Published online by Cambridge University Press: 07 August 2001
There is evidence that faecal egg counts of Schistosoma mansoni vary considerably from day to day, which results in poor sensitivity of single stool readings. Intra-specimen variation of S. mansoni egg counts may also be considerable, but has previously been considered as the less important component. We quantified the relative contribution of these two sources of variation among 96 schoolchildren from an area in Côte d'Ivoire highly endemic for S. mansoni. Stool specimens were collected over 5 consecutive days, and 5 egg-counts were made in each specimen by the Kato–Katz technique. The point prevalence of the first sample was 42.7% and the cumulative prevalence after the maximum sampling effort was 88.5%. Using generalized linear mixed models we found that the presence of S. mansoni eggs in a stool sample varied much more between days than within specimens, indicating that stool sample examination over multiple days is required for accurate prevalence estimates. However, using the same approach, we found that among infected children intra-specimen variation in egg counts was 4.3 times higher than day-to-day variation. After praziquantel administration, day-to-day variation was more important than before, since most infections were very light and thus likely to be missed altogether by stool examination on a single day. We conclude that diagnostic sensitivity in high transmission areas is maximized by making several stool readings on several days, but examining 1 stool specimen several times can make reasonable estimates of infection intensity.
Diagnosis of Schistosoma mansoni infections in epidemiological studies is usually accomplished by examination of faecal samples for the presence of ova, typically using the Kato–Katz thick smear technique (Katz, Chaves & Pellegrino, 1972). This method is relatively straightforward, allows a large number of samples to be examined within a short time, provides a quantitative measure of infection intensity, and subsequent treatment is based upon (WHO, 1993). However, diagnosis in epidemiological studies is complicated by 3 main sources of variation in faecal egg counts, which together reduce the sensitivity of the diagnostic test (Knight et al. 1976; Sleigh et al. 1982; De Vlas et al. 1992; Engels, Sinzinkayo & Gryseels, 1996). First, a small proportion of the infected population is responsible for a large amount of the total egg excretion, probably due to a variation in numbers of worms per person caused by individual variations in exposure, immune status, and individual susceptibility (Butterworth et al. 1985; Wilkins, 1989). This results in an aggregated frequency distribution (Bradley, 1972; Polderman, 1979; Anderson & May, 1985). Second, egg counts in the same individual vary from day-to-day (Martin & Beaver, 1968; Barreto et al. 1978; Polderman, 1979; Barreto, Smith & Sleigh, 1990; Gryseels, Nkulikyinka & Engels, 1991; Engels et al. 1996). Third, egg density varies as a function of location of sampling within a single stool specimen (Barreto et al. 1990; Engels, Sinzinkayo & Gryseels, 1997a). The latter 2 factors are also of great relevance in a clinical setting.
There is a general agreement that inter-individual variation in a given population is more important than day-to-day variation in a single individual, and it has been suggested that the latter is more important than intra-specimen variation (Barreto et al. 1990; Engels et al. 1997a). On this basis, a stochastic mathematical model has been developed to estimate the ‘true’ infection prevalence of S. mansoni, accounting for only individual and day-to-day sources of variation (De Vlas et al. 1992; De Vlas & Gryseels, 1992). Predictions of this model have shown good agreement with observed cumulative prevalences obtained after the examination of multiple stool specimens (Engels et al. 1996; De Vlas et al. 1997). However, sampling of stool specimens over several days adds considerably to the amount of labour and materials required for diagnosis, and is operationally not feasible for large-scale surveys conducted with scarce resources. A sampling effort that minimizes the number of sampling days without compromising sensitivity would therefore be valuable.
