of their intensity of infection; (2) these individuals produce infectious particles that survive longer or are more infectious than others; (3) these hosts shed more infectious particles than others in the population (Anderson and May, Reference Anderson and May1991; Matthews et al. Reference Matthews, McKendrick, Ternent, Gunn, Synge and Woolhouse2006; Chase-Topping et al. Reference Chase-Topping, Gally, Low, Matthews and Woolhouse2008). Heterogeneity in any one or combination of these processes can result in an increase in the basic reproductive number (R0) and hence the spread of infection (Woolhouse et al. 1991, Reference Woolhouse, Dye, Etard, Smith, Charlwood, Garnett, Hagan, Hii, Ndhlovu, Quinnell, Watts, Chandiwana and Anderson1997; Smith et al. Reference Smith, McKenzie, Snow and Hay2007).
Our study pertains specifically to the third mechanism, with the aim to identify the relative contribution of host characteristics and parasite co-infection status in generating variation in fecundity of the nematode H. polygyrus. Variation in egg output per infected host need not be linearly related to variation in the nematode intensity, particularly if worm growth and fecundity are constrained by density dependence via intra-specific competition or immune-mediated host responses, and perhaps exacerbated by co-infection (Goater, Reference Goater1992; Tompkins and Hudson, Reference Tompkins and Hudson1999; Dezfuli et al. Reference Dezfuli, Giari and Poulin2001; Paterson and Barber, Reference Paterson and Barber2007). Hence, we used in utero egg count as an estimate of nematode fecundity (Ractliffe and LeJambre, Reference Ractliffe and LeJambre1971; McClelland, Reference McClelland1980; Smith, Reference Smith1988; Szalai and Dick, Reference Szalai and Dick1989; Marcogliese, Reference Marcogliese1997; Tompkins and Hudson, Reference Tompkins and Hudson1999). This parameter is arguably more reliable than the alternative approach of counting worm eggs expelled by the host in the faeces, owing to the variability in faecal egg counts that can occur within and between days (Anderson and Schad, Reference Anderson and Schad1985; Keymer and Slater, Reference Keymer and Slater1987; Stear et al. Reference Stear, Bishop, Doligalska, Duncan, Holmes, Irvine, McCririe, McKellar, Sinski and Murray1995; Harvey et al. Reference Harvey, Paterson and Viney1999). As such, eggs per gram (EPG) data from a few time-points may not accurately reflect a host's infectiousness. Assuming that the rate of egg production (i.e., the nematode's lifetime reproductive or shedding rate) is linearly related to the number of eggs in the nematode uterus, the number of eggs in utero serves as a reliable estimate of the number of eggs shed into the environment (Michel, Reference Michel1963; Ractliffe and LeJambre, Reference Ractliffe and LeJambre1971). Previous work has shown this to be indeed the case in nematodes, where a positive association was found between the number of eggs in utero and the faecal egg count in sheep infected with Teladorsargia circumcinta (Stear et al. Reference Stear, Bishop, Doligalska, Duncan, Holmes, Irvine, McCririe, McKellar, Sinski and Murray1995; Stear and Bishop, Reference Stear and Bishop1999). Here, we assume that in utero egg count is a reliable measure of worm fecundity in H. polygyrus, which serves as a proxy for host infectiousness.
Hosts harbouring highly fecund worms are likely to shed more infectious particles than others in the population, and hence contribute more to transmission events than the average host. Studies on H. polygyrus but also on other nematode and tick vectors have demonstrated that male hosts, particularly those in breeding condition may be responsible for the majority of parasite transmission (Perkins et al. Reference Perkins, Cattadori, Tagliapietra, Rizzoli and Hudson2003; Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004; Luong et al. Reference Luong, Grear and Hudson2009). Furthermore, variation in the intensity of nematode infection between individuals has been related to host body mass and sex (Viney, Reference Viney2002; Paterson and Viney, Reference Paterson and Viney2002; see review by Poulin, Reference Poulin1996) and will also have a major influence on variation in individual host infectiousness. In this study, we examined the relationship between host characteristics and nematode fecundity by quantifying the number of in utero eggs of individual worms in wild mice. We asked, ‘Do inherent variations among individual hosts contribute to the variation in nematode fecundity and hence production of parasite transmission stages’? We hypothesized that intrinsic differences between individual hosts with regard to sex, breeding status and body mass generate variation in nematode fecundity. Since males generally harbour a higher intensity of parasites, we expected that breeding males harboured nematodes with higher fecundity than other hosts. As a contrast to the relationship between worm fecundity and host characteristics, we also examined the relationship between worm intensity and host characteristics.
Co-infecting parasites and pathogens have also been proposed to affect the number of infective stages produced (Bassetti et al. Reference Bassetti, Bischoff and Sherertz2005; Matthews et al. Reference Matthews, McKendrick, Ternent, Gunn, Synge and Woolhouse2006). The hypothesis here is that individuals that carry co-infections are more susceptible than single infected hosts, and that these nematodes are also more fecund. For example, co-infection with the poxyvirus myxoma has been shown to be positively associated with increased susceptibility to the nematode, Trichostronglyus retortaeformis (Cattadori et al. Reference Cattadori, Albert and Boag2007). This interaction is thought to be often mediated by the host immune system whereby pathogens exert a suppressive effect on the cytokine response, stemming the control of nematode establishment, expulsion and fecundity (Cox, Reference Cox2001; Lello et al. Reference Lello, Boag, Fenton, Stevenson and Hudson2004; Graham, Reference Graham2008; Montes et al. Reference Montes, Sanchez, Verdonck, Lake and Gonzalez2009). Thus, we asked ‘How do inter-specific interactions between the parasite community influence nematode fecundity and intensity’? We proposed that co-infecting microparasites indirectly generate variation in nematode fecundity such that worms from hosts not previously exposed to microparasites are expected to have fewer eggs in utero than worms collected from hosts that were exposed.
