Introduction
Eimeria maxima invades and undergoes asexual and sexual development in the middle intestine altering intestinal nutrient uptake by disrupting gut absorptive capacity. The release of large numbers of merogonic stages causes sloughing of the gut epithelium that in turn sets up an ideal environment for colonization by microbes such as Clostridium perfringens leading to necrotic enteritis. Eimeria maxima is distinctive among the seven Eimeria species infecting chickens because its oocysts and male gamonts are the largest (up to 39 × 35 µm) among the avian Eimeria species (Levine, Reference Levine1973). During our efforts to compare two E. maxima strains (APU1, APU2, Jenkins et al. Reference Jenkins, Dubey, Miska and Fetterer2017), it became obvious that there were discrepancies in the literature concerning E. maxima schizont development and the number of generations of schizogony. Almost 90 years ago, Tyzzer (Reference Tyzzer1929) described endogenous development of E. maxima, as well as naming the species based on the large size of its oocysts. In this seminal study, Tyzzer (Reference Tyzzer1929) found that schizonts were distributed throughout the small intestine, but that the greatest numbers were present in jejunum–ileum. Tyzzer (Reference Tyzzer1929) observed E. maxima schizonts at 72, 96 and 120 h, but not at 24 or 48 h p.i. The small size and presence at the surface epithelium of schizonts at 72 and 96 h has generally been confirmed by other authors (Long, Reference Long1959; Scholtyseck, Reference Scholtyseck1963). Subsequently, Challey and Johnson (Reference Challey and Johnson1968) and Millard et al. (Reference Millard, Bradley and Long1972) reported on the first-generation schizonts that were missed previously. However, there is uncertainty concerning the number of schizogonic generations and details of development are lacking because of the small size of schizonts.
The purpose of this study was to identify the primary site of E. maxima invasion and development, and resolve discrepancies in the literature concerning intracellular sporozoite morphology and schizogony, particularly the number of schizont stages and site(s) in the intestinal epithelium where each stage of development occurs.
Materials and methods
Eimeria maxima strain and experimental infections of chickens
All studies of the E. maxima life cycle described in this study utilized the E. maxima APU1 strain, which had been propagated in susceptible chickens every 3–4 months for 10 years after initial isolation (Fetterer and Barfield, Reference Fetterer and Barfield2003; Jenkins et al. Reference Jenkins, Dubey, Miska and Fetterer2017). The purity of E. maxima oocysts was confirmed by microscopy and ITS1-PCR using procedures described elsewhere (Jenkins et al. Reference Jenkins, Miska and Klopp2006). Broiler chickens (male, Hubbard-Ross 708, Longeneckers Hatchery, Elizabethtown, PA, USA) were each inoculated at 1 week of age orally with 5 × 106 E. maxima APU1 oocysts, and intestinal tissues were harvested in two separate studies.
Experiment 1
Chickens inoculated with E. maxima oocysts were necropsied at 12, 24, 36, 48, 60, 72, 96 or 120 h p.i. The small intestine was divided into three segments: segment #1 consisted of the entire duodenal loop (14–19 cm long), segment #2 consisted of the jejunum from the end of duodenum to Meckel's diverticulum (34–49 cm long) and segment #3 consisting of Meckel's diverticulum to the just anterior of the caeca (23–37 cm long). Each segment was flushed with 10% buffered formalin (BF) from both ends and immersed in 10% BF. After 1–2 h in BF, 12 cross-sections (3–4 mm thick at equal distances) were cut and left in BF for at least 1 day. Formalin fixed tissues were then embedded in paraffin for sectioning (American HistoLabs, Rockville, MD, USA). The remainder of intestinal segments were saved in 10% formalin for further studies. Histological sections were cut at 3–5 µm thick, stained with haematoxylin and eosin and examined for coccidian stages at 1000× magnification. In addition, 60 cross-sections were prepared from segment #2 of each of two chickens euthanized at 48 and 60 h after E. maxima APU1 inoculation
Experiment 2
The objective of this experiment was to obtain additional details of the life cycle in two chickens inoculated with APU1 strain of E. maxima. These chickens were euthanized at 72 or 96 h p.i. and only jejunum (segment #2) was studied. A 5 cm section of intestine was slit open, spread on paper towel and the surface epithelium was very gently scraped with a glass slide, mashed and a 4 × 2 cm area was smeared on 7·5 × 2·5 cm glass slides. The smears (100 each at 72 and 96 h) were fixed with methanol, air dried and examined after staining with Giemsa. All smears were examined for coccidian stages at 1000× magnification.