We approach this issue in the present study by quantifying day-to-day and intra-specimen variation of egg counts in schoolchildren from an area highly endemic for S. mansoni in western Côte d'Ivoire. Stool specimens were collected from the same child on consecutive days, and multiple examinations were made on each specimen, under standardized conditions. This procedure was repeated after the administration of praziquantel, thus enabling us to assess the relative influence of day-to-day and intra-specimen variation on the apparent efficacy of the drug.
The present study was carried out between November 1997 and February 1998 in 2 neighbouring villages located near the town of Man in western Côte d'Ivoire. Gbatongouin (school coordinates: N = 7.525; W = 7.625) is located 19 km north-west of Man, and Mélapleu (N = 7.538, W = 7.654) is some 6 km further west. Both villages are accessible throughout the year. Our earlier work in the same area showed that S. mansoni infection is highly endemic (Utzinger et al. 1998, 2000a,b).
Ethical clearance for this study was obtained from the Ministry of Public Health of Côte d'Ivoire. All 107 schoolchildren attending Standard 3 were enrolled. The study aims were discussed with the village chiefs, the school directors and the teachers of Standard 3, who all agreed to the study. After explaining the objectives to the children, the teachers prepared lists giving name, sex and age. The afternoon before the first survey, all children in Standard 3 were issued with a 500 ml vol. plastic container and invited to return the containers the following day with a large portion of their morning stools. Containers were collected between 08.00 and 09.00 h, and immediately identified with numbers attached to the containers. At the end of stool collection, children were issued with new containers for the following day. This procedure was repeated over 5 consecutive days.
Stool specimens were transferred to the central laboratory in Man. From each specimen, 5 samples were taken at randomly selected locations, and 42 mg Kato–Katz thick smears were prepared on microscope slides according to the method described by Katz et al. (1972). The slides were allowed to clear for 30–45 min before they were analysed within a maximum delay of 45 min under a light microscope at low magnification. The slides were quantitatively examined by 1 of 4 experienced technicians. The total egg count of S. mansoni, hookworm, Ascaris lumbricoides and Trichuris trichiura was recorded separately. For the purpose of quality control, 10% of the slides were randomly selected and re-examined for the presence of S. mansoni eggs the following day by the senior technician.
Four weeks after praziquantel treatment, the stool collection and examination procedure was repeated. In this case, for organizational reasons, 4 consecutive stool specimens were collected and 3 Kato–Katz thick smears were examined from each specimen.
The day after the last stool collection in the first survey, all children were treated with a single oral dose of 40 mg/kg praziquantel (WHO, 1993). Those children who still had S. mansoni eggs in their stool 4 weeks after praziquantel administration, received another single oral dose of 40 mg/kg praziquantel. At this time, children with geohelminth infections were also treated with a single oral dose of albendazole (400 mg).
All data were double entered and cross-checked using EpiInfo software (version 6.04; Centres for Disease Control and Prevention, Atlanta, GA, USA). Only those children with complete data sets before and after the administration of praziquantel were included in the final analysis; 96 out of 107 children.
Analysis focused on assessing the effects of increased sampling effort on cumulative infection prevalence and geometric mean egg count. Our approach explicitly took into account the chronological order of the survey, with the smallest sampling effort defined as the first sample derived from the first stool specimen. The sampling effort increased with additional samples from the same stool specimen and additional stool specimens. Before praziquantel administration, a total of 25 sampling efforts per individual were considered, corresponding to 5 samples per day over 5 days. After praziquantel treatment there were 12 sampling efforts, corresponding to 4 days and 3 samples per day.
The maximum sampling efforts before and after the administration of praziquantel were taken as the ‘gold standard’ for the purpose of further analysis. Mean infection intensities were estimated in a 2 step process for each sampling effort. First, egg counts were averaged by the arithmetic mean at the individual level. The distribution of these averaged egg counts yielded the mean egg count for the population for any particular sampling effort. The mean egg counts of each individual were then transformed to percentage deviations from the same individual's mean egg count obtained after 25 (before treatment) or 12 observations (after treatment).
Before the administration of praziquantel, the different components of the variability of egg counts were investigated using generalized linear mixed models (GLMM). The low prevalence of S. mansoni infections, combined with a lower number of stool specimens collected and samples examined, precluded such an analysis after treatment. In the first model, the cumulative prevalence data from those children with complete data sets were treated as being binomially distributed (using a logit link), by distinguishing between the presence or absence of S. mansoni eggs in any particular sample. In the second model, only those children in whose stool S. mansoni eggs had been identified were included, and we assumed an overdispersed Poisson distribution (log link) for egg counts. We estimated the variance components between children (child-to-child variation), between days of stool collection (day-to-day variation, adjusted for difference among children), and between samples taken from the same stool specimen (intra-specimen variation, i.e. the residual variation). The random effects in both models were therefore child, day-within-child and specimen-within-day-within-child (i.e. the residual variance), while the fixed effects included village of origin, sex and age of a child. The child and the day-within-child variance components were directly estimated, while the residual variance was determined by estimating the dispersion parameters from the model. Model analysis was conducted using GLMM procedure of the GENSTAT software, based on the method of Schall (1991).
At the time of the study, 107 schoolchildren attended Standard 3 in the 2 schools and were eligible for enrolment. During the first survey, 101 children (94%) provided 5 consecutive stool specimens, and 5 samples were examined from each specimen. Four weeks after the administration of praziquantel, 96 children provided 4 consecutive stool specimens with 3 samples examined, resulting in an overall compliance of 90%. The median age of these children was 10 years with a large range between 7 and 14 years, but with 90% of the children being 8–11 years old. There were significantly more boys (62) than girls (34) (χ2, 1 D.F. = 4.2, P = 0.041), which resulted in a high sex-ratio of 1.82. After examination of 25 samples, in 85/96 children S. mansoni eggs were found in at least 1 sample. The cumulative infection prevalences of hookworm, A. lumbricoides and T. trichiura were 75, 43 and 9%, respectively.
Before praziquantel administration, the point prevalence of S. mansoni infection derived from a single Kato–Katz reading ranged from 32.3% to 53.1%. Fig. 1 illustrates the influence of increased sampling effort on the prevalence of S. mansoni infections. As expected, there was a considerable difference in the prevalence of infection when the minimum effort of the first sample on the first day (point prevalence: 42.7%) was compared with the maximum effort of 25 samples (cumulative prevalence: 88.5%), suggesting a mean single-test sensitivity of only 48.2%. Sampling over 2 days produced the largest relative increase in cumulative prevalence. When the first 2 stool specimens were examined with a single sample, the prevalence was higher (61.5%) than when the first stool specimen was examined 5 times (55.2%). However, the first 2 samples from the first 3 days resulted in a slightly higher prevalence (75.0%) than a single sample from all 5 days (74.0%). As shown in Fig. 1, an even greater sampling effort than 5 samples over 5 days would probably have resulted in higher infection prevalences, albeit in ever decreasing increments for each additional effort.

