INTRODUCTION
Harbour porpoises, Phocoena phocoena, are the most abundant cetacean in Northern European waters (Hammond et al. 1995) and they are also the most frequently stranded marine mammals in United Kingdom waters (DETR, 2000). Evans (1991) produced evidence suggesting a declining density of harbour porpoises in the English Channel and Southern North Sea and proposed a range of possible causes which included increased mortality due to entanglement in fishing gear (by-catch), habitat degradation due to increased shipping activity, noise, changes in prey availability and pollution. Jepson (2003) documented by-catch as the most common cause of death in stranded harbour porpoises in England and Wales. However, infectious disease was shown to be an important contributing factor to mortality in many studied porpoises (Jepson et al. 2000; Jepson, 2003), most of which were pneumonias associated with various combinations of parasitic, bacterial and mycotic agents. Around the UK, many stranded porpoises, including juveniles, are found to be heavily parasitized by nematodes and trematodes (Baker and Martin, 1992; Kuiken et al. 1994; Jepson et al. 1999) which is consistent with recent post-mortem findings in harbour porpoises from the waters of Scotland (DETR, 2000), Germany (Siebert et al. 2001) and Belgium/Northern France (Jauniaux et al. 2002).
Parasites have been implicated in both strandings and disease processes of marine mammals and are, in many cases, opportunistic as they tend to cause disease in physically and immunologically debilitated animals (Dierauf, 1990). Most notably, virtually all UK-stranded individuals that died of pneumonia consistent with bacterial infection, were concurrently infected with pulmonary parasites (Kirkwood et al. 1997), suggesting that pulmonary parasites might play a role in the aetiology of, or act as a vector for, the bacteria involved in bacterial pulmonary disease in harbour porpoises (Jepson et al. 2000). Pulmonary pathology in harbour porpoises is predominated by parasitic lesions with the nematode Family Pseudaliidae considered the most important (Dailey, 1985; Baker and Martin, 1992, Jepson et al. 2000, Siebert et al. 2001, Jauniaux, 2002). Although little is known about their life-cycles, severe pneumonias and pulmonary vascular lesions have been associated with Pseudalius inflexus and Torynurus convolutes, commonly resulting in disease and mortalities in harbour porpoises (Baker and Martin, 1992; Kuiken et al. 1994; Kirkwood et al. 1997). Further still, Anisakis species nematodes are frequently found in the stomachs of Odontecetes where they are found free or attached to the mucous membrane of the cardiac stomach in which they are often associated with ulcers and, in rare cases, perforation (Dhermain, Soulier, and Bompar, 2002).
A major factor found to be strongly associated with porpoise mortality is bioaccumulation of immunosuppressant chemical pollutants such as polychlorinated biphenyls (PCBs), consistent with an adverse effect on the overall health of porpoises (Jepson et al. 1999, 2005). Various studies have documented high levels of polychlorinated biphenyls (PCBs) and other persistent organic contaminants in harbour porpoises from the Bay of Fundy/Gulf of Maine (Westgate et al. 1997), the Baltic Sea (Falyandysz et al. 1994), the North Sea (van Scheppingen et al. 1996), Scandinavia (Kleivane et al. 1995) and UK waters (Kuiken et al. 1993, 1994; Jepson et al. 1999; Bennett et al. 2001, Jepson et al. 2005). There are concerns regarding the role of persistent, bioaccumulative and immunosuppressive pollutants in disease causation and health of the entire harbour porpoise population in the UK (Law et al. 1991, 1998, 2002; Law and Whinnet, 1992; Bennett et al. 2001). The immunosuppressive nature of PCBs and some other organochlorine compounds is well documented (Safe, 1994). Due to their high trophic level, marine mammals are vulnerable to high level bioaccumulation of these contaminants (Tanabe, Iwata and Tatsukawa, 1994, Aguilar, Borrell and Pastor, 1999) as their lipid-rich blubber acts as a reservoir for lipophilic chemicals and they have a limited capacity for metabolism and excretion of PCBs. Duinker et al. (1989) suggested that the ability to metabolize PCBs may be even lower in harbour porpoises than in many other small cetaceans.