MATERIALS AND METHODS
Animal trapping and pathogen sampling
Extensive trapping of Apodemus flavicollis, the yellow-necked mouse was carried out in July 2002 using multi-capture live traps (Ugglan type 2, Graham Sweden) at 6 study sites for 3 nights, with a total trapping effort of 3456 trap nights. The study sites were located in mixed broadleaf woodlands of the Italian Dolomitic Alps. The methods followed standard trapping procedures and are described elsewhere (Ferrari et al. Reference Ferrari, Rosa, Pugliesa and Hudson2007). For each mouse we recorded the host sex, body mass and breeding condition. Individuals with descended testes, perforated vagina or pregnancy were recorded as animals in breeding condition. Mice with a body mass above 15 g were euthanized following the animal procedures of the European Commission Directive 86/609/EEC implemented by Italy. The gastro-intestinal tract was removed and all helminths extracted using a filtration-sedimentation technique (Euzeby, Reference Euzeby1982). All helminths were counted and identified to species level. The dominant helminth species was Heligmosomoides polygyrus, a directly transmitted nematode common in A. flavicollis (Gregory et al. Reference Gregory, Montgomery and Montgomery1992; Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004). From the larger dataset we randomly selected a subset (25%, n=59 mice) to carry out in utero egg counts. From these mice each female H. polygyrus was mounted onto an individual slide and the eggs in utero were visually counted under a stereomicroscope. A subsample of nematodes (n=24) from this group was measured to assess the relationship between worm length and in utero egg count. Individual H. polygyrus were photographed under a stereomicroscope (Leica® S6E) with a digital camera (Nikon® Coolpix 4500). The length of each nematode was measured using the ImageJ software package (Rasband, Reference Rasband2007).
Blood and tissue samples were taken from each individual to test if the mice had been exposed to the following pathogens: arenavirus, cowpox, Ehrlichia spp., murine-herpes gammavirus (MuHV), and tick-borne encephalitis (TBE). Serum samples were analysed using standard enzyme-linked immunosorbent assay (ELISA) and haemaglutination inhibition (HI) assay techniques for TBE as described in Perkins et al. (Reference Perkins, Cattadori, Tagliapietra, Rizzoli and Hudson2006) and Rizzoli et al. (Reference Rizzoli, Neteler, Rosa, Versini, Cristofolini, Bregoli, Buckley and Gould2007). Serum samples were tested for the arenavirus and MuHV using an indirect immunofluorescent (IF) antibody assay as described in Tagliapietra et al. (Reference Tagliapietra, Rosa, Hauffe, Laakkonen, Voutilainen, Vapalahti, Vaheri, Henttonen and Rizzoli2009) and Telfer et al. (Reference Telfer, Bennett, Carslake, Helyar and Begon2007), respectively. Cowpox virus antibody was also determined in an IF antibody assay as described in Crouch et al. (Reference Crouch, Baxby, McCracken, Gaskell and Bennett1995). Standard PCR and DNA sequencing were used for the identification of Ehrlichia from liver and spleen samples taken at necropsy (Liz et al. Reference Liz, Anderes, Sumner, Massung, Gern, Rutti and Brossard2000). We recognize that animals that were seropositive for a given pathogen may not have been actively infected with the microparasite at the time of necropsy (see Discussion section).
Host characteristics and H. polygyrus intensity
The relationship between nematode intensity and host factors was analysed with a generalized linear model using a negative binomial error distribution. Nematode intensity per host was modelled as the response with host mass, sex, breeding condition, co-infection of any microparasite, and their interactions as explanatory variables. We used backwards model selection starting with the full model with all interaction terms to determine a minimum best-fit model. We removed explanatory terms if a drop-in-deviance χ2 test comparing a full model to a reduced model was not significant (P>0·05). We first tested the most complex interaction terms and proceeded with less complex interaction terms until we reached a minimum model.
Host characteristics and H. polygyrus fecundity
To investigate the relationship between host characteristics and nematode fecundity, we used the logarithm of in utero egg counts of female H. polygyrus from a subset (n=59 hosts) of the full data as the response variable in a linear mixed effect (LME) regression model. Host body mass, host sex, and host breeding condition were entered into the model as fixed-effect explanatory variables. To account for the replicated effect of measuring multiple worms from the same host, the unique identification code of each host was included as a random effect. We again used backwards model selection starting with the full model with all interaction terms to determine a minimum best-fit model. A posteriori contrasts were employed to test for differences in mean nematode fecundity between specific functional host-groups, which were defined as non-breeding females, breeding females, non-breeding males, and breeding males.
Age and co-infection relationship with H. polygyrus intensity
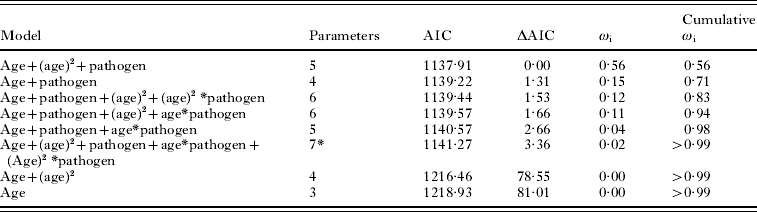
Body mass was used to estimate the age class of each non-juvenile (⩾19 g; Bajer et al. Reference Bajer, Behnke, Pawelczyk, Kulis, Sereda and Sinski2005; where 19 g was the smallest animal recorded in breeding condition). We created 6 equal-sized age classes with increments of 3·85 g. The relationship between age class, H. polygyrus intensity and co-infection was investigated by fitting the observed data to hypothetical age-intensity curves and determining the best fit. The fits investigated included linear and second order polynomial, which were chosen according to previously described age-intensity distributions (Wilson et al. Reference Wilson, Bjornstad, Dobson, Merler, Poglayen, Randolf, Read, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2001). Models were built using pathogen co-infection as an additive factor and as an interaction factor between the linear and polynomial age variables; giving 8 competing models to compare (Table 3). We conducted a Pearson χ2 goodness-of-fit test on the full model. Model comparisons were carried out using Akaike's information criterion (AIC) value and relative importance of variables in the candidate models was evaluated by summing the model weight (ωi) of the models containing a parameter of interest (Burnham and Anderson, Reference Burnham and Anderson2002). The individual (ωi) indicates the probability that a given model is the best model of the candidate set given the data, the cumulative (ωi) refers to the probability that a subset of models contains the best fit model among competing models, and variable weight is an index of the relative importance of an individual explanatory variable among the candidate set of models.