Fig. 1. Pre-treatment cumulative infection prevalences of Schistosoma mansoni. Each point on a curve represents a cumulative prevalence value, derived from a subset of data corresponding to a particular sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.
Using a generalized linear mixed model approach, we estimated that day-to-day variation was 5.2-fold more important than intra-specimen variation in terms of determining the presence or absence of infection (Table 1). Variation between individual children was even more important than day-to-day variation. School and sex effects were insignificant, but age had a borderline effect, resulting in an odds ratio of 1.57 (95% C.I.: 1.00–2.45).

The effect of sampling effort on cumulative prevalence after the administration of praziquantel is shown in Fig. 2. The prevalence estimate derived from the lowest sampling effort (point prevalence: 3.1%) was almost 9-fold lower than the estimate derived from the maximum possible effort (cumulative prevalence: 27.1%). The shape of the curve in Fig. 2 suggests that further sampling might still have had a significant effect on the final prevalence estimate. Considering the maximum sampling effort as ‘gold standard’, both before and after praziquantel administration, the apparent parasitological cure rate 4 weeks after treatment was 69.4% (95% C.I.: 58.5–79.0%), but was apparently much higher after a single examination: 92.7% (95% C.I.: 80.1–98.5%).

Fig. 2. Post-treatment cumulative infection prevalences of Schistosoma mansoni. Each point on a curve represents a cumulative prevalence value. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3.
Fig. 3 illustrates the results of increased sampling effort on the geometric mean egg count of all S. mansoni-infected children before praziquantel treatment. Comparatively little influence was observed – the lowest possible effort yielded an estimate of 85.6 eggs per gram of stool (epg), whereas the maximum effort yielded an estimate of 67.8 epg. Post-treatment geometric mean infection intensities of S. mansoni are shown in Fig. 4. Although egg counts were much lower after praziquantel treatment, there was a tendency for higher mean egg counts to be associated with an increasing sampling effort. After reading the first sample on the first day, the mean egg count was 3.8 epg, but after reading 3 samples on 4 days this had risen to 7.8 epg. The shapes of the curves in Fig. 4 indicate that further sampling might have led to higher estimates of the geometric mean egg count. Again considering our maximum sampling effort as ‘gold standard’ both before and after praziquantel administration, the apparent egg-reduction rate was 88.5%.

Fig. 3. Pre-treatment geometric mean egg counts of Schistosoma mansoni for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.
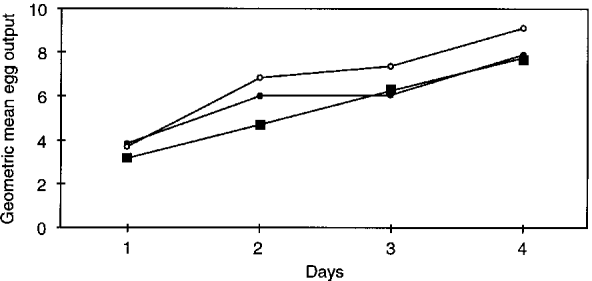
Fig. 4. Post-treatment geometric mean egg counts of Schistosoma mansoni for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3.
Applying the generalized linear mixed modelling procedure to egg count data from infection-positive children before treatment yielded the results shown in Table 2. The effect of age was of borderline significance, with a trend towards higher egg counts at higher ages (odd ratio 1.27, 95% C.I.: 0.99–1.62). As expected, there was a strong individual egg count variation (variance coefficient: 2.272, S.E.: 0.397). Intra-specimen variation was also observed to be high (variance coefficient: 2.085, S.E.: 0.070), and was 4.3-fold higher than day-to-day variation (variance coefficient: 0.483, S.E.: 0.063).

The strong effect of both day-to-day and intra-specimen variation are evident in Fig. 5. Deviations from ‘gold standard’ values decreased sharply with increasing numbers of samples analysed, either when samples were derived from different stool specimens, or when different samples were taken from the same specimen. The effect of analysing more samples per stool specimen was most apparent in the change from 1 to 2 samples.