Recently, attempts have been made to collate existing toxicological data from aquatic mammals in order to derive dose-response relationships, particularly for the adverse health effects of PCB exposure (Kannan et al. 2000). The purpose of this study was to investigate the role of PCBs in harbour porpoise mortalities in association with levels of nematodiasis. Further, we sought to identify any biologically attributable relationships between host factors, blubber PCB levels and nematode burdens observed in post-mortem examinations, based on analysis of porpoises stranded around the coast of Britain.
MATERIALS AND METHODS
Post-mortem examination
All harbour porpoise data were obtained from a long-term UK national programme of stranded marine mammals, utilizing detailed systematic post-mortem examination and sample collection protocols (Law, 1994). We recognize that although stranded porpoises may represent a biased data-set, animals diagnosed to have died due to physical trauma were usually found to be in good overall health and nutritional status prior to the stranding event. This category of animals has been used as ‘healthy’ controls in case-control studies (Jepson et al. 1999, 2005; Bennet et al. 2001) and so are likely to be representative of healthy porpoises in the wild (Jepson, 2003). Blubber samples for toxicological analysis were collected and preserved using standardized methodology (Aguilar et al. 1999). Freshly dead or slightly decomposed individuals were selected for toxicological analyses to reduce errors in blubber organochlorine levels associated with decomposition (Borrell and Aguilar, 1990). Most carcasses were kept between 0 °C and ambient temperature prior to post-mortem examination, although 41 were stored at −20 °C.
For the present study, the concentrations of 25 chlorobiphenyl (CB) congeners (Hutchinson and Simmonds, 1994; Jepson, 2003) were determined using standardized and fully validated methodology with associated analytical quality control procedures (Allchin, Kelly and Portmann, 1989; Law, 1994; Jepson et al. 2005) and the sum of the congeners' concentrations in blubber (Σ25CBs) expressed as mg/kg lipid weight. Sex and age were determined for all individuals. Where possible, age was determined by quantification of growth layer groups from analyses of decalcified tooth sections, or from body length according to Lockyer (1995). Furthermore, the cause of death (c.o.d) of each porpoise was placed into 1 of 3 broad categories: ‘physical trauma’ (n=117), including by-catch, dystocia, impact by boats and attack by bottlenose dolphins (Tursiops truncatus); ‘infectious disease’ (n=72), including a range of pneumonias and other infections; and ‘other’ causes of death (n=35, starvation in the majority of cases and some individuals in which cause of death could not be determined). A detailed description of all sampling procedures has recently been published by Jepson et al. 2005. Of the 340 individuals for which Σ25CBs data were available, 224 individuals were included in this research. Individuals were necessarily excluded where advanced state of decomposition prevented determination of sex, age or cause of death.
Classification trees
One of the main hurdles faced in investigating the link between nematode burden as a response variable with Σ25CBs levels, porpoise age, sex and cause of death as explanatory variables is the semi-quantitative nature of the parasite data. Historically, post-mortem records have classed nematode burdens from each porpoise as being one of ‘absent’, ‘light’, ‘moderate’ or ‘heavy’. This scoring makes traditional analysis of variance or modern generalized linear modelling inappropriate, as these techniques rely on having a continuous response variable.
Therefore, initial inspection of the data was undertaken by using classification trees generated through recursive partitioning. Here, the models were fitted using binomial recursive partitioning, whereby the data are successively split along coordinate axes of the explanatory variables so that, at any node, the split which maximally distinguishes the response variable (based on minimizing observed deviance) in the left and right branches is selected (for an introductory explanation see Crawley (2002)). This technique readily identifies important features in the structure of the data and provides a valuable tool to guide the construction of further statistical models.
Linear modelling
Based on trends suggested by the creation of classification trees with nematode burden levels as the response variable, a range of generalized linear and linear mixed effects models were fitted to the data. In all cases, Σ25CBs levels were taken as the response variable along with 4 fixed effects: nematode burden (4 factor levels: ‘absent’, ‘light’, ‘moderate’ and ‘heavy’), cause of death (3 factor levels: ‘physical trauma’, ‘infectious disease’ or ‘other’) and porpoise sex as categorical explanatory variables and age at death as a continuous explanatory variable. All possible interactions between these fixed effects were also included in the maximal models.