RESULTS
Parasite community
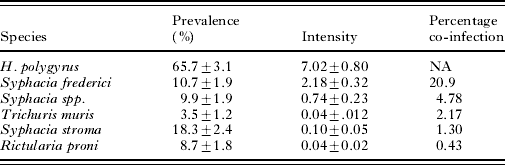
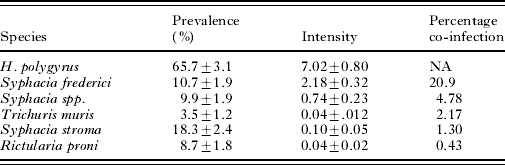
We captured a total of 230 A. flavicollis, from which 6 species of nematode parasites were recovered (Table 1). The directly transmitted nematode H. polygyrus was most prevalent at 66% and also reached the highest mean intensity at 7·02±0·80 (Table 1). This intensity distribution followed a negative binomial distribution (goodness-of-fit test P>0·1) with mean intensity=7·02 (95% CI [5·70, 8·84] and with an estimated dispersion parameter from the negative binomial, k=0·34 (95% CI [0·30, 0·44]). H. polygyrus body length was correlated with the number of eggs in utero per female worm (Coef.=0·107, s.e.=0·034, P=0·002). The most common nematode co-infection occurred with H. polygyrus and Syphacia frederici at 20·9% (Table 1).
Table 1. Summary of the prevalence and intensity (±s.e.) of infection for six species of parasitic nematodes recovered from the wild rodent, Apodemus flavicollis
(Co-infection indicates the percentage of the population which is simultaneously co-infected with Helgimosomoides polygyrus and another given species of gastro-intestinal parasitic nematode.)
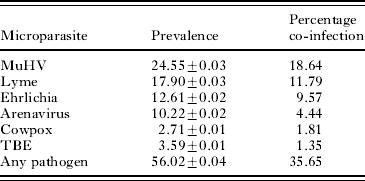
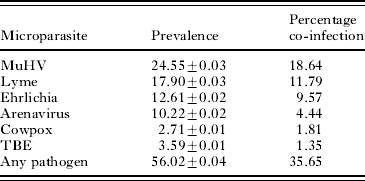
Sera from mice were analysed for antibodies to 5 different pathogens. Of the pathogens tested, mice had the highest antibody-positive sero-prevalence against murine herpes gammavirus (MUHV) (24·5±0·03%), which also had the highest percentage of co-infection (18·64%) with H. polygyrus (Table 2). The majority of animals (56·0±0·04%) had antibodies against at least 1 of the pathogens screened. Co-infection with H. polygyrus and any given pathogen occurred at a prevalence of 35·6% (Table 2).
Table 2. Summary of the prevalence of antibodies to six different pathogens from a free-living population of Apodemus flavicollis
(The percentage co-infection indicates the frequency at which the given pathogen co-occurs with Heligmosomoides polygyrus.)
Host characteristics and H. polygyrus intensity
A generalized linear model (GLM) analysis of the intensity of H. polygyrus and host factors identified a weak but significant positive association between the intensity of infection and host body mass (Coef.=0·041, s.e.=0·017, P=0·019). The intensity of infection (uninfected mice included) was significantly (Coef.=−1·85, s.e.=0·446, P<0·001) lower among mice in breeding condition (mean±s.e.=3·74±1·16) than those not in breeding condition (mean±s.e.=8·88±0·75). Host sex (Coef.=0·037, s.e.=0·272, P=0·891) and the interaction with breeding status and sex were not significant (Coef.=0·832, s.e.=0·522, P=0·111). The relationship between H. polygyrus intensity and S. frederici intensity was not significant (Coef.=0·009, s.e.=0·028, P=0·754). There were no statistically significant differences in the intensity of H. polygyrus in relation to the presence or absence of microparasite antibodies (Coef.=0·149, s.e.=0·237, P=0·53).
Host characteristics and H. polygyrus fecundity
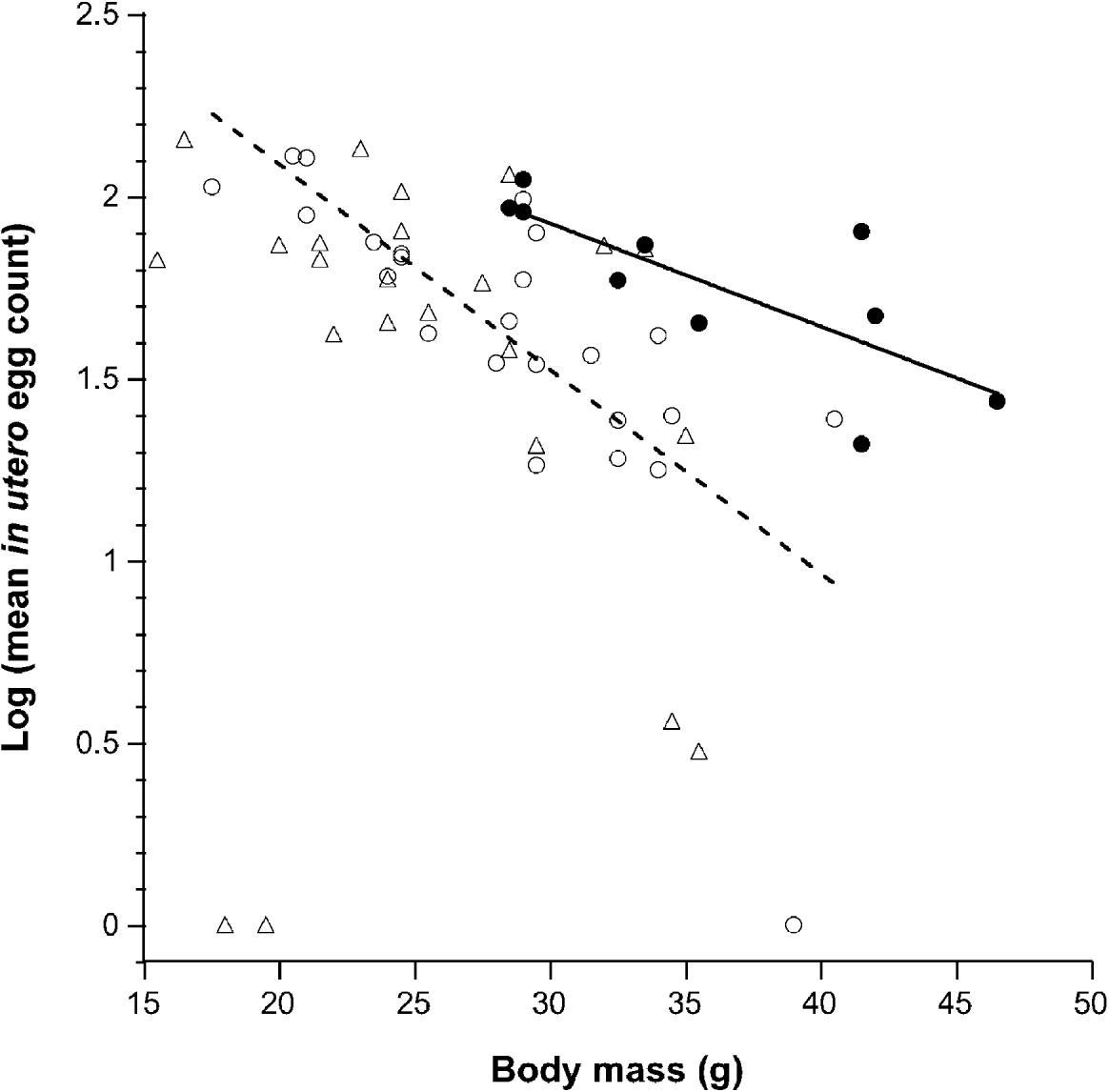
We measured in utero egg counts from a total of 59 A. flavicollis hosting a total of 652 nematodes, from which 417 intact female worms were sampled. The mean number of eggs in utero was not correlated with the intensity of H. polygyrus (Coef.=−0·681, s.e.=0·420, P=0·112). A linear-mixed-effects regression analysis of host factors revealed that host body mass was significantly negatively associated with the number of eggs per worm (Coef.=−0·039, d.f.=327, P<0·0001; Fig. 1). Host sex (Coef.=0·084, s.e.=0·065, P=0·203) or breeding status alone was not significant (Coef.=−0·156, s.e.=0·151, P=0·308), but the interaction between sex and breeding status was significantly associated with nematode fecundity (Coef.=0·521, d.f.=53, P=0·004). Co-infection with another dominant nematode parasite, S. frederici did not influence H. polygyrus fecundity (Coef.=0·005, s.e.=0·011, P=0·681). The presence or absence of antibodies to any pathogen did not significantly alter H. polygyrus fecundity (Coef.=0·125, s.e.=0·076, P=0·10).
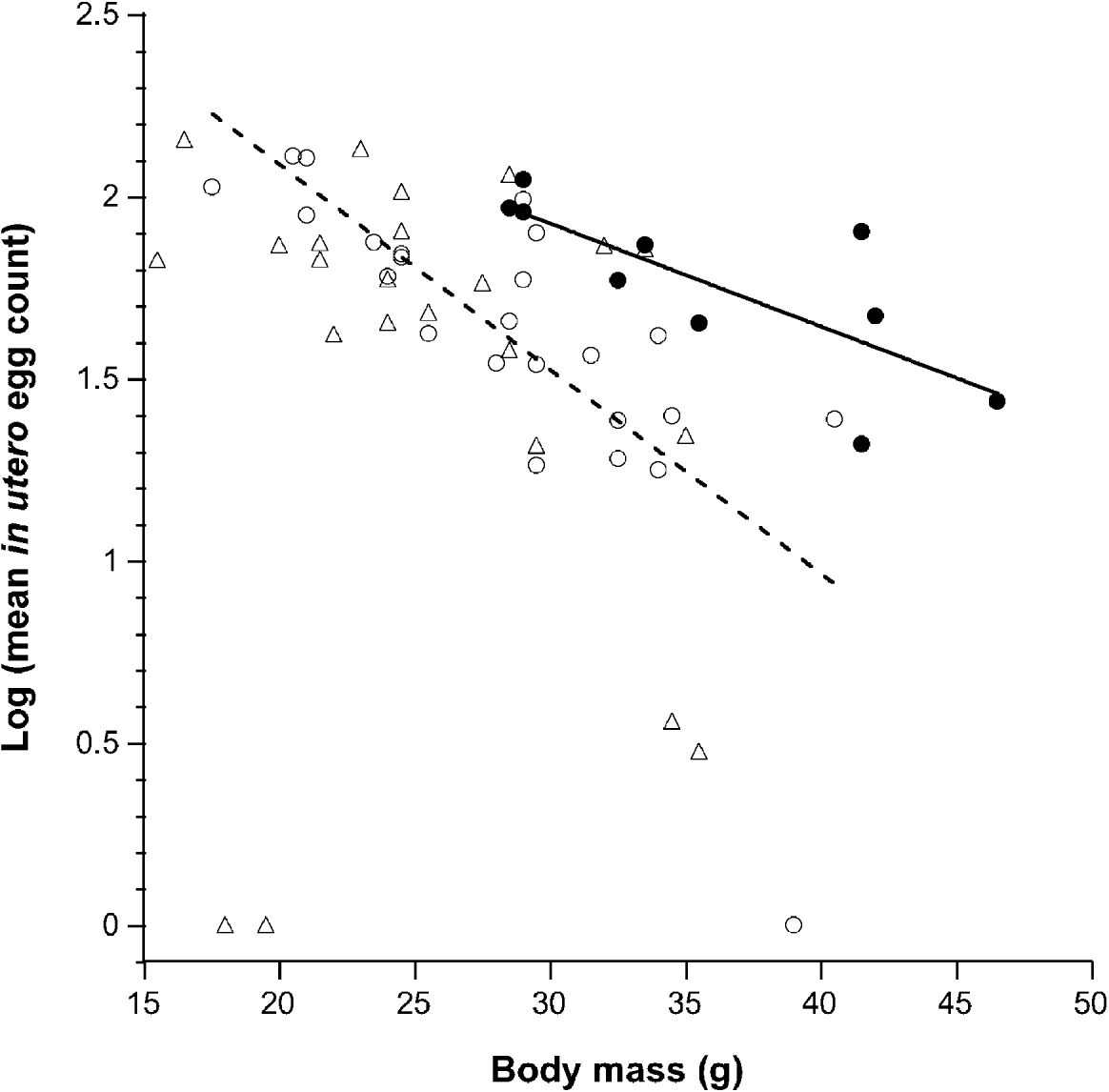
Fig. 1. The relationship between host body mass of wild rodents and the nematode fecundity. Breeding (solid circles) and non-breeding (open circles) males have a negative relationship between worm fecundity and body mass (black line, breeding males; dashed line, non-breeding males). Note that for a given mass, breeding males harboured more fecund nematodes on average compared to non-breeding males. The association between egg count and body mass was not significant for non-breeding females (open triangle); 2 females, both pregnant have been omitted. The 3 individuals with no eggs are likely to be immature nematodes.