Fig. 5. Pre-treatment mean relative difference from the ‘gold standard’ (25 samples) of Schistosoma mansoni egg counts for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.
S. mansoni diagnosis has been the subject of some scrutiny in recent years, with particular emphasis placed on the difficulties faced by health workers when trying to estimate prevalence and mean infection intensities as a prelude to intervention (WHO, 1999). These difficulties are primarily a result of the extreme levels of variation in faecal egg counts that are characteristic of schistosome infections. By identifying and quantifying the components of this variation we can improve the prospects for both accurate diagnosis and effective treatment.
The results of the present study, carried out in an area of western Côte d'Ivoire where S. mansoni infections are endemic (Utzinger et al. 1998, 2000), confirm that individuals are the main source of egg-count variation. Our analysis also supports the general assumption that the distribution of S. mansoni egg counts in a given population is overdispersed, with only a few individuals excreting the bulk of the eggs (reviewed by Anderson & May, 1985).
The main objective of the analysis was to assess sources of variation within individuals, and specifically to assess the relative importance of day-to-day and intra-specimen components. By modelling the hierarchical structure of the data, we found that intra-specimen variation in S. mansoni egg counts was significantly more important. Interestingly, a similar conclusion was recently drawn for estimating infection intensities of hookworm and Oesophagostomum bifurcum infections, since repeated coprocultures from a single stool specimen produced appropriate intensity measures (Pit et al. 1999). However, our results are in contrast with previous observations from a study carried out in north-east Brazil (Barreto et al. 1978, 1990), where intra-specimen variation explained 31.5% of the total egg count variance, and the day-to-day variation accounted for 46.1%. Although we used exactly the same diagnostic technique, and the same commercially available testing kits with 42 mg templates, it is difficult to compare our results with those from Brazil due to important differences in the epidemiological settings and different sampling efforts. First, we analysed egg counts from 85 S. mansoni-infected children, aged between 7 and 14 years, whereas Barreto and colleagues collected stools from only 23 S. mansoni-positive subjects with an age of 8–78 years. Second, the mean egg count in Brazil was higher than in our study. Third, our sampling effort consisted of collecting stools on 5 consecutive days and preparing 5 smears per specimen, whereas in Brazil stool specimens were collected over 3 consecutive days with 2 smears per specimen.
Our results indicate that it may not be necessary to investigate several consecutive stool specimens for obtaining an adequate measurement of infection intensity, but examining a single stool repeatedly may suffice. On the other hand it is clearly indicated to collect repeated stool specimens, rather than to perform multiple readings on a single specimen, if determination of ‘all’ infected subjects is the major aim. This conclusion is in agreement with those of Engels et al. (1997a), who, via a different analytical approach, concluded that sampling fewer times on different days would give the most accurate estimate of the infection status in their population studied. However, care is needed in comparing our results with those from Engels and collaborators, since they worked in another epidemiological setting (Burundi), collected stools from only 20 individuals with an age range of 6–60 years, and only on 3 days, but examined 10 samples from each stool specimen. It is therefore possible that conclusions regarding the best sampling-effort compromise will depend partly on characteristics of the study subjects as well as the design.
Previously published observations and suggestions support the idea that intra-specimen variability is likely to be more important. First, it is assumed that eggs are not randomly distributed within a stool sample because mixing of intestinal contents is not uniform (Hall, 1981). We attempted to compensate for possible unevenness of distribution by asking for large portion of stools (in 500 ml vol. containers) and taking samples from different areas. Although the results from earlier studies suggested a random distribution of S. mansoni eggs in the stool (Martin & Beaver, 1968; Ratard et al. 1990), these findings have to be treated with caution, as the sample size of study participants was very small. More recently, the distribution of S. japonicum eggs was found to be non-random, with an aggregation of eggs on the stool surface and with egg counts decreasing from the beginning of the stool to the end (Ye et al. 1998; Yu et al. 1998). Poor mixing of eggs with other stool material leads to clustering, which will affect the number of eggs on each slide even in the absence of any other source of variation. In addition, it has been shown that though the volume of stool samples prepared by the Kato–Katz technique is constant, the amount of solid matter varies considerably, because of differences in stool consistency (Teesdale, Fahringer & Chitsulo, 1985; Engels et al. 1997b). This can also lead to variation in the number of eggs on each slide. Given this diversity of sources of variation, our results are not surprising, despite contradicting previous studies. We therefore suggest that factors which affect day-to-day concentrations of eggs, such as stool consistency and variation in egg production of female worms, are perhaps less important in providing reliable information on intensity of infection than hitherto proposed.
Although the relative importance of different sources of variation on egg count variability can be further debated, the effect of limited sampling effort on values for infection prevalence is well established. Our results confirm previous reports regarding the underestimation of S. mansoni infection prevalence (De Vlas et al. 1992, 1997; De Vlas & Gryseels, 1992; Engels et al. 1996; Utzinger et al. 1998, 2000b) and emphasize the need to examine stools taken on different days rather than making multiple readings from a single specimen, when a definitive diagnosis of infection status is required and the first sample is negative. This recommendation is of particular importance for areas of low infection intensity (De Vlas et al. 1992; Engels et al. 1996).