Results
Distribution of E. maxima stages in intestine
Depending on the duration of infection, stages were seen throughout the small intestine but the jejunum was the most parasitized (Table 1). The entire life cycle was completed by 120 h.
Table 1. Summary of endogenous development of Eimeria maxima in small intestine of chickens orally inoculated with five million oocysts

+, Positive; −, negative.
There was no host reaction to the parasite in histological sections.
Sporozoites
Sporozoites excysted in vitro and stained with Giemsa were approximately 12 × 2·5 µm (Fig. 1A). They contained a nucleus and one or two large refractile bodies. In histological sections of small intestine, sporozoites were detected 12–72 h p.i. and were located singly in parasitophorous vacuoles (PV). At 12 h, sporozoites were in the lamina propria and the epithelium of the villus. At 24 h, sporozoites were intracellular in enterocytes of the glands of Lieberkühn (here after referred as glands). Sporozoites were 6–7 × 3–3·5 µm and contained prominent RB that were eosinophilic. The nucleus was pushed to side or at the non-conoidal end. Of all sections, only one sporozoite was seen at 72 h.

Fig. 1. Eimeria maxima sporozoites and the development of first-generation schizonts. (A) Sporozoites released from oocyst in vitro. Giemsa stain. (B–I) Stages in histological sections of glands of Lieberkühn of the jejunum of chickens orally inoculated with oocysts, haematoxylin and eosin stain. The luminal part of glands is oriented on the top. In B–G, the refractile body/bodies (RB) are eosinophilic (arrows), the nucleus and nuclei are basophilic and indicated by arrowheads. (A) Two sporozoites. Arrows point to RB. (B) Two sporozoites in separate parasitophorous vacuoles (PV). The nucleus in the sporozoite on the left is located terminally, whereas it is centrally located in the sporozoite on the right. (C) Elongated sporozoite-shaped immature schizont with nuclei around RB. (D–G) Different shaped immature schizonts. (H) Merozoites (arrowhead) arising from the central mass (arrow) of a schizont. (I) Two small schizonts (arrow, arrowhead) with small PV. (J) Mature schizont with radiating merozoites (arrow).
First-generation schizonts
All development occurred in variable-sized PV in enterocytes of glands at 36–72 h (Fig. 1). Early stages were sporozoite-shaped; round uninucleated stage was not seen. The RB from sporozoites was fragmented into several pieces (Fig. 1C–G). The nucleus was divided into irregular-shaped nuclei that were arranged throughout the schizont, and were often obliterated by RB. Immature schizonts were often irregular in appearance ranging from elongated to pear-shaped or oblong. At 36 h, most organisms seen were sporozoites and only few had multiple nuclei. The details of nuclear division were masked by the RB. Merozoites were observed arising asynchronously from the main mass. Thus, some merozoites were free in the PV, while others were still attached to the main mass (Fig. 1H). Because of the variability of the PV, the schizont size varied containing a maximum of 20 merozoites. Merozoites were 6–7 × 1 µm and contained a centrally located nucleus (Fig. 1). The lumens of infected glands were dilated and free merozoites were seen in the glandular lumen (Fig. 2A and B). Among many recuts of the jejunum of chicken (D224, Table 1) euthanized 72 h, a single mature schizont was found in gland enterocyte at 72 h; it had 10 merozoites and was located at the base of gland touching the muscular mucosae. The measurements are from tissue sections.

