Introduction
In 2015, there were 212 million new cases of malaria and 429 000 deaths (WHO, 2016a). Malaria is caused by a protozoan of genus Plasmodium sp. and Plasmodium falciparum is the most pathogenic species causing severe form and high mortality rate. Clinical manifestation of severe malaria can include damages on central nervous system (Idro et al. Reference Idro, Jenkins and Newton2005), severe anaemia (Stoute et al. Reference Stoute, Odindo, Owuor, Mibei, Opollo and Waitumbi2003), kidney failure (Plewes et al. Reference Plewes, Kingston, Ghose, Maude, Herdman, Leopold, Ishioka, Hasan, Haider, Alam, Piera, Charunwatthana, Silamut, Yeo, Faiz, Lee, Mukaka, Turner, Anstey, Roberts, White, Day, Hossain and Dondorp2017), pulmonary dysfunction (Maguire et al. Reference Maguire, Handojo, Pain, Kenangalem, Ric, Tjitra and Anstey2008), disseminated intravascular coagulation, hepatic dysfunction and shock (Jain et al. Reference Jain, Kaushik and Kaushik2016), and also cardiovascular abnormalities (Costenaro et al. Reference Costenaro, Benedetti, Facchin, Mengoli and Pellizzer2011). There are different pathways which P. falciparum infection can trigger cardiac disorders. Parasitized red blood cells can occlude myocardial capillaries, leading to ischaemic cardiomyopathy and dilated heart (Mohsen et al. Reference Mohsen, Green, McKendrick and West2001; Costenaro et al. Reference Costenaro, Benedetti, Facchin, Mengoli and Pellizzer2011); high levels of tumour necrosis factor (TNF-α) may play a role in inflammatory process at heart, impairing myocardial function (Torre-Amione et al. Reference Torre-Amione, Kapadia, Benedict, Oral, Young and Mann1996); and hypoxia induced by severe anaemia may cause ischaemic myocardial injury with prolonged QTc interval of electrocardiogram (ECG), as frequently observed in children (Sadoh and Uduebor, Reference Sadoh and Uduebor2016).
In addition to cardiovascular complications inherent to P. falciparum infection, currently available antimalarial drugs may aggravate and trigger additional cardiotoxic effects. Hypotension was observed with quinine and quinidine (Mecca et al. Reference Mecca, Elam, Nash and Caldwell1980) and chloroquine caused cardiac conduction disorders and hypotension leading to sudden death (Looareesuwan et al. Reference Looareesuwan, White, Chanthavanich, Edwards, Nicholl, Bunch and Warrell1986). Prolonged PR, QRS and QTc intervals of ECG were induced by amodiaquine (Ngouesse et al. Reference Ngouesse, Basco, Ringwald, Keundjian and Blackett2001) and halofantrine (Leite et al. Reference Leite, Grabe-Guimarães, Guimarães, Machado-Coelho, Barratt and Mosqueira2007). Artemisinin derivatives caused QT and QTc interval prolongation (Classen et al. Reference Classen, Altmann, Gretener, Souppart, Skelton-Stroud and Krinke1999) and Torsade de Pointes (TdP), which was observed in dogs specifically for ATM (Yin et al. Reference Yin, Wang, Wang, Dong, Han, Guan, Zhao, Qu, Yuan, Gao, Jing and Ding2014).
According to WHO (2016b), malaria treatment should be performed with antimalarial drugs combination therapy, including artemisinin derivatives, in order to reduce P. falciparum resistance. Semisynthetic derivatives of artemisinin, such as artesunate, artemether (ATM), arteeter and dihydroartemisinin significantly reduce parasitaemia and they are used against resistant strains of P. falciparum as artemisinin-based combination therapy (ACT) (WHO, 2016b). Despite its effectiveness as antimalarial by schizonticide and gametocide activities (Brossi et al. Reference Brossi, Venugopalan, Dominguez-Gerpe, Yeh, Flippen-Anderson, Buchs, Luo, Milhous and Peters1988), ATM is used in ACT, mainly with lumefantrine, but presents short half-life (Silamut et al. Reference Silamut, Newton, Teja-Isavadharm, Suputtamongkol, Siriyanonda, Rasameesoraj, Pukrittayakamee and White2003), significant general toxicity (Efferth and Kaina, Reference Efferth and Kaina2010), including cardiotoxicity (Yin et al. Reference Yin, Wang, Wang, Dong, Han, Guan, Zhao, Qu, Yuan, Gao, Jing and Ding2014). Thus, investigation of artemisinin derivatives toxicological profile, particularly its cardiotoxicity, is a theme of special interest in chemotherapy of life-threatening diseases such as malaria.
Considering that new safer and simple strategies to improve the efficacy of malaria treatment is an urgent demand, new pharmaceutical formulations to reduce the toxicity of the current used drugs and to increase their efficacy require investigation. Nanostructured lipid carriers were already developed to enhance the oral efficacy of ATM-lumefantrine and was able to complete parasite clearance with a reduced daily dose (Prabhu et al. Reference Prabhu, Suryavanshi, Pathak, Sharma and Patravale2016). Another study was conducted for the development of self-nanoemulsifying drug delivery systems (SNEDDS) containing ATM, with significant absorption and permeation increases and also, P. berghei parasitaemia reduction (Tripathy et al. Reference Tripathy, Das, Chakraborty, Sahu, Pramanik and Roy2012). Polymeric nanocapsules (NC) have been proved to be a useful tool to treat parasitic diseases, increasing efficacy of drugs by controlling the release into the body (Mosqueira et al. Reference Mosqueira, Loiseau, Bories, Legrand, Devissaguet and Barratt2004), protecting sesquiterpene lactone from degradation (Branquinho et al. Reference Branquinho, Pound-Lana, Marques, Saúde-Guimarães, Vilela, Spangler, de Lana and Mosqueira2017a), and dramatically reducing their toxic effects by parenteral and oral routes (Leite et al. Reference Leite, Grabe-Guimarães, Guimarães, Machado-Coelho, Barratt and Mosqueira2007; Branquinho et al. Reference Branquinho, Roy, Farah, Garcia, Aimond, Le Guennec, Saude-Guimarães, Grabe-Guimaraes, Mosqueira, Lana and Richard2017b). NC are polymeric drug nanocarriers that vary from 100 to 400 nm in size and have a vesicular structure with the polymeric wall surrounding an oily cavity where hydrophobic drugs can be dissolved (Legrand et al. Reference Legrand, Barratt, Mosqueira, Fessi and Devissaguet1999). To the best of our knowledge, a nanocarrier containing ATM modifying or reducing cardiotoxicity was not evaluated or reported until now. Thus, the present work investigated an experimental malaria pharmacotherapy to improve oral efficacy of ATM and to avoid or reduce its cardiovascular toxic effects. NC containing ATM (ATM-NC) was prepared to evaluate the oral treatment efficacy against P. berghei infection in mice and cardiovascular safety, using the analyses of ECG signal of uninfected and infected mice.