In addition, we incorporated the calendar year in which the stranding occurred in a number of ways. It should be stressed that, regardless of the exact model specification in terms of how ‘year’ was included, the minimum adequate models were the same with respect to all other explanatory variables. Initially, we included ‘year’ as an additional continuous fixed effect, just as ‘age’ is treated. We further analysed the data by fitting a number of linear mixed effect models (Pinheiro and Bates, 2000) with ‘year’ as a random effect. This class of model allows for improved model fit by treating the integer years of stranding as a sample taken from a hypothetical population of years – effectively as blocks – thus taking into account variance attributable to differences between years, without reducing the power of the model by constraining degrees of freedom in annual parameter estimation. With improved partitioning of variance, resulting models are more instructive and mixed effects models also allow investigation into the correlation structure of parameters, which were assumed to be independent in the initial generalized linear models. Initially, no correlation structure was assumed for residuals. This was compared to a number of autoregressive (AR), moving average (MA) and autoregressive-moving average (ARMA) temporal autocorrelation forms (Pinheiro and Bates, 2000).
Comparison of Akaike Information Criteria (Akaike, 1973) showed natural-log link functions with Gamma error distributions to be the best fitting model structure for generalized linear models. The response variable was natural-log transformed for mixed effects modelling. Models were assessed by stepwise deletion of highest order terms (or reduction of factor levels) and minimum adequate models were fitted with only significant main effects or interactions. Mixed effects model fits, where fixed effects were either identical or nested, were compared by AIC and likelihood ratio (LR) tests (Burnham and Anderson, 2000).
All classification tree and linear modelling was performed using the R statistical software package (www.r-project.org).
RESULTS
Classification trees
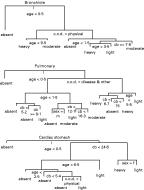
Separate classification trees were constructed for each of the 3 nematode burdens measured (Fig. 1). The first split in all of the trees was between porpoises less than 6 months old and those older. This was the main explanation for the presence or absence of parasites, suggesting that porpoises acquire parasites at a very early age. Subsequently, the trees for nematodes located in the lungs (bronchioles and pulmonary artery) appear similar, but a different picture arises from nematodes found in the cardiac stomach. Splits in the bronchiole and pulmonary nematode trees indicate that cause of death is the next most important factor. Subsequently, Σ25CBs levels feature on the trees, with the highest Σ25CBs levels associated with intermediate (‘light’ or ‘moderate’) parasite burden. Porpoise sex and various interactions between age, sex and Σ25CBs levels account for much of the remainder of the observed deviance in parasite burden.

Fig. 1. Classification trees generated by recursive partitioning of deviance. These are followed from top (‘root’) to bottom (‘terminal node’ or ‘leaf’). At each split, if the stated criterion is met (e.g. ‘age <0·5’ or ‘c.o.d.=physical’), proceed down the left-hand branch, otherwise proceed down the right-hand branch. Highest bifurcations show partitioning of explanatory variables which explain the greatest amount of observed deviance (with vertical branch length indicating changes in deviance) in nematode burdens. (c.o.d.=‘cause of death’, cb=Σ25CBs).
The cardiac stomach nematode burden tree shows a different pattern. After the initial split based on age, younger or older than 6 months, Σ25CBs level was the next most important split, with a threshold value below which parasite burden is ‘absent’ or ‘light’ and above which, parasite burden is ‘light’ to ‘heavy’. Further deviance in parasite burden is explained by the age structure, Σ25CBs levels, cause of death and, at Σ25CBs levels higher than 24·8 mg/kg, porpoise sex.
Relationship between Σ25CBs levels and the different nematode burdens
Taking the initial generalized linear model approach with ‘year’ as an additional continuous fixed effect, no interaction terms involving year of stranding were retained and ‘year’ was not found to explain a statistically significant amount of observed deviance (F1,210=2·180, P=0·141) as a main effect. However, inclusion of ‘year’ as a random effect provided a substantially improved fit (AIC=1723 without ‘year’ as a random effect, AIC=587 with ‘year’ as a random effect) and allowed investigation of stochastic fluctuations between years of stranding. Whilst model fit was marginally improved with first order autoregressive (AR(1)) annual autocorrelation, likelihood ratio tests show this to be not significant (likelihood ratio (LR)=2·236, P=0·135). A linear mixed effect model with no correlation structure assumed was therefore used to assess the importance of the remaining explanatory variables.
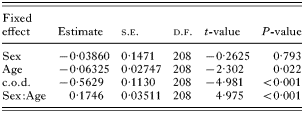
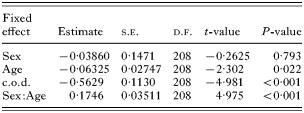
The highest levels of Σ25CBs corresponded with moderate worm burdens for both bronchiole and pulmonary nematodes (mean Σ25CBs levels for given nematode burdens: bronchiole nematodes –‘absent’=18·5 mg/kg, ‘light’=20·5 mg/kg, ‘moderate’=22·7 mg/kg, ‘heavy’=21·3 mg/kg; pulmonary nematodes – ‘absent’=18·0 mg/kg, ‘light’=20·0 mg/kg, ‘moderate’=24·3 mg/kg, ‘heavy’=23·8 mg/kg), as suggested by their respective classification trees (Fig. 1). However, nematode burdens were found not to explain Σ25CBs levels in a linear mixed effect model (bronchiole: F3,205=0·8089, P=0·490, pulmonary: F3,205=0·4822, P=0·695), and illustrated by box plots (Fig. 2A and B). Removal of the cause of death (F2,207=13·52, P<0·001) or the sex[ratio ]age interaction terms (F1,207=24·98, P<0·001) resulted in significant increases in observed deviance. The minimum adequate model to explain Σ25CBs levels where bronchiole or pulmonary nematodes were considered is summarized in Table 1.

Fig. 2. Box-whisker plots showing central tendency (median), skew and kurtosis in the relationship between nematode burdens and natural log-transformed Σ25CBs. Explanatory variables: (A) bronchiole nematodes; (B) pulmonary nematodes; (C) cardiac stomach nematodes.
Table 1. Summary table of treatment contrasts for the minimum adequate model necessary (based on F tests from stepwise deletion of terms – see Results section) to explain observed deviance in Σ25CBs levels, where bronchiole and pulmonary nematodes were considered (Estimates are for the difference in parameter values between categorical factor levels or gradients of continuous categorical variables.)
In contrast, for parasites found in the cardiac stomach, the highest Σ25CBs levels were observed with the heaviest burdens of nematodes (Fig. 2C). However, cause of death (‘physical trauma’, ‘infectious disease’ or ‘other’) was also found to be highly significant in explaining observed deviance in Σ25CBs levels: but further analysis showed that Σ25CBs levels were not significantly different between porpoises stranded as a result of ‘infectious disease’ or ‘other’ causes (t=0·4347, P=0·664). Therefore, cause of death was condensed into a 2- level factor, where porpoises stranded as a result of ‘physical trauma’ were found to have significantly lower Σ25CBs levels than those stranded as a result of a single, new factor level comprising ‘infectious disease and other’ causes of death (t=4·773, P<0·001).
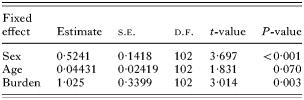
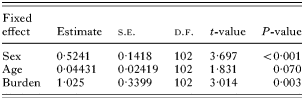
When this is taken into account, cardiac stomach nematode burden was only found to be statistically significant as a main effect explaining Σ25CBs levels in porpoises which died of ‘physical trauma’ (F1,101=8·999, P=0·0034) and not in porpoises assigned to the new factor level comprising ‘infectious disease and other’ causes of death (F3,86=2·026, P=0·116). In the ‘physical trauma’ dataset, AR(1)MA(1) correlation structure provided a significantly better fit than when no correlation structure was assumed (LR=8·256. P=0·0041). Other significant explanatory variables in explaining deviance in observed Σ25CBs levels are summarized in Table 2.
Table 2. Summary table of treatment contracts for the minimum adequate model necessary (based on F tests from stepwise deletion of terms – see Results section) to explain observed deviance in Σ25CBs levels for porpoises stranded through physical trauma (‘Burden’ refers to nematodes recovered from the cardiac stomach and comprises a 2- level factor of ‘absent, light and moderate’ or ‘heavy’. Estimates are for the difference in parameter values between categorical factor levels or gradients of continuous categorical variables.)
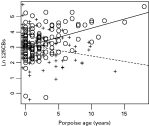
Concentrations of Σ25CBs increased with age in male porpoises whilst decreasing in females (Fig. 3). In addition, removal of the cardiac stomach nematode burden and porpoise sex interaction from the physical trauma strandings model reduced the model fit by an amount very close to significance at the 5% level (LR=3·806, P=0·0511).