Fig. 2. Eimeria maxima schizonts in histologic sections of the jejunum of chickens. Haematoxylin and eosin. (C–I) Second generation of schizonts in the jejunum of a chicken 60 h p.i. Scale bar in B–I is 5 µm. (A) A dilated gland with a ruptured schizont (arrow), 48 h p.i. (B) Higher magnification of Fig. 2A showing merozoites at the periphery of a central mass (arrow) and free merozoites (arrowhead) away from the main mass. Double arrowheads point to an intracellular schizont. (C) A group of immature schizonts (arrow). Arrowhead points to parasitized parasitophorous vacuole above the nucleus of enterocyte. (D) Two multinucleated amoeboid-shaped immature schizonts (arrows). (E) An elongated immature schizont (arrow) on the left and a mature schizont with merozoites (arrowhead) on the right. (F) A sausage-shaped multinucleated schizont (arrow). (G) Three schizonts in one field. The schizont on far right is immature and sausage-shaped (double arrowheads), the schizont in the middle is mature and has a central residual body (arrow) and the schizont on the far left is mature. Note centrally located nucleus in merozoites (arrowhead). (H) Two merozoites above the nucleus of the enterocyte. (I) Mature schizont (arrow) below the enterocyte nucleus.
Second-generation schizonts
Mature second-generation schizonts were seen in the jejunum at 60 h. Schizonts were located exclusively in enterocytes at the villar tips (Fig. 2C–I). Immature schizonts were amoeboid to sausage-shaped (Fig. 2D–G), were 10 µm in maximum diameter and contained up to 12 merozoites, sometimes arranged around a residual body (Fig. 2G). The merozoites were 4–5 µm long and contained a centrally located nucleus (Fig. 2G). The measurements are from tissue sections. The merozoites appeared to differ in size (Fig. 2); however, smears were not available to confirm the size differences.
Third-, fourth- and fifth-generation schizonts
The number of generations could not be determined with certainty because of small-sized schizonts and hyperinfection. Therefore, schizogony is described separately at 72, 96 and 120 h.
Seventy two hours
Schizogony was seen in enterocytes, not only at the tips of the villi but also along the villus, up to the opening of glands, but not in glands. In sections, schizonts were approximately 5 µm in diameter and contained up to 10 merozoites that were approximately 4 µm long, based on measurements in sections (Fig. 3A). Some merozoites were arranged around a central residual body (Fig. 2G).
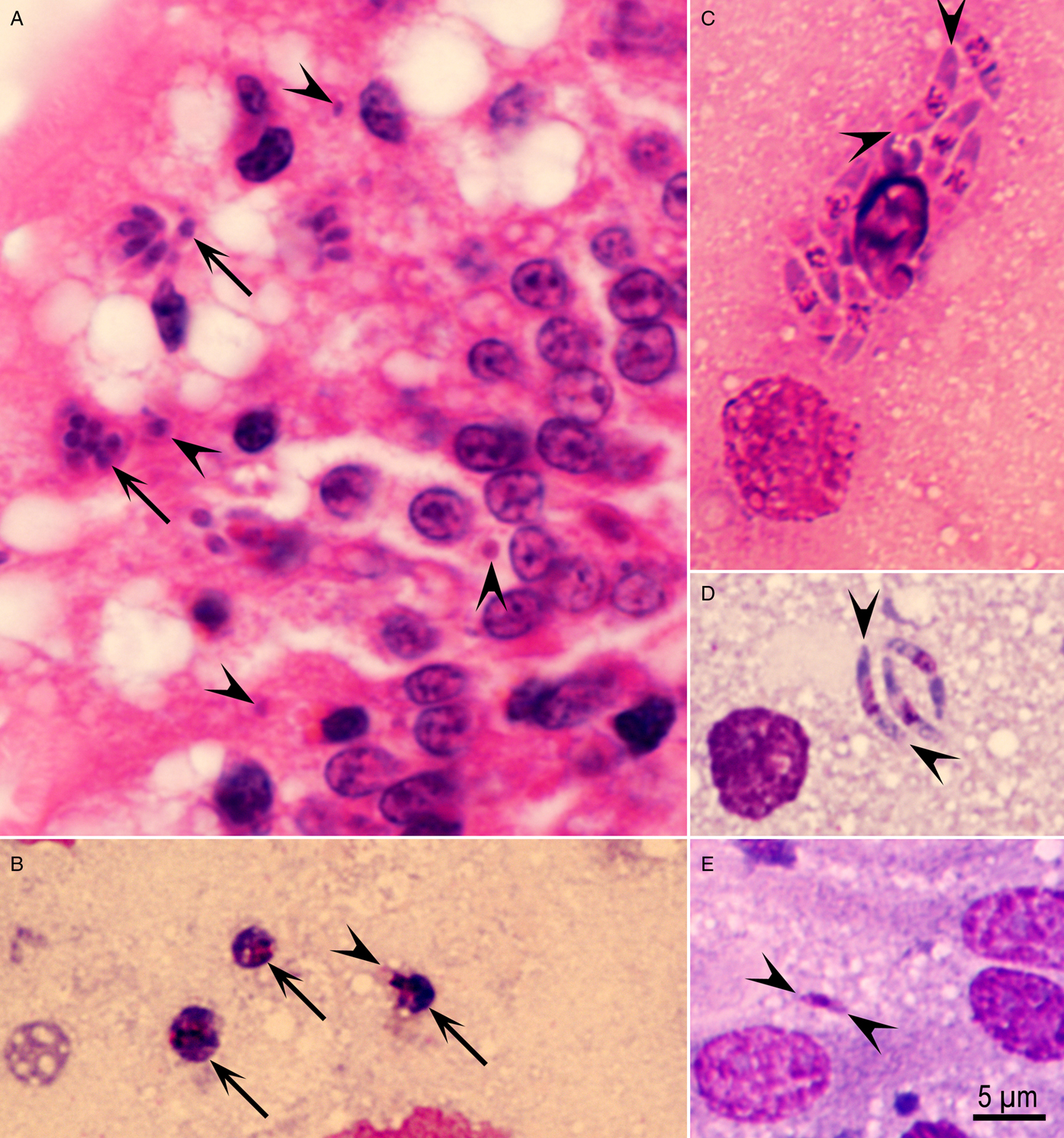
Fig. 3. Schizogony of Eimeria maxima in the jejunum of chicken, 72 h p.i. (A) Histological section showing mature schizonts (arrows) and single organisms (arrowheads) at the villar tips. Haematoxylin and eosin stain. (B–D) Smears of the jejunum stained with Giemsa. (B) Three immature schizonts (arrows) with two or three nuclei. Arrowhead points to a merozoite arising at the 2–3 nucleated stage. (C) Mature schizont containing eight banana-shaped 7·5 µm long (arrowheads) merozoites. (D) A group of three slender merozoites (arrowheads). (E) Small (arrowheads) 3 µm long merozoite.
Only a few schizonts were seen in smears of the jejunum. The immature schizonts were <5 µm in diameter and merozoites were forming even at the three-nucleated stage (Fig. 3B). Two sizes of merozoites were seen – the long merozoites were 7 × 1·5 µm, whereas small ones were 3·5 × 1 µm (Fig. 3C–E).
Ninety-six hours
Profuse schizogony occurred at 96 h with at least two morphologically distinct types of schizonts (Fig. 4A and B). Most schizonts did not have a residual body and contained long merozoites. Some schizonts had a large residual body and short merozoites (Fig. 4B). Based on histological sections, schizonts were up to 10 µm in diameter and the merozoites were 5 µm (long merozoites) and 2 µm (short merozoites).
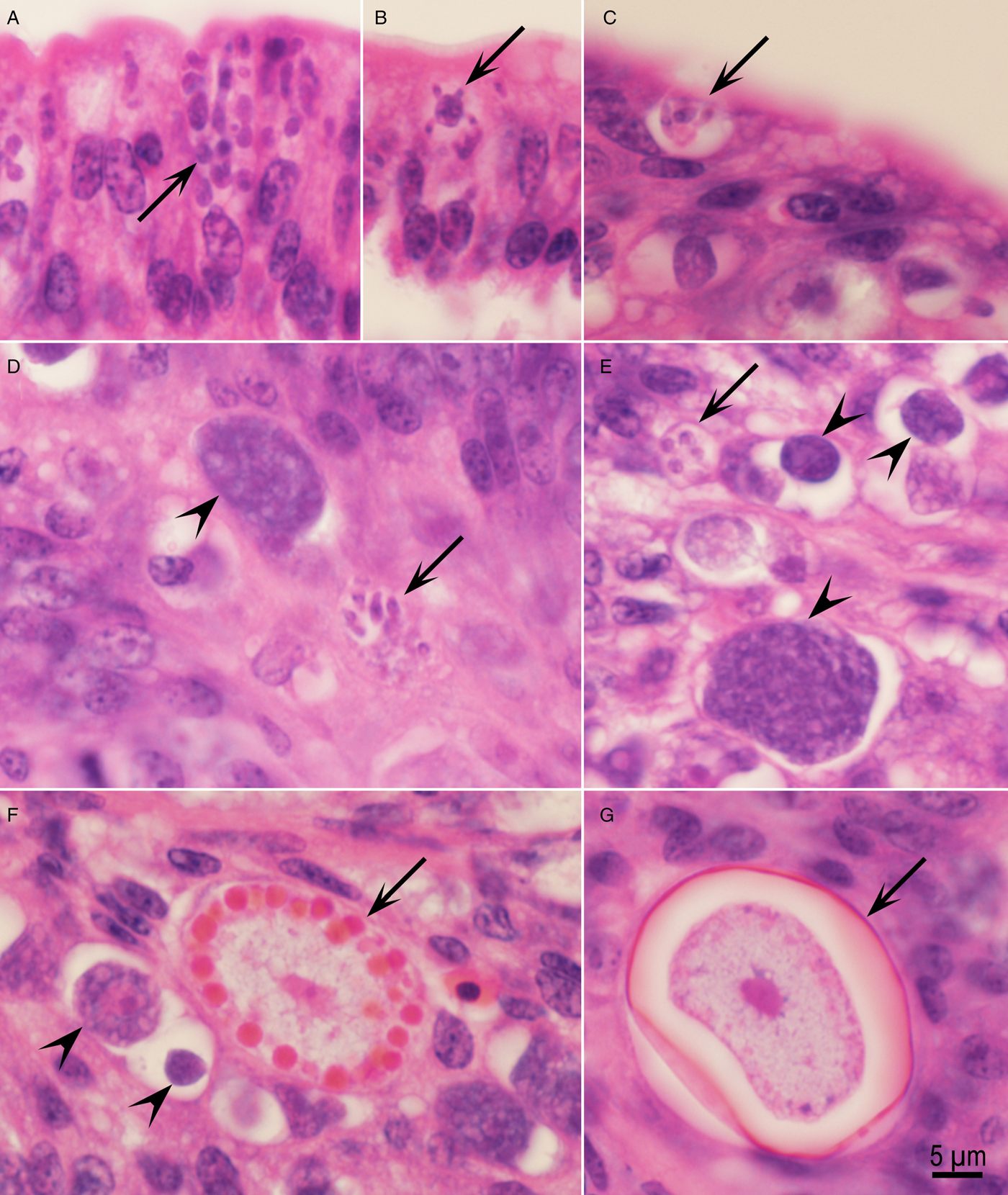
Fig. 4. Asexual and sexual development of Eimeria maxima in the jejunum of chickens. Haematoxylin and eosin stain. (A) Numerous schizonts (arrow) above enterocyte nucleus, 96 h p.i. (B) Arrow points to a schizont with small merozoites attached to a large residual body, 96 h p.i. (C) A schizont with four merozoites in the superficial epithelium at the villar tip, 120 h. (D, E) Schizont (arrows) in the lamina propria adjacent to a microgametocyte (arrowheads), 120 h p.i. (F) Mature (arrow) and immature macrogamonts (arrowheads) in the lamina propria, 120 h. (G) Unsporulated oocyst with a central nucleus and oocyst wall (arrow) partly peeled away from the parasitophorous vacuole.
More details of parasite development were clear in smears (Fig. 5). Relatively few schizonts and many merozoites were seen in smears. Immature schizonts were round, oblong, elongated and contained up to 10 nuclei (Fig. 5A–I). Merozoite formation began as early as at the two- or three-nucleated stage (Fig. 5C and E). Two merozoites were seen once within a PV (Fig. 5D). Schizonts with small merozoites (3–4 µm long) had a large residual body (Fig. 5J–L); some merozoites were seen in the residual body (Fig. 5K). Different sizes and shapes of merozoites seen are shown in Fig. 5M–P. Clearly, there were two distinct sizes of merozoites; small 3–4 µm vs. long 7·5–8·0 µm (Fig. 5P). These measurements are based on smears.
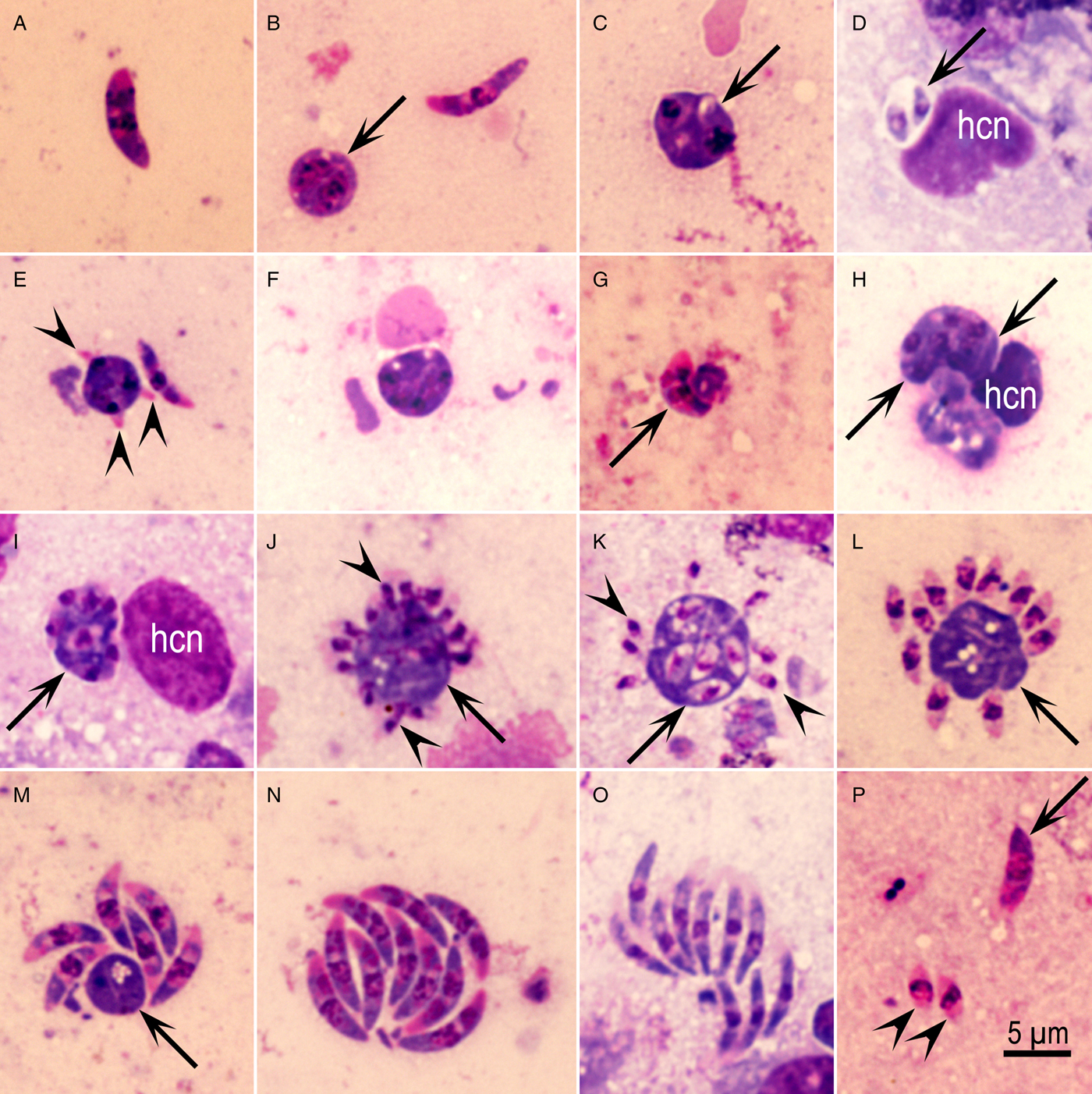
Fig. 5. Asexual Eimeria maxima stages in the jejunum of chicken, 96 h p.i. Giemsa stain. (A) Merozoite with two nuclei-like structures. (B) Binucleated small schizont (arrow) and an 8 µm long merozoite. (C) An organism (arrow) with two developing merozoites/nuclei. (D) Two merozoites within one parasitophorous vacuole, apparently binary/endodyogeny division. (E) A trinucleated schizont with developing merozoites (arrowheads). (F) Schizont with four nuclei. (G) Merozoite-shaped schizont with six nuclei. (H) Elongated multinucleated (arrowheads) schizont. (I) Schizont with seven or more nuclei (arrow). (J) Schizont with a large residual body (arrow) and small merozoites (arrowheads) forming at the periphery. (K) Small merozoites (arrowheads) arising from a central undivided mass (arrow) that has fully formed merozoites. (L) Small (3·5 µm long) merozoites arranged around a large undivided mass (arrow). (M) Large (7·5 µm) six merozoites around a small residual body (arrow). (N) Group of 10 stout merozoites. (O) Group of 10 slender merozoites. (P) Two small (arrowheads) and a large merozoite (arrow) in one field.
One hundred and twenty hours
At 120 h, both schizogony and gametogony were seen in histological sections of jejunum. Only a few schizonts were detected in both the villar epithelium (Fig. 4C) and the lamina propria (Fig. 4D–E). Schizonts were small (5 µm in maximum diameter) and contained up to eight 4 × 1 µm merozoites; they were present both in the epithelium (Fig. 4C) and in the lamina propria (Fig. 4C–E). In histological sections, longitudinally cut merozoites were 4 × 1 µm. These merozoites could be distinguished from the earliest gamonts that were each located in a separate PV.
Male and female gamonts, and fully formed oocysts were seen in the subepithelial tissue, apparently in the lamina propria (Fig. 4D–G).
Discussion
The present study provides details of the schizogony of E. maxima, such as the number of schizogonous stages and their location in the gut. In the present study, immature first- and second-generation schizonts were sporozoite-/merozoite-shaped and contained multiple nuclei. In most Eimeria species, the asexual development is considered by a divisional process of schizogony where sporozoites round up after invasion of host cells (mostly enterocyte) leading to a uninucleated organism called the trophozoite (Levine, Reference Levine1973). Subsequent development occurs by schizogony, wherein the parasite nucleus divides in to four or more nuclei (in some species, such as Eimeria bovis into more than 1000 nuclei) before merozoite formation occurs (Levine, Reference Levine1973). In our study of E. maxima, we did not see the trophozoites after sporozoite invasion. Instead, the young schizont remained sporozoite-shaped and the nuclei were distributed throughout the schizont. Additionally, merozoite formation was not synchronous in that some merozoites were fully formed, whereas others were still forming. A similar process was observed for the second-generation schizonts where immature schizonts were sausage-shaped or elongated. Additionally, some organisms at 96 h appeared to divide into merozoites at the 2–3-nucleated stage, not previously reported for eimerian species.
Based on the present study and review of the literature, there are more than three generations of schizonts in the life cycle of E. maxima. First-generation schizonts are distinctive because of their morphology and exclusive location in glands. In general, the first-generation schizonts here appear similar to those reported by Millard et al. (Reference Millard, Bradley and Long1972). However, Millard et al. (Reference Millard, Bradley and Long1972) did not provide details of the development. The differences in the gut region parasitized by E. maxima are apparent between our present study (in the jejunum) and that of Millard et al. (Reference Millard, Bradley and Long1972) in the duodenum, probably due to several factors. For instance, Millard et al. (Reference Millard, Bradley and Long1972) examined only 1 cm of the duodenum, whereas we examined all regions of the small intestine. Millard et al. (Reference Millard, Bradley and Long1972) dosed 11 chickens with two million oocysts of the Houghton strain of E. maxima in two breeds of chickens. Two Brown leghorn chickens each were killed at 24, 48, 72 and 96 h p.i. and three Light Sussex chickens were killed at 63 h. Weather the dose, parasite strain or breed of chickens affected results has not been investigated. The size of first-generation schizonts in both studies is similar. Challey and Johnson (Reference Challey and Johnson1968) in an oral presentation at a regional meeting first alluded to first-generation schizonts in the glands of chickens; details of this paper were never published.
With respect to the location, the first-generation schizonts of E. maxima resemble first-generation schizonts of Eimeria acervulina that is often found mixed with E. maxima in naturally infected chickens. Eimeria acervulina first-generation schizonts also occur in gland enterocytes of duodenum and are of the same size as E. maxima (Vetterling and Doran, Reference Vetterling and Doran1966). However, unlike E. acervulina, E. maxima first-generation schizonts are located in large PV.
The second generation of E. maxima was completed between 48 and 60 h; these were absent at 48 h but had matured by 60 h. Unlike the first generation, the second generation occurred at the villar tips. Additionally, some second-generation schizonts were located below the host cell nucleus. Millard et al. (Reference Millard, Bradley and Long1972) also reported small immature schizonts at 63 h p.i. and mature schizonts at 72 h.
The number of generations beyond the first two could not be conclusively identified. The number of parasites seen at 60, 72 and 96 h suggests that schizonts at 72 h were the third generation and those at 96 h were the fourth generation. A possible fifth generation of schizonts was seen at 120 h. Unlike, the previous generations, the fifth generation schizonts were also located in subepithelium along with gamonts. The significance of these schizonts is unknown because by 120 h gamonts had formed.
In the present study, unsporulated oocysts were seen in histological sections at 120 h but not in feces. However, prepatent period for APU1 E. maxima is 138 h. Long (Reference Long1959) mentioned a prepatent period of 121 h for the Houghton strain of E. maxima, and in chickens fed single oocyst, the prepatent period was 7 days. These differences may be related to the location of oocysts in the subepithelium and their slow release in to the lumen or the strain of the parasite.
Avian coccidiosis is an intestinal disease caused by protozoa in the genus Eimeria. Each year, the disease incurs over $350 million loss in the USA alone, and over $1 billion worldwide due to poor weight gain in infected chickens and the cost of administering anticoccidial drugs and vaccines. The most costly parasite is E. maxima because it not only depresses weight gain and feed utilization, but also predisposes chickens to other diseases such as necrotic enteritis, a bacterial disease that causes high mortalities in young chicks. Contrary to previous studies conducted over the last century, our studies revealed that the parasite localizes to an area of the gut involved in nutrient absorption. Although there were no obvious histologic lesions, the extensive replication probably affects absorption. This information will be valuable to poultry and pharmaceutical companies that are considering control measures against avian coccidiosis caused by E. maxima.
Acknowledgements
We would like to thank Celia O'Brian, Carolyn Parker and Oliver Kwok for technical assistance. We are indebted to Dr Shiv Kumar Verma for assembling photographs.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.