Materials and methods
Materials
Dihydroartemisinin methyl ether (ATM), poly-ε-caprolactone (PCL); poloxamer 188, carboxymethylcellulose sodic, and HPLC grade acetone were purchased from Sigma-Aldrich (Sigma-Aldrich Co., St. Louis, MO, USA); sorbitol (aqueous solution 70%, Synth, Brazil). Paluther® intramuscular parenteral formulation was granted by the National Malaria Control Program Ministry of Health, Brazil, that contains 80 mg mL−1 of ATM dissolved in sesame oil. Soy lecithin at ~75% phosphatidylcholine (Epikuron™170) was a gift from Cargill (Germany). Miglyol® 812N was purchased from Sasol Germany GmBH. MilliQ water was purified using a Symplicity® System (Millipore, Bedford, USA) and used throughout experiments.
ATM formulations preparation
Blank-NC and ATM-NC were prepared by the interfacial deposition method of preformed polymer followed by solvent displacement, as previously described by Fessi et al. (Reference Fessi, Puisieux, Devissaguet, Ammoury and Benita1989). Briefly, to prepare 2·0 mL of ATM-NC it was used an organic phase containing 24 mg of PCL, 30 mg of soy lecithin, 100 µL of Paluther® (80 mg mL−1), 2·0 mL of acetone and an aqueous phase (8 mL) containing 30 mg of poloxamer 188. After complete dissolution of all components in each phase, the organic phase was poured, into aqueous phase using a 3 mL syringe at 60 mL min−1 flowrate. NC formed instantly and the dispersion was maintained under agitation during 10 min. Then solvents were evaporated under reduced pressure (Heildolph Rotary Evaporator Instruments, Germany) until small volumes of NC is obtained. The formulation volume was measured in a calibrated test tube and the volume completed with MilliQ water up to 2 mL final volume to obtain the final concentration of 4 mg mL−1 of ATM in an NC colloidal suspension. To prepare blank-NC it was used the same procedure in absence of ATM using 100 µL of Miglyol 812N as NC oily core. An oral dosage form of the free-ATM suspension was prepared mixing 0·09 g of ATM with 0·3 g of carboxymethylcellulose and 2·0 mL of 70% w/v sorbitol solution that was further dispersed in 15 mL of water. The vehicle was prepared same way in the absence of ATM. This suspension was freshly prepared on the day of treatment beginning and protected from light. Free-ATM solution and ATM-NC formulations were maintained at controlled temperature protected from direct light.
NC characterization: size and zeta potential
Mean hydrodynamic diameter, polydispersity index (PDI) and zeta potential of NC were determined using Zetasizer NanoZS equipment (Malvern Instruments, UK), using the dynamic light scattering technique (DLS) and zeta potential DLS coupled to microelectrophoresis. Zeta potential is a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles in the aqueous medium. The measurements were performed at 25 °C with samples previously diluted 1000-fold in water, using three distinct formulations and in triplicate. The results are expressed as the mean ± standard deviation (s.d.).
Animals
Male and female black C57BL/6 mice aged 6–8 weeks (20–22 g) were used. The experimental protocols were approved by Ethical Committee of Federal University of Ouro Preto under protocol number 2014/14. All experiments were in compliance with the guidelines established by the Brazilian College of Animal Experimentation (COBEA). The animals were maintained under environmental conditions of 12 h day/night cycles, room temperature 22 ± 2 °C, standard diet and water ad libitum. Female mice were used only to evaluate ATM antimalarial efficacy, as performed before (Khoury et al. Reference Khoury, Cromer, Elliott, Soon, Thomas, James, Best, Aogo, Engel, Gartlan, Akter, Sebina, Haque and Davenport2017).
Treatment protocol
Male mice were distributed into two groups of uninfected and Plasmodium berghei-infected animals. Mice were treated with: (1) vehicle (n = 6), (2) blank-NC (n = 6), (3) free-ATM at doses 40, 80 or 120 mg kg−1 per administration (n = 6 each) or (4) ATM-NC at doses 40, 80 or 120 mg kg−1 per administration (n = 6 each). Treatments were given by oral route, administered by gavage, twice daily (every 12 h), during 4 days, totalizing eight administrations per dose. For infected mice, treatment started at the 4th day after infection to evaluate the antimalarial efficacy and ECG parameters, respectively to female and male mice.
In vivo antimalarial efficacy
Female mice were infected with an intravenous inoculum of 1 × 106 red blood cells (RBCs) infected with P. berghei NK-65 strain diluted in 0·2 mL of saline. The treatment was performed as described before (Peters et al. Reference Peters, Ze-Lin, Robinson and Warhurst1986), with modifications. Mice were considered infected after established infection with parasitaemia higher than 25% (three days), and treatment was initiated as described in treatment protocol (n = 6 per group at the beginning). Parasitaemia was monitored at day zero (before infection) and at 3, 6, 11, 14, 21, 30 days after infection, considering the day of infection as day one. Blood samples from mice tail vein were used for the direct parasitaemia analysis in Giemsa-stained thin blood smears. Percentage of parasitaemia (% of infected RBCs) was determined microscopically at 1000× magnification, by examining a minimum of 3000 cells, for each mouse.
ATM general toxicity evaluation
General toxicity of ATM, in free or NC dosage form, was evaluated using the % survival of uninfected mice treated with the highest used ATM dose. Mice were treated as described in treatment protocol with vehicle, blank-NC, free-ATM (120 mg kg−1) or ATM-NC (120 mg kg−1) (n = 6, each group). Survival was evaluated during 30 consecutive days of mice observation.
ECG signal
A total of 16 experimental groups of male uninfected and P. berghei-infected mice were treated as described in the protocol. For ECG signal record, mice were anaesthetized with ketamine (100 mg kg−1) and xilasin (14 mg kg−1), intraperitoneally. ECG signals were obtained before (baseline) any procedure, and 2, 6 and 24 h after the end of treatment for uninfected mice. Since for uninfected mice main electrocardiographic alterations were at 6 and 24 h after last free-ATM dose administration, for the infected mice ECG was obtained only at baseline and these two times.