Fig. 3. The relationship between stranded porpoise age (years) and sex-specific, accumulated, natural log-transformed Σ25CBs. ‘o’ shows male porpoises (solid line: ln Σ25CBs=3·08615+0·11261×age), ‘+’ shows female porpoises (dashed line: ln Σ25CBs=3·08615−0·06017×age).
Porpoise age is clearly an important explanatory factor associated with nematode burden in all cases. Classification trees suggest that the oldest porpoises are associated with intermediate worm burdens, regardless of whether they occur in the lungs or the stomach. This pattern is more clearly demonstrated with box plots (Fig. 4) and may reflect a genuine biological relationship between host age and parasite burden. However, there are possible sampling bias effects associated with this data-set that also need to be considered and which are discussed later.

Fig. 4. Box-whisker plots showing central tendency (median), skew and kurtosis in the relationship between nematode burdens and stranded porpoise age (years). Explanatory variables: (A) bronchiole nematodes; (B) pulmonary nematodes; (C) cardiac stomach nematodes.
DISCUSSION
Determining the mechanisms underlying parasite infections in the wild is crucial for our understanding of the factors affecting host health and the design of effective conservation programmes. However, many wild animal populations cannot be experimentally manipulated or even measured directly, making informative epidemiological studies virtually impossible. This is particularly true for marine mammals, where the logistical difficulties associated with population sampling mean that the factors affecting individual health and population dynamics largely remain a mystery. However, using appropriate statistical tools and extensive, stratified data, it is possible to gain considerable insight into how different processes interact to affect individual worm burdens and subsequent host health. Here we adopt such an approach, using a 15-year database of harbour porpoises stranded around the coast of Britain to explore the causes and consequences of parasite infections in this wild population of marine mammals.
A number of interesting associations between nematode burdens, Σ25CBs levels and host factors are apparent. The change in nematode burden with porpoise age is clearly important and yet not completely straightforward. Classification trees consistently point to the fact that porpoises acquire parasites very early in life. Initially, increasing age appears to be associated with increasing nematode burdens, however, the oldest porpoises examined (up to 18 years old when stranded) were found to have predominantly intermediate nematode burdens. Such ‘convexities’ in age-intensity profiles have been observed in many epidemiological studies of helminth infections in human and wild animal populations (Anderson and Gordon, 1982; Gregory, Montgomery and Montgomery, 1992; Anderson and May, 1995) and may be evidence of parasite-induced host mortality, age-related changes in host behaviour leading to reduced exposure with age, or acquired immunity, such that older animals are better equipped to limit nematode burdens (Anderson and Gordon, 1982). However, we note that a drop in sample size with increasing age can lead to underestimates of parasite burden in older hosts (Pacala and Dobson, 1988), such that the apparent fall off in nematode burden in the oldest porpoises may be artefactual. These competing hypotheses could be tested with statistical models incorporating appropriate weighting for host sample size and maximum likelihood best fit techniques if parasite burden was a continuous variable (Pacala and Dobson, 1988; Wilson et al. 2003). However, such fine-grain analyses are not possible for this dataset, since only qualitative categories of worm burdens are available. This result therefore highlights the need for detailed, quantified recording of parasite burden, which is currently the focus of further work.
Careful inspection of classification trees suggests that the highest levels of Σ25CBs may be associated with intermediate levels of both bronchiole and pulmonary nematodes and with high levels of nematodes found in the cardiac stomach. However, due to the confounding effects of porpoise age, it is doubtful whether bronchiole and pulmonary nematode burdens can really be explained by measured levels of Σ25CBs and no statistically significant associations were identified. We therefore suggest that PCBs are not a major determinant of harbour porpoise lungworm burdens in the UK. However, the observed positive link between Σ25CBs levels and nematodes recovered from cardiac stomachs is more promising and deserves elaboration.