To further examine the interaction between sex and breeding condition, an a posteriori contrast analysis on the functional groups was conducted. On average H. polygyrus that infected breeding males were 52% more fecund than those infecting non-breeding males (Coef. est.=0·411, s.e.=0·148, P=0·006). Worms from breeding males were also more fecund on average than those infecting non-breeding females (Coef.=−0·567, s.e.=0·194, P=0·003). The mean in utero egg count for worms from non-breeding males exhibited a tendency to be higher than for non-breeding females, but this was not statistically significant (Coef.=0·256, s.e.=0·136, P=0·060). Worm fecundity was not significantly different for breeding and non-breeding female mice (Coef.=−0·193, s.e.=0·273, P=0·479). The sample size of breeding females was low (n=2) and therefore lacked sufficient statistical power to detect differences among groups.
Age and co-infection relationship with H. polygyrus intensity
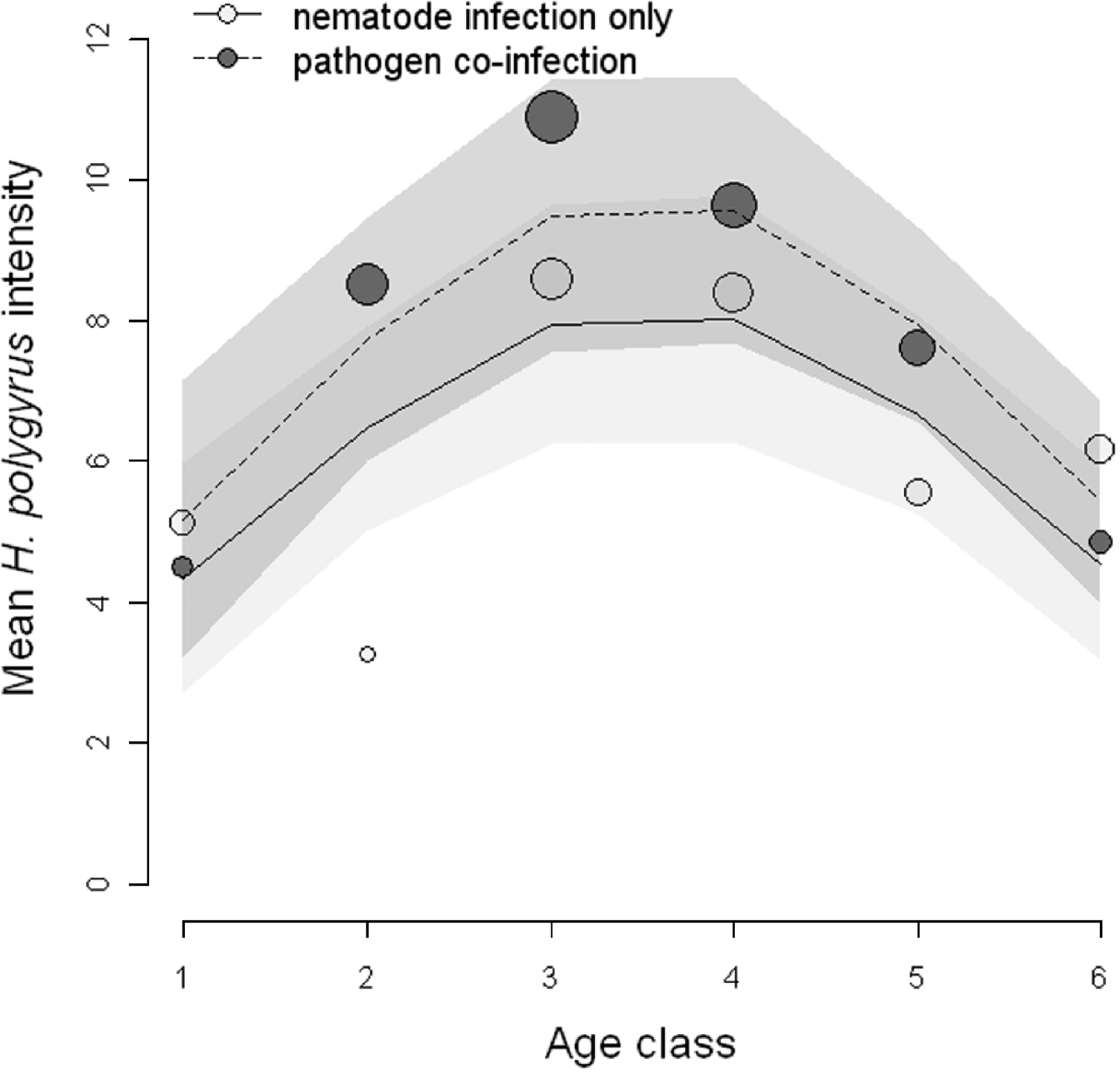
Next, we tested the hypothesis that co-infection with a microparasite would influence the intensity of H. polygyrus infection. Out of the 8 competing models, the top 4 candidate models explained a cumulative 94% of the model weights indicating that there is a high probability that one of the top 4 models is the best model of the candidate models (Table 3). A Pearson goodness of fit test showed that the full model was an adequate fit to the data (P=0·84). However, no single model had strong support as the best model. We calculated the variable weights in order to assess which explanatory variables contained in the top 4 models were important predictors of H. polygyrus intensity and the linear predictor of age, as well as the polynomial predictor of age was important in our candidate set of models (0·94 and 0·79 variable weights, respectively). Variable weights of the interaction terms between age (linear component) and pathogen co-infection, as well as age2 (polynomial component) and pathogen co-infection were low (0·11 and 0·12, respectively). Hence, nematode intensity increased with host age, peaked in the middle age classes before decreasing in older animals. Although the additive effect of pathogen co-infection was an important component of the candidate models (variable weight 0·98), the age-intensity profile did not differ greatly between mice singly-infected with H. polygyrus and mice co-infected with H. polygyrus and a pathogen (Fig. 2).
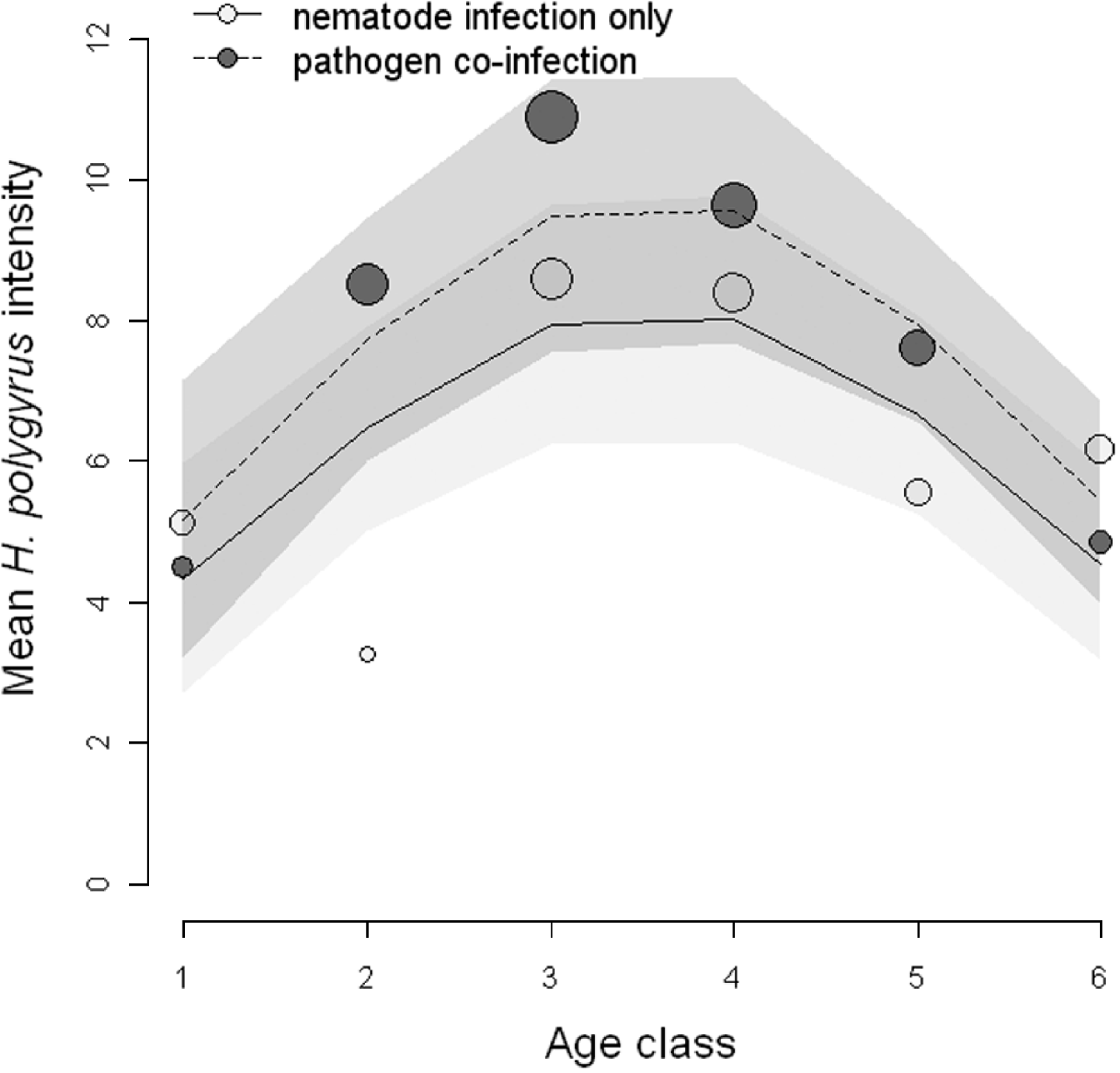
Fig. 2. Age-intensity curves showing the relationship between intensity of Heligmosomoides polygyrus and host age class. The observed mean H. polygyrus intensity for mice with the nematode infection only (dark circles) and mice with both nematode and antibodies to pathogen (light grey circles) are shown with size indicating relative sample size. The predicted values for nematode-infected (solid line) and co-infected (dashed line) mice are shown based on a model with the 3 most important relative variable weights, age+age2+pathogen co-infection, with 95% prediction confidence limits (shaded areas).
Table 3. Candidate models, AIC values, and model weights for 8 generalized linear models testing for linear versus the polynomial fit of the age – Heligmosomoides polygyrus intensity profile, as well as interaction between age-intensity and pathogen co-infection
(The value ωi refers to the probability that the model is the best model given the data and the term cumulative ωi is the probability that the ascending set of candidate models contains the best model given the data.)
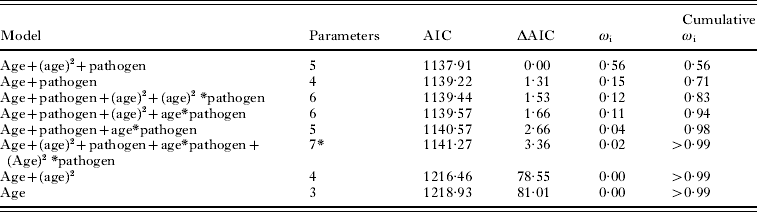
* Full model, Pearson goodness-of-fit test (P=0·84).
DISCUSSION
We examined the relative importance of intrinsic host factors and microparasite co-infection in generating variation in H. polygyrus fecundity, a proxy for infectiousness. Studies on other parasite-host systems have suggested that a small proportion of the population is responsible for the majority of transmission events (Woolhouse et al. Reference Woolhouse, Dye, Etard, Smith, Charlwood, Garnett, Hagan, Hii, Ndhlovu, Quinnell, Watts, Chandiwana and Anderson1997; Perkins et al. Reference Perkins, Cattadori, Tagliapietra, Rizzoli and Hudson2003; Galvani and May, Reference Galvani and May2005). There are several possible mechanisms underlying this heterogeneity in transmission, including variation in contact rates between infected and susceptible hosts, and the quality and/or quantity of infective stages shed into the environment (Keeling et al. Reference Keeling, Woolhouse, Shaw, Matthews, Chase-Topping, Haydon, Cornell, Kappey, Wilesmith and Grenfell2001; Matthews et al. Reference Matthews, McKendrick, Ternent, Gunn, Synge and Woolhouse2006; Chase-Topping et al. Reference Chase-Topping, Gally, Low, Matthews and Woolhouse2008). In our study, we investigated the relative contribution of intrinsic differences between hosts and co-infection status to variation in the quantity of infective stages shed, measured here in terms of nematode fecundity. Our results show that sex and breeding status interacted such that breeding males harboured more fecund nematodes than other hosts. In contrast, we did not find any evidence to support the hypothesis that co-infecting pathogens generated variation in worm fecundity. The presence or absence of antibodies to any pathogen did not significantly affect worm fecundity, and the age-intensity profiles for these two groups did not differ significantly from each other. Taken together, these results suggest that intrinsic differences between hosts, specifically with regard to sex and breeding condition, contribute relatively more to the variation in H. polygyrus fecundity than co-infection status.
Interestingly, animals of higher body mass harboured nematodes that had a lower fecundity than those of smaller animals. H. polygyrus fecundity has been shown to decrease with worm age and eventually cease at 8–10 weeks post-infection (Keymer and Hiorns, Reference Keymer and Hiorns1986; Gregory et al. Reference Gregory, Keymer and Clarke1990; Quinnell, Reference Quinnell1992). Given that host body mass serves as a proxy for age, the decrease in worm fecundity in larger animals is likely due to the senescence of worms acquired earlier in life. An alternative explanation for the decline in worm fecundity with host age is the development of acquired immunity in older animals (Gregory et al. Reference Gregory, Keymer and Clarke1990). Gregory et al. (Reference Gregory, Montgomery and Montgomery1992) showed that in repeated nematode infections, nematode egg production decreased over time at a faster rate than in primary infections, suggesting that acquired immunity acts on worm fecundity to constrain parasite population growth. Other model systems such as T. retortaeformis have also demonstrated a reduction in worm fecundity with host age as a consequence of acquired immune response (Chylinski et al. Reference Chylinski, Boag, Stear and Cattadori2009).
However, for a given body mass, male hosts in breeding condition harboured significantly more fecund worms than non-breeding males. Possible mechanisms underlying this pattern include host-mediated effects such as immuno-suppression by testosterone, which may be elevated during breeding (Poulin, Reference Poulin1996; Zuk and McKean, Reference Zuk and McKean1996). Such immuno-suppression may allow worms to grow larger in the breeding males, thus leading to greater worm fecundity. Indeed, H. polygyrus body length was a predictor of the number of eggs in utero in our study. Moreover, Swanson et al. (Reference Swanson, Falvo and Bone1984) and Harder et al. (Reference Harder, Wunderlich and Marinovski1992) showed that lab mice treated with testosterone implants display a positive relationship between testosterone levels and nematode growth and egg production. An alternative explanation for the higher egg counts among male mice is that breeding males may be subject to greater stress levels as a result of territorial defence and agonistic encounters, compared to non-breeding males (Anderson, Reference Anderson1994; Zuk, Reference Zuk1990). Stress hormones produced by the host under these circumstances including cortisol or corticosterone have been shown to be immuno-suppressive (Noble, Reference Noble1961, Reference Noble1962, Reference Noble1966; Stein and Schleifer, Reference Stein, Schleifer and Zales1985; Zuk and McKean, Reference Zuk and McKean1996; Elftman et al. Reference Elftman, Norbury, Bonneau and Truckenmillar2007).
In contrast to the nematode fecundity results, the intensity of infection was lower among breeding mice compared to non-breeding mice. These results suggest that hosts in breeding condition harbour fewer but more fecund nematodes than non-breeding hosts, which is consistent with our findings for male hosts. These contrasting results also illustrate the advantage of using fecundity as a more direct measure of infectiousness than intensity. The general negative relationship between intensity and fecundity may arise as a consequence of intra-specific competition, limiting nematode establishment, growth, or egg production as competition for resources increases. However, the mean number of H. polygyrus eggs in utero was not correlated with the intensity of infection, suggesting a lack of density-dependent constraint on per capita female fecundity in this system. Alternatively, a host-mediated mechanism, such as the immune response, may be controlling parasite growth and fecundity (Stear et al. Reference Stear, Bishop, Doligalska, Duncan, Holmes, Irvine, McCririe, McKellar, Sinski and Murray1995, Reference Stear, Bairden, Duncan, Holmes, McKellar, Park, Strain, Murray, Bishop and Gettinby1997; Zuk and McKean, Reference Zuk and McKean1996; Poulin, Reference Poulin1996). Indeed, Keymer and Hiorns (Reference Keymer and Hiorns1986) showed that lab mice subject to repeated infections exhibited an immune-mediated decrease in worm fecundity (also see Gregory et al. Reference Gregory, Keymer and Clarke1990). Therefore, breeding animals harbour fewer and more fecund nematodes than non-breeding animals, and this difference can result in higher levels of infectiousness among the mice in breeding condition.
We found no evidence for differences in worm fecundity or intensity of infection between the host sexes. The lack of sex differences conflicts with comparative studies in which male mammals were found to consistently harbour larger and presumably more fecund nematodes (Poulin, Reference Poulin1996; Dare and Forbes, Reference Dare and Forbes2008). Both laboratory and field studies of H. polygyrus infections in wood mice (Apodemus sylvaticus) show that males have a higher prevalence and intensity of infection than females (Elton, Reference Elton1931; O'Sullivan et al. Reference O'sullivan, Smal and Fairley1984; Gregory et al. Reference Gregory, Keymer and Clarke1990; Ferrari et al. Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004). Our inability to detect a significant difference between the sexes may be due to the small sample size among females, particularly those in breeding condition.
The overall age-intensity profile for H. polygyrus was convex such that the intensity of H. polygyrus peaked in the middle age classes before turning over and declining in the older hosts. This convex shape has also been documented in wild and enclosed populations of A. sylvaticus infected with H. polygyrus (Gregory, Reference Gregory1991; Gregory et al. Reference Gregory, Montgomery and Montgomery1992). The initial rise in the curve is likely due to an increase in exposure or force of infection with age. Several possible mechanisms may explain the decline in intensity among older age classes, including parasite-mediated host mortality, acquired immune response, and/or age-related changes in host exposure to parasite infective stages (Anderson and Gordon, Reference Anderson and Gordon1982; Anderson and May, Reference Anderson and May1985; Hudson and Dobson, Reference Hudson, Dobson, Grenfell and Dobson1995; Wilson et al. Reference Wilson, Bjornstad, Dobson, Merler, Poglayen, Randolf, Read, Skorping, Hudson, Rizzoli, Grenfell, Heesterbeek and Dobson2001). Parasite-induced host mortality has been demonstrated in lab mice (Keymer and Hiorns, Reference Keymer and Hiorns1986; Scott Reference Scott1987, Reference Scott1990) and in enclosed wood mice populations infected with H. polygyrus (Gregory, Reference Gregory1991). There is also ample evidence for acquired immunity from a number of laboratory and semi-natural studies of H. polygyrus infections in lab Mus (Keymer and Hiorns, Reference Keymer and Hiorns1986; Keymer and Tarlton, Reference Keymer and Tarlton1991; Tanguay and Scott, Reference Tanguay and Scott1992) and Apodemus species (Gregory et al. Reference Gregory, Keymer and Clarke1990; Quinnell, Reference Quinnell1992). For example, wood mice infected with H. polygyrus exhibited exposure-dependent constraints on parasite population growth, with higher variability in population dynamics between individuals exposed to repeated infections compared to those in primary infection (Gregory et al. Reference Gregory, Montgomery and Montgomery1992; Quinnell, Reference Quinnell1992).
Neither the shape nor magnitude of the age-intensity curve was statistically different between single- and co-infected animals. Also, co-infection with at least one microparasite did not influence the intensity or fecundity of H. polygyrus compared to hosts infected with the nematode alone. In contrast, laboratory mice experimentally co-infected with H. polygyrus and a respiratory bacterium have been shown to be chronic shedders of nematode eggs (Lass et al., personal communication). The failure to detect a difference in our study may be because other naturally occurring pathogens were not considered in the ELISA and HI tests, but may have played a potentially important role in what was categorized as an uninfected animal this study. Furthermore, hosts that tested sero-positive for a pathogen may not be infected at the time of dissection since the ELISA tests merely detected the presence/absence of antibodies, possibly from past infections. If the microparasite had been cleared, then the strength of the pathogen-induced immune response may be diminished, and any immune-mediated interaction between H. polygyrus and a microparasite infection could be below the threshold of detection (Janeway and Travers, Reference Janeway and Travers1996).
In summary, the present results suggest that intrinsic differences between hosts, specifically the interaction between sex and breeding condition, had a relatively greater impact on nematode fecundity than co-infection with a microparasite. Given that worm fecundity is a proxy for host infectiousness, our results suggest that heterogeneity in the infectiousness of hosts can create super-spreaders, potentially independent of infection intensity. In this case, breeding males were more infectious than other hosts, which supports the findings of Perkins et al. (Reference Perkins, Cattadori, Tagliapietra, Rizzoli and Hudson2003) and Ferrari et al. (Reference Ferrari, Cattadori, Nespereira, Rizzoli and Hudson2004) that breeding males are driving transmission of several Apodemus parasites. Optimal control of infectious diseases should target treatment of those individuals that account for the majority of the transmission (Anderson and May, Reference Anderson and May1991; Woolhouse et al. Reference Woolhouse, Dye, Etard, Smith, Charlwood, Garnett, Hagan, Hii, Ndhlovu, Quinnell, Watts, Chandiwana and Anderson1997). Future studies that incorporate seasonal data and the experimental manipulation of nematode infections are needed to provide better insight into the role of intrinsic host factors and parasite-community in generating variation in host infectiousness.
We thank I. Cattadori, A. Pathak, B. Vigliotti for insightful discussions on host immunity, and B. Bozick for assisting with the in utero egg counts. This work was funded by NSF grant EF-0520468 as part of the joint NSF-NIH Ecology of Infectious Disease program, and in part by The Autonomous Provincie of Trento, under grant ECODIS. We also thank the anonymous reviewers for their helpful comments.