Our results also indicate the pivotal importance of examining consecutive stool specimens after praziquantel administration, in order to assess the parasitological cure rate of this drug accurately (Utzinger et al. 2000b. Since prevalence and infection intensity decrease sharply after praziquantel treatment (Gönnert & Andrews, 1977), single Kato–Katz thick smears are likely to miss a large proportion of residual infections. Recently, the question was raised of whether current schistosomicidal chemotherapy protocols may be subcurative, a situation which could lead, at least in the long-term, to drug resistance (Doenhoff, 1998). This argument was based on the fact that dose-finding studies for praziquantel were based on clinical trials that were generally evaluated with single parasitological diagnoses, so that the cure rate could have been overestimated. Our data clearly confirm that such overestimation was likely, as the apparent cure-rate after examination of a single sample was 92.7%, but was only 69.4% after examination of 12 samples. We can reasonably assume that increasing the sampling effort still further would have revealed more residual infections, so even the 69.4% cure rate is probably an overestimate. Since praziquantel has a low efficacy against juvenile worms (Xiao, Catto & Webster, 1985; Sabah et al. 1986), it is possible that some individuals were excreting eggs from newly patent worms, and this could partly explain the presence of eggs in some stool samples after praziquantel treatment.
The present study was conducted in an area of high transmission, and our conclusions may be valid in other areas of similar endemicity. Further work using a similar intensity of sampling needs to be conducted in areas of low transmission. It is likely that day-to-day variation will be more important in such areas, because the clustering of low numbers of eggs within stools (which causes intra-specimen variation) will have a less pronounced impact on egg-count variation. This was evident in our analysis of post-treatment data, making the assumption that the post-treatment situation is a proxy for low transmission areas.
Although we used 25 stool examinations per person as a gold standard for the purpose of analysis, it was evident that additional sampling would have allowed us to make more precise estimates of infection intensities and prevalence. However, it is also clear that examining 5 samples over 5 days is unfeasible in most situations, and as the number of samples increases so does the probability of diagnosing false-positives. Since several examinations of 1 stool requires less time and labour than analysing consecutive stool samples, it may therefore be a reasonable compromise in high transmission areas to reduce the number of days of sampling whilst increasing the number of examinations of each stool sample. Our analyses suggest that this approach can be equally valid as sampling fewer times over more days in identifying heavily infected individuals, by virtue of more accurately estimating individual infection intensities. Since WHO recommendations for schistosome control programmes at the community level are partly based on the prevalence of heavily infected individuals (WHO, 1993, 1999), our suggested approach may be particularly appropriate wherever morbidity control efforts are undertaken and resources for parasitological surveys are limited. However, if the objective is transmission control or elimination of S. mansoni, our analyses confirm that examination of single stool specimens will never suffice. In this case, alternative diagnostic tools with a higher sensitivity are required, perhaps application of serological methods.
We are grateful to the village chiefs, school directors, teachers of Standard 3 and children from the 2 primary schools of Gbatongouin and Mélapleu. Thanks are addressed to Dr Y. A. Ossey and Dr A. N'Dri for their support of the study. Our work would not have been possible without the excellent assistance of L. A. Ahiba and the four laboratory technicians A. Allangba, A. Fondio, L. K. Lohourignon and M. Traoré. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), the Swiss Agency of Development and Cooperation (SDC) and the Roche Research Foundation. J. Utzinger was supported by the Rudolf Geigy Foundation and the Swiss National Science Foundation; M. Booth by the Wellcome Trust; E. K. N'Goran by the Swiss Academy of Natural Science; I. Müller by the Swiss National Science Foundation; and C. Lengeler was in receipt of the PROSPER grant 32-41632.94 from the Swiss National Science Foundation.

Fig. 1. Pre-treatment cumulative infection prevalences of Schistosoma mansoni. Each point on a curve represents a cumulative prevalence value, derived from a subset of data corresponding to a particular sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.

Table 1. Overall results of generalized linear mixed model (GLMM) analysis for presence or absence of Schistosoma mansoni eggs in stool samples among 96 children with complete data sets

Fig. 2. Post-treatment cumulative infection prevalences of Schistosoma mansoni. Each point on a curve represents a cumulative prevalence value. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3.

Fig. 3. Pre-treatment geometric mean egg counts of Schistosoma mansoni for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.

Fig. 4. Post-treatment geometric mean egg counts of Schistosoma mansoni for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3.

Table 2. Generalized linear mixed model (GLMM) analysis of egg counts among all 85 Schistosoma mansoni-positive children

Fig. 5. Pre-treatment mean relative difference from the ‘gold standard’ (25 samples) of Schistosoma mansoni egg counts for each possible sampling effort. The number of consecutive days of sampling is indicated on the x-axis. The number of stool readings per-person-per-day is as follows: ([bull ]) = 1; (○) = 2; ([squf ]) = 3; (□) = 4; ([utrif ]) = 5.