The direction of cause-and-effect underlying the positive association between Σ25CBs levels and cardiac stomach nematodes is not clear at present and may be a simple result of increased food intake resulting in both greater exposure to infective stages and greater accumulation of pollutants. However, contrary to this hypothesis is the observation that the stomachs from heavily infected porpoises were generally found to be emptier than those from parasite-free porpoises (Jepson, personal observation). An alternative explanation is that the observed relationship between PCB levels and cardiac stomach worm burdens reflects a genuine underlying biological mechanism, such as PCB-related immnuosuppression (Safe, 1994). Closer inspection of the classification tree for cardiac stomach nematodes, box plots of Σ25CBs against parasite burden and collapsing factor levels of nematode burden in linear modelling shows there is very little change in Σ25CBs level associated with ‘absent’, ‘light’ and ‘moderate’ cardiac stomach nematode levels, but significantly higher Σ25CBs levels associated with ‘heavy’ nematode burden. This is suggestive of a threshold level of Σ25CBs of approximately 25 mg/kg lipid, as indicated by our classification tree, below which there is no association between Σ25CBs levels and cardiac stomach worm burdens, and above which, considerably higher parasite burdens are found. The idea of a PCB threshold has been suggested previously (Kannan et al. 2000), where a threshold for total blubber PCB levels (based on the Aroclor 1254 formulation) of 17mg/kg lipid was proposed. It should be noted that the threshold value proposed by Kannan et al. (2000) is for total PCBs. In our present study, we recorded a restricted set of 25 CB congeners, hence ‘Σ25Cbs’, and previously Jepson et al. (2005) showed that it was possible to convert total PCB levels to Σ25CBs levels with a conversion factor of 0·5×total PCBs. This suggests that the threshold value we observed (25 mg/kg Σ25CBs, equivalent to 50 mg/kg total PCBs) is considerably higher than that suggested by Kannan et al. (2000). However, the 17 mg/kg total PCB threshold proposed by Kannan et al. (2000) was for the initial onset of adverse effects, and it may be expected that the biological consequences of these effects would only be detectable at higher PCB levels. Therefore, the higher threshold value observed from our statistical analysis may be consistent with the previous studies and is likely to reflect a genuine factor affecting harbour porpoise health in UK waters. Clearly, our analysis does not provide a mechanistic explanation of this phenomenon. However, one possibility is that the observed PCB threshold may reflect the degree of PCB-related immunosuppression (Safe, 1994) which results in a significant and observable increase in the cardiac stomach nematode burden. Further empirical studies examining the functional relationship between PCB levels and host immunocompetence would prove invaluable in our understanding of the factors affecting the dynamics of the harbour porpoise-parasite community.
The decrease in Σ25CBs levels with porpoise age in females is presumably due to maternal off-loading (Borrell, Bloch and Desportes, 1995), whereby adult females redistribute a large part of their body organochlorine burden to their progeny via gestation and lactation (Aguilar and Borrell, 1994, Tanabe et al. 1994; Kleivane et al. 1995, van Scheppingen et al. 1996). Linear modelling points to a likely association between Σ25CBs levels, porpoise sex and cardiac stomach nematode burdens, with the corresponding classification tree suggesting that this link is strongest in porpoises above the threshold level for Σ25CBs.
Whilst the impact of annual variations in Σ25CBs levels was not a primary focus of this study, especially as there was no evidence for a long-term drift in Σ25CBs levels over successive years, we note that stochastic fluctuations from year to year appear to be important when modelling the association between Σ25CBs levels and cardiac stomach nematodes, for porpoises stranded through physical trauma. There may be a number of contributory factors behind this; both demographic and environmental stochasticity can operate to control populations (Bonsall and Hastings, 2004) and the nature of stochasticity is highly dependent on spatial scale (Bonsall et al. 2005). Further investigation into the underlying physiological processes, the role of host immunity and the significance of threshold effects in harbour porpoise populations, along with detailed study into temporal and spatial heterogeneity and the effects of stochasticity, would be invaluable in determining a causal link between environmental pollution, parasite abundance and host population dynamics.
The UK marine mammal strandings project is funded by the UK Department for Environment, Food and Rural Affairs. A.F. was funded by a fellowship from NERC. R.K.S. would like to thank Ms Debby Cox of the Jane Goodall Institute. In addition, we wish to thank J. Kirkwood, T. Kuiken, T. Patterson, V. Simpson, H. Ross, A. Collof, B. Monies, R. Penrose, S. Macgregor, N. Davison, C. Iskajaer-Ackley, G. Bell, C. Lockyer, E. Rogan, S. Northridge, S. Moss, R. Sabin, D. George, A. Muir, N. Evans, C. Spurrier, J. Chimonides, and S. Turk for their contributions to the marine mammal strandings project.