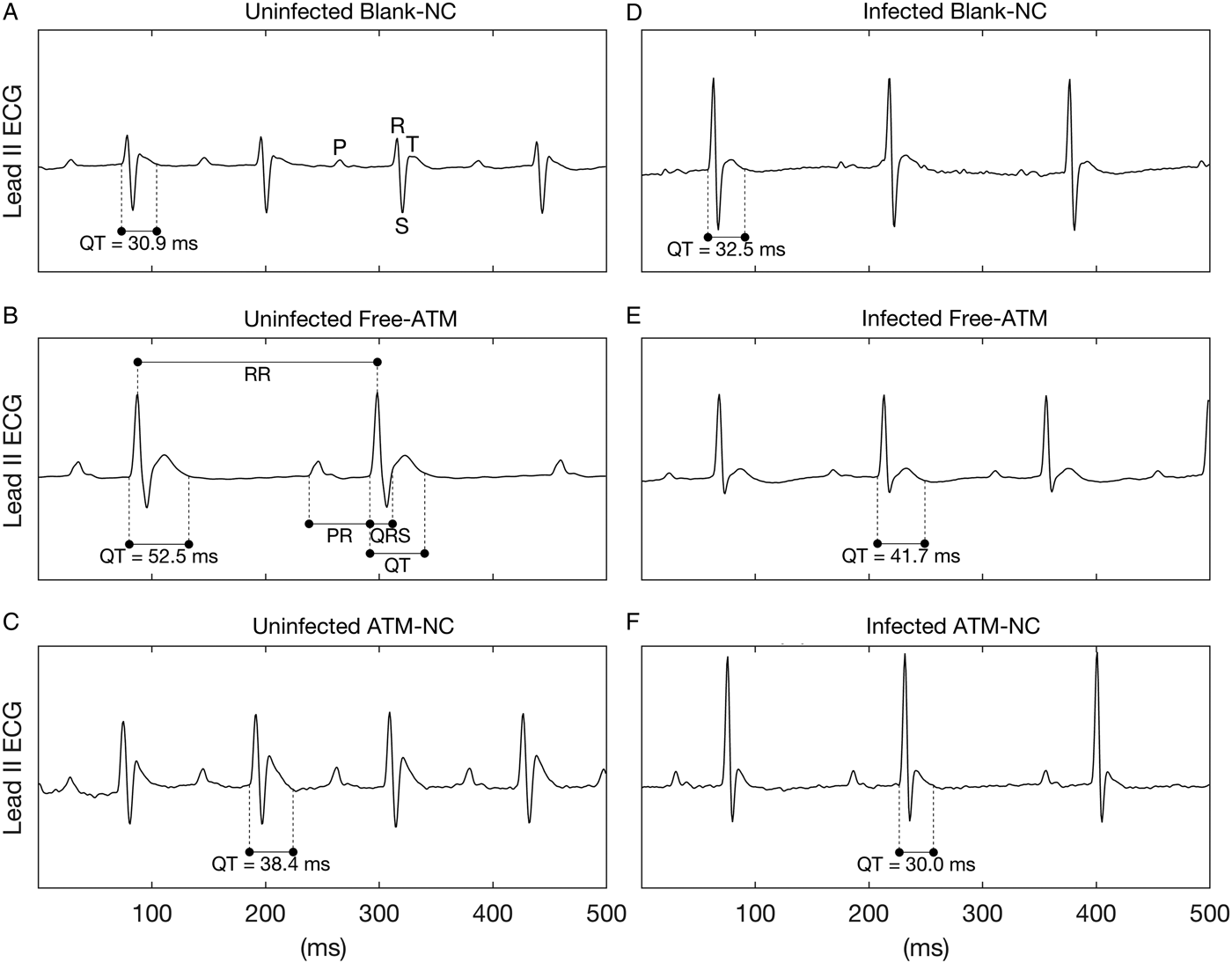
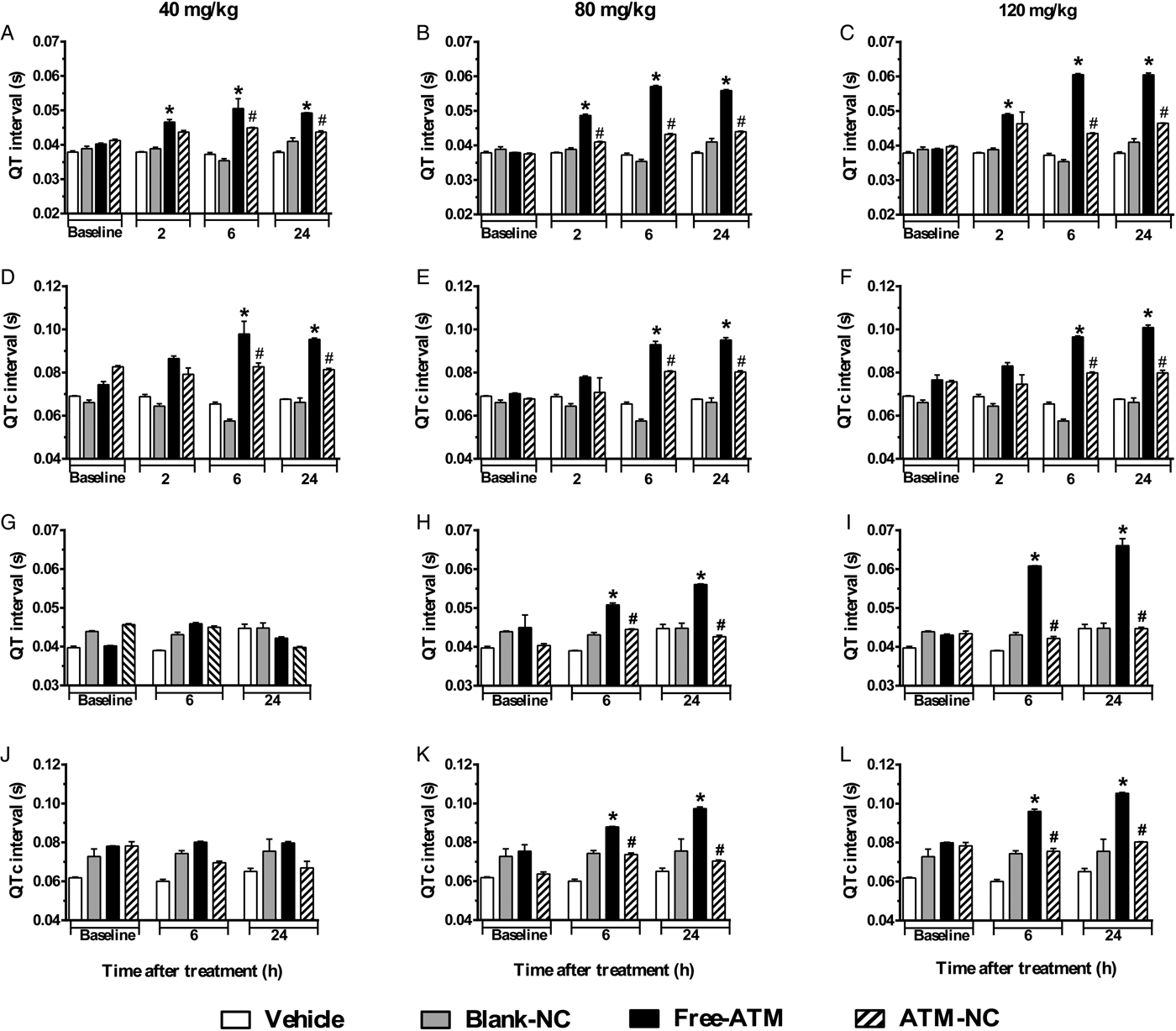
Limb lead II ECG was continuously recorded during 5 min using subcutaneous stainless steel needle electrodes, connected by a shielded cable to a biopotencial amplifier with a bandpass of 0·5–100 Hz, and sampled at 1200 Hz with an A/D conversion board of 16 bits resolution (DaqBoard/2001, IOtech, USA). From raw ECG records, three segments of 2 s were randomly extracted, and in these waveforms all cardiovascular parameters were measured and averaged to generate a single value each. Figure 2A shows the waves P, R, S and T of an ECG. The measured ECG parameters were RR, PR, QRS, QT intervals (Fig. 2B). RR interval was measured between two successive R-waves, determining a complete cardiac cycle, and used to calculate the heart rate (HR) in beats-per-minute (bpm), where HR = 60/RR. PR interval was measured from the beginning of P-wave to the onset of QRS complex, meaning the interval between the onset of atrial depolarization and the onset of ventricular depolarization. QRS interval was measured between the start of R-wave and the end of S-wave and represents the ventricular depolarization. QT interval was measured between the onset of QRS complex and the end of T-wave, which represents a complete ventricular systole, including depolarization and repolarization (Farraj et al. Reference Farraj, Hazari and Cascio2011). In addition to the measured intervals, the QTc interval was calculated, which is the QT interval corrected by HR by the Fridericia's formulae (QTc = QT/RR1/3) and it is an improved marker (with less dependence on HR) of cardiac arrhythmia risk. It should be noted that despite the lack of Q-wave in mice, it still appears in the definition of intervals, such as QRS, QT (and QTc), where the beginning of Q-wave must be replaced by the beginning of R-wave. ECG intervals are expressed as absolute values (mean ± s.e.m.) in seconds, except for Fig. 2 were they are shown in ms.
Statistical analysis
ECG and area under the curve of parasitaemia were analysed using One-way ANOVA followed by Tukey's post-test. For comparison of percentual survival it was used Kaplan–Meier to estimate Cox regression. Graph Pad Prism® 5.0 (Graph Pad Software, USA) software was used as a tool for statistical analysis. Data are expressed as the mean ± standard error of the mean (s.e.m.). A P value below 0·05 was considered statistically significant.
Results
NC characterization
ATM-NC were previously developed and characterized in detail by our group (Vidal-Diniz, Reference Vidal-Diniz2014; unpublished data). Encapsulation efficiency (EE) was >90% at concentration of 4·0 mg mL−1. Table 1 shows mean size, PDI and zeta potential of blank-NC and ATM-NC. NC were monodisperse in size (PDI <0·3) and presented negative zeta potential values at NC surface, which maintains colloidal stability by electrostatic repulsion, preventing particles aggregation. ATM reduced slightly the surface charge in modulus and increased NC mean size, evidencing its association with the oily core of NC. No ATM precipitation was observed until three months after preparation stored at 4 °C. Considering these physicochemical characteristics, NC formulations were considered suitable for in vivo studies in mice model.
Table 1. Physicochemical characteristics of nanocapsules formulations

a Poly-ε-caprolactone (PCL) nanocapsules (NC); ATM-NC, artemether in NC (4 mg mL−1); PDI, Polydispersity index; s.d., standard deviation. Measurement after dilution 1:100 in MilliQ water (n = 3).
Survival and antimalarial efficacy of free-ATM and ATM-NC
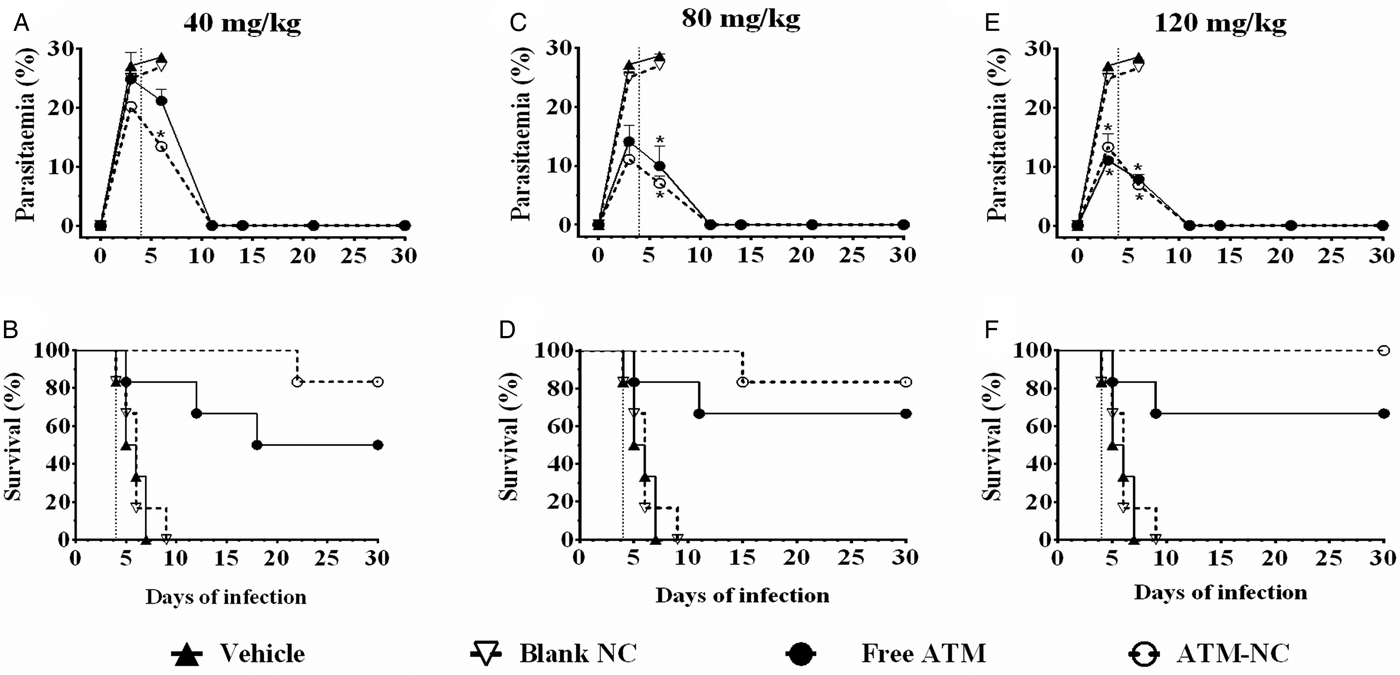
At all three ATM doses, parasitaemia was reduced and survival was increased for both formulations, free and NC, compared with control groups, vehicle and blank-NC (Fig. 1). Parasitaemia reduction at 6th day was about 63% after oral treatment with free-ATM and about 69% after treatment with ATM-NC, both at dose 120 mg kg−1, when compared with respective control groups (Fig. 1E). ATM-NC 40 mg kg−1 was more efficient than free-ATM in reducing parasite number at the 6th day after infection (Fig. 1A). From the 11th day, efficacy was similar for three ATM doses, free and NC (Fig. 1A, C and E). Until the 9th day after infection (Fig. 1B, D and F), all animals treated with vehicle or blank-NC went to death. The areas under the curve comparison using the Kaplan–Meier survival estimator were not significantly different (P > 0·05) between 40 and 80 mg kg−1 for ATM-NC in treated infected mice.
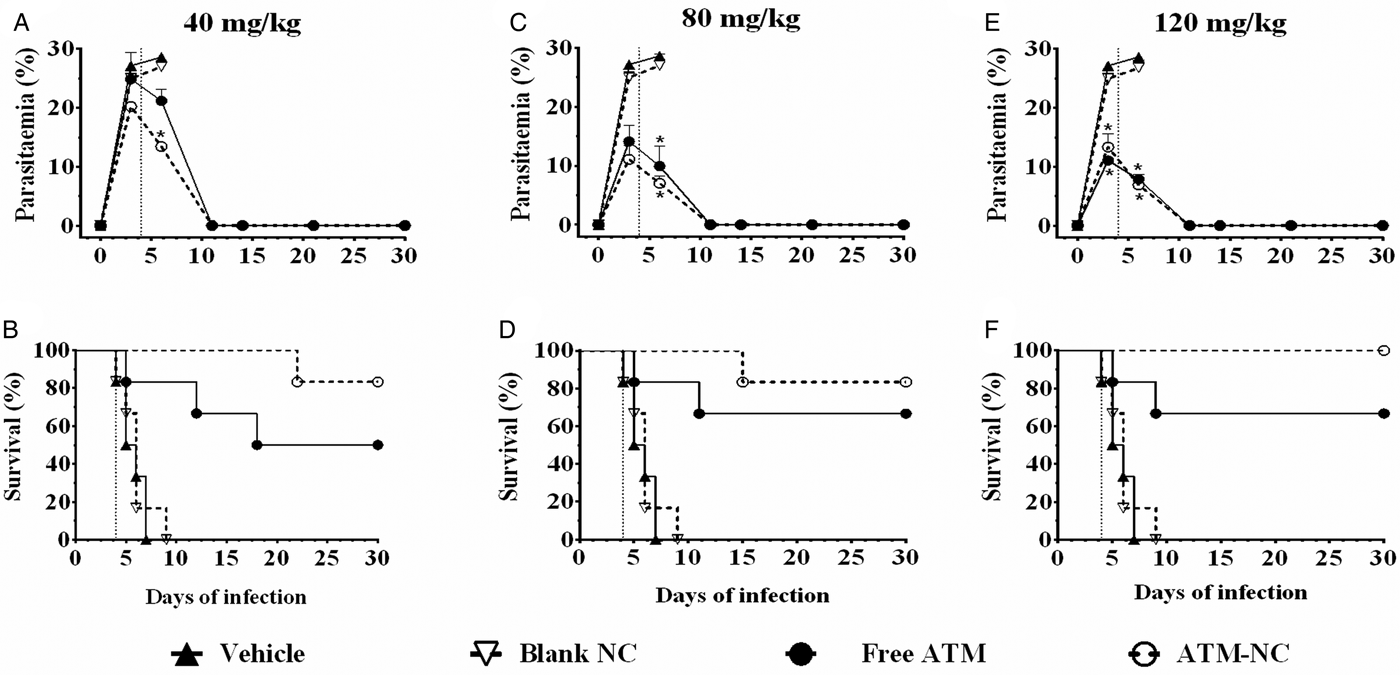
Fig. 1. Parasitaemia and survival rate until 30th day of infection with Plasmodium berghei. Percentual of parasitaemia and survival rate of infected mice after treatment with free-ATM, ATM-NC or control solutions (vehicle or blank-NC). (A and B) 40 mg kg−1; (C and D) 80 mg kg−1; E and F: 120 mg kg−1. Vertical dotted lines indicate first day treatment. *P < 0·05. ANOVA followed by Tukey's post-hoc test compared with vehicle treated mice.
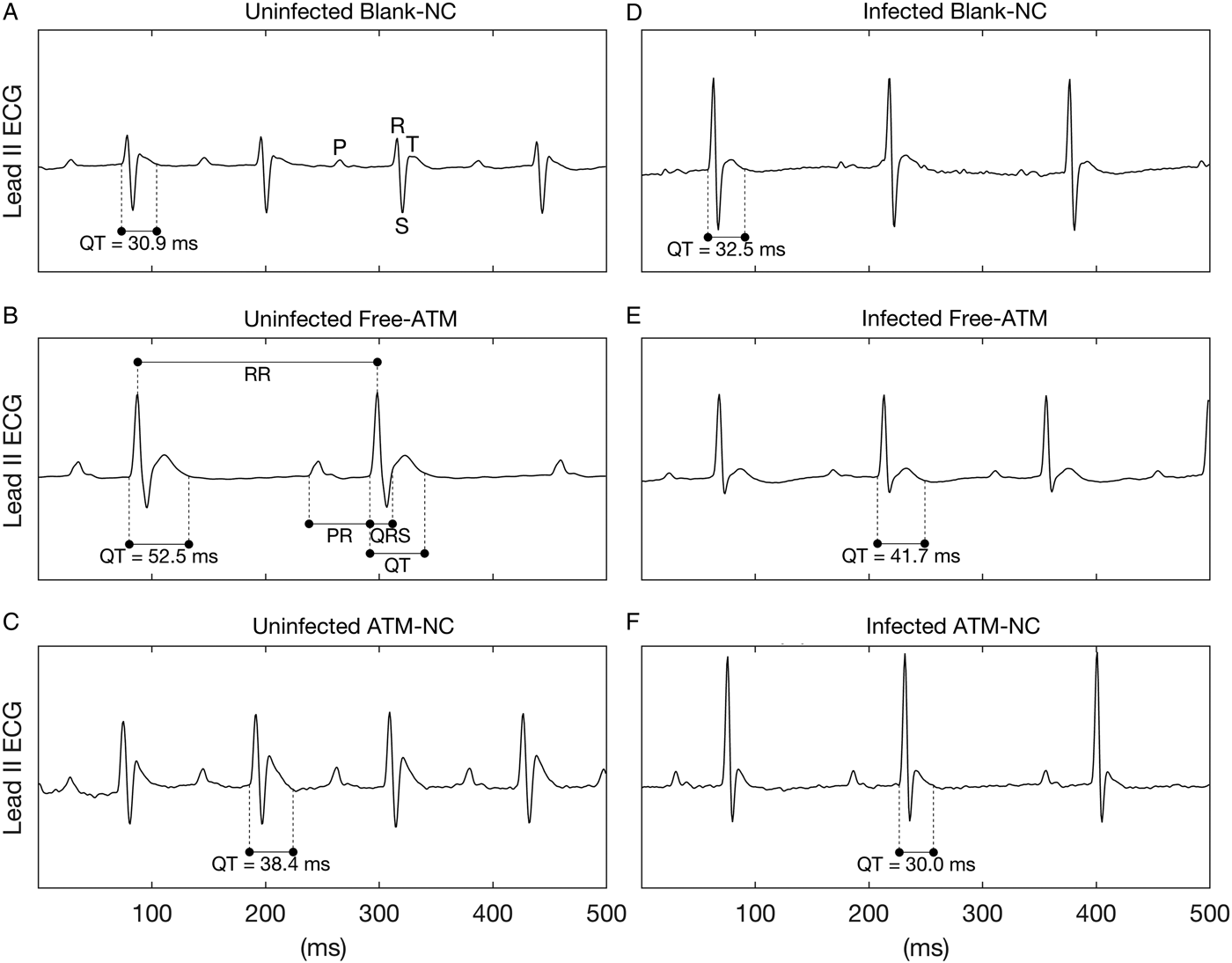
Fig. 2. Representative ECG records of uninfected and Plasmodium berghei-infected mice. ECG records showing the effects of treatment with blank-NC (A, D), free-ATM (B and E), and ATM-NC (C, F), both 120 mg kg−1, on QT interval of uninfected (A–C) and infected mice (D–F). Panel (A) shows the waves P, R, S and T, while (B) shows the intervals RR, PR, QRS and QT.
For ATM toxicity evaluation and its influence on % survival of uninfected animals, none of the mice treated with vehicle, blank-NC or ATM-NC went to death (100% survival), indicating the absence of toxic effect that could be related to pharmaceutical excipient toxicity or ATM delivered by NC. However, 50% of mice treated with free-ATM (120 mg kg−1) went to death after 4 days of treatment, indicating high toxicity. Encapsulation of ATM abolished its toxicity even with the highest dose.
Cardiovascular parameters
Figure 2 shows representative ECG segments of uninfected and P. berghei-infected mice treated with blank-NC, free-ATM or ATM-NC at 120 mg kg−1 24 h after the last oral dose administration.
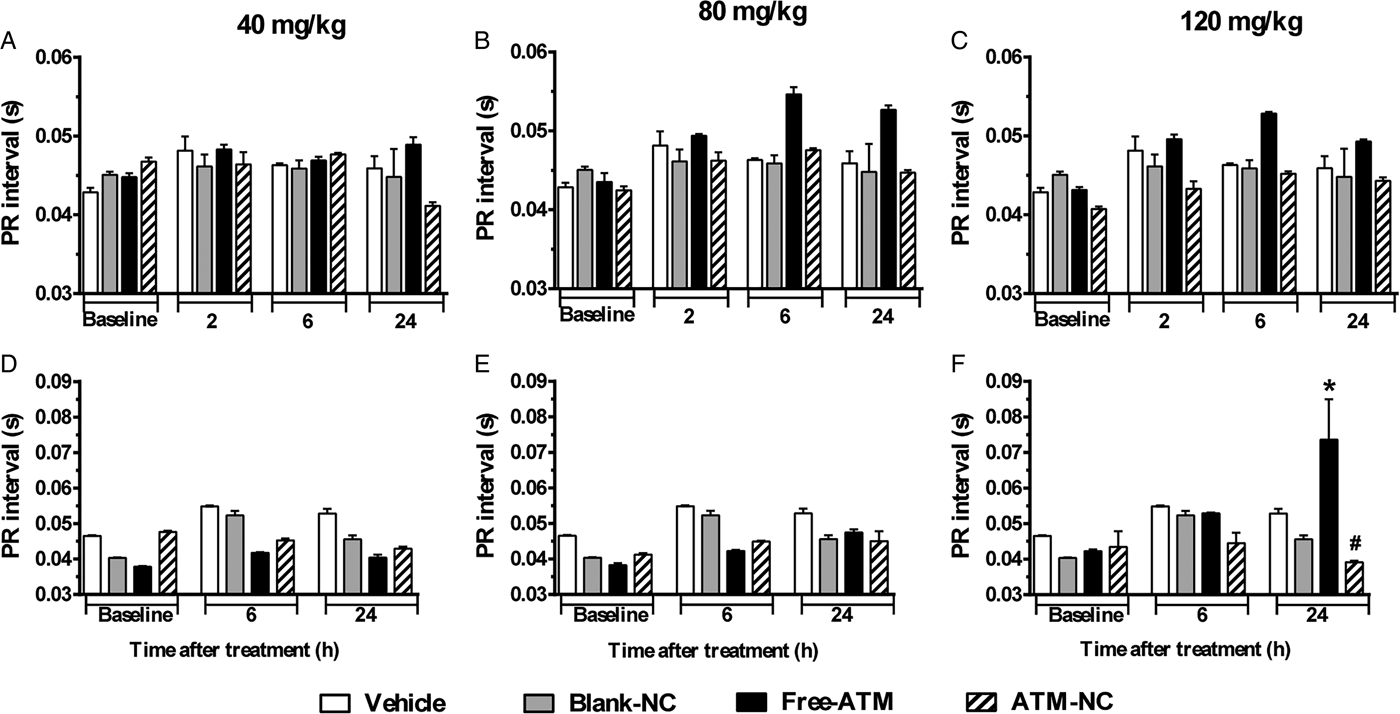
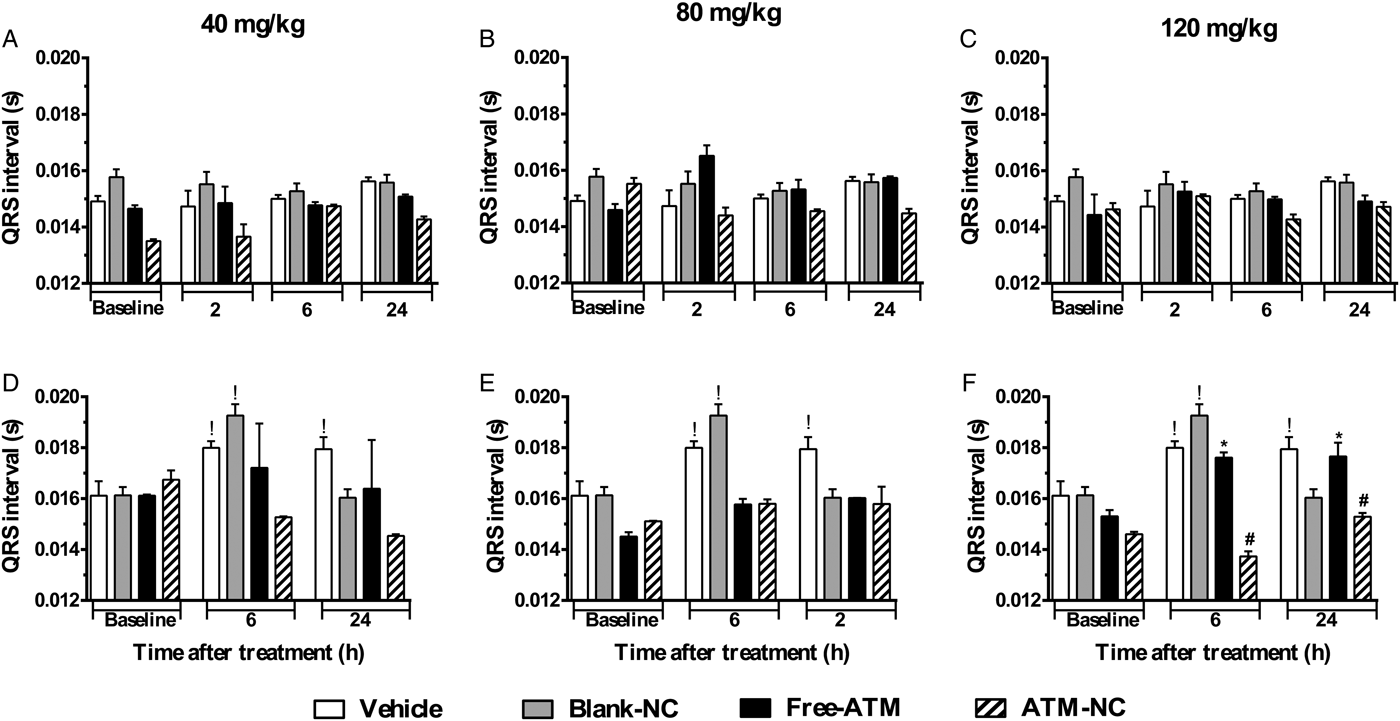
For uninfected mice, PR (Fig. 3A–C) and QRS (Fig. 4A–C) intervals were similar for all groups. For P. berghei-infected mice, PR interval showed a significant increase (74%), related to vehicle treatment, 24 h after the last dose with free-ATM 120 mg kg−1 (Fig. 3F). QRS interval was significantly higher (Fig. 4) for infected mice at 6 (20%) and 24 h (15%) after treatment with vehicle (Fig. 4D–F), compared with uninfected mice at same time (Fig. 4A–C). QRS interval also increased (Fig. 4D–F) in infected mice treated with free-ATM at dose 120 mg kg−1. These alterations of PR and QRS intervals were not observed for ATM-NC treatment of infected mice. This effect strongly shows the influence of malaria, caused by P. berghei, causing a cardiac electrophysiological disturbance, beyond free-ATM toxicity.
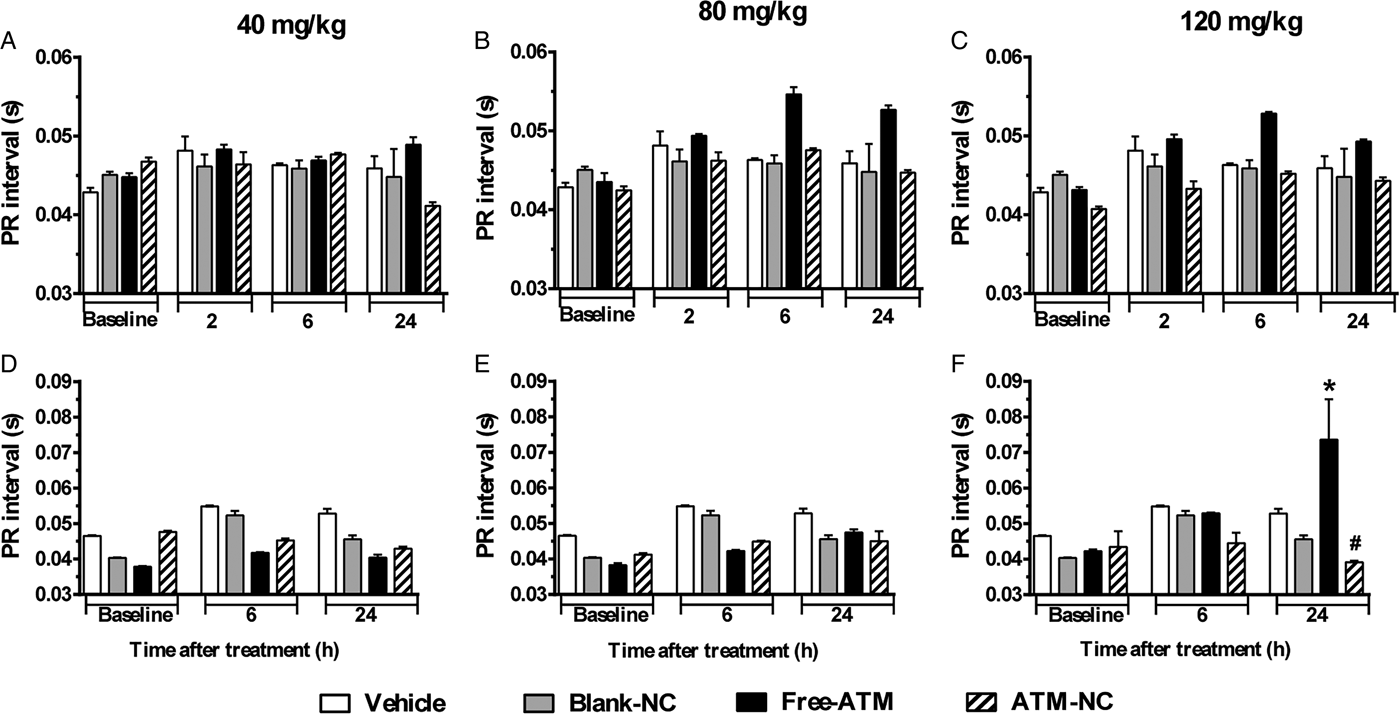
Fig. 3. PR interval (mean ± s.e.m.) of ECG of uninfected and P. berghei-infected mice. Effect of ATM on PR interval 2, 6 and 24 h for uninfected mice, 6 and 24 h for infected mice, after last oral dose treatment with free-ATM, ATM encapsulated in nanocapsules (ATM-NC), both at 40, 80 and 120 mg kg−1, or control solutions (vehicle or blank-NC). (A–C) Uninfected mice. (D–F) Infected mice. *P < 0·05 compared with vehicle and blank-NC, #P < 0·05 compared with free-ATM. One-way ANOVA followed by Tukey's post-hoc test.
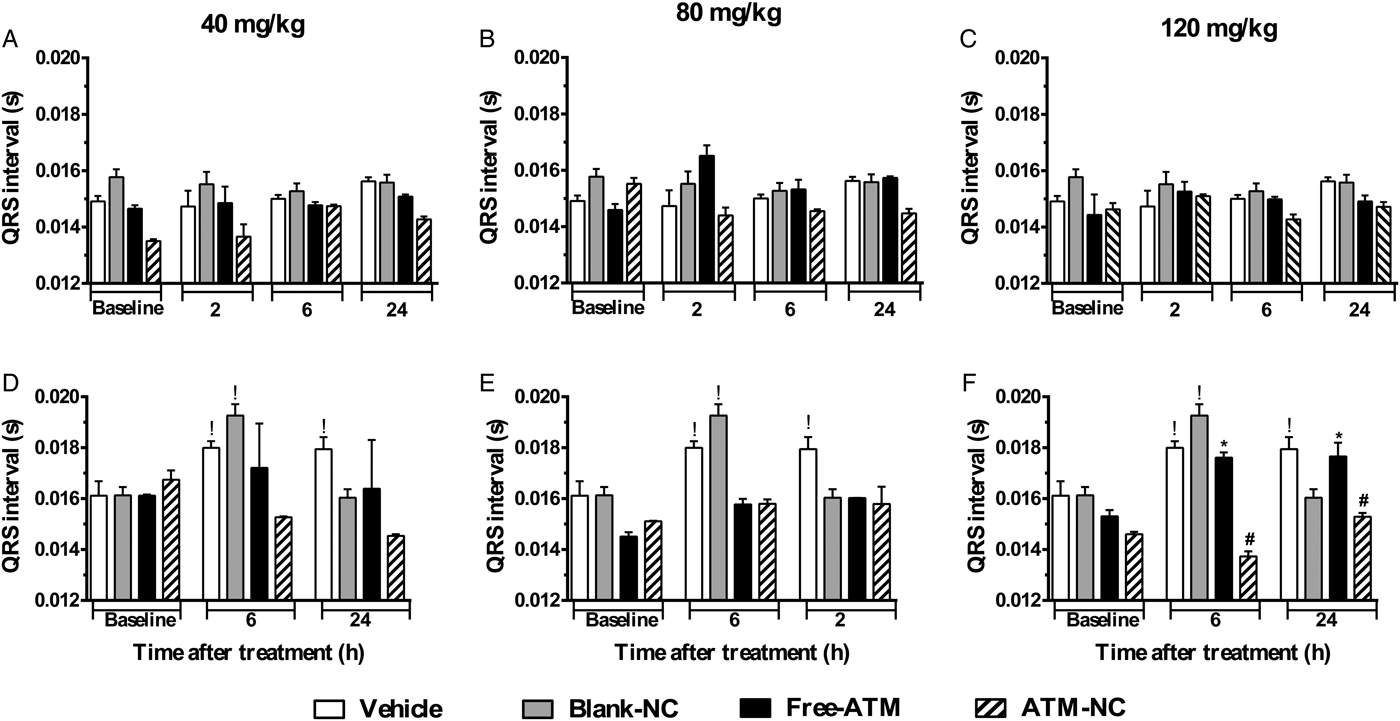
Fig. 4. QRS interval (mean ± s.e.m.) of ECG of uninfected and P. berghei-infected mice. Effect of ATM on QRS interval 2, 6 and 24 h for uninfected mice, 6 and 24 h for infected mice, after last oral dose treatment with free-ATM, ATM encapsulated in nanocapsules (ATM-NC), both at 40, 80 and 120 mg kg−1, or control solutions (vehicle or blank-NC). (A–C) Uninfected mice. (D–F) Infected mice. *P < 0·05 compared with vehicle and blank-NC, #P < 0·05 compared with free-ATM, !P < 0·05 compared with baseline of the same group. One-way ANOVA followed by Tukey's post-hoc test.
Analysis of QT (Fig. 5A–C) and QTc (Fig. 5D–F) intervals of uninfected mice compared with control groups showed significant increase after free-ATM, mainly for 120 mg kg−1, such as 55 and 41%, respectively, at 24 h. ATM-NC did not cause significant increases in QT and QTc intervals on uninfected mice (Fig. 5A–F). ATM-NC (120 mg kg−1) given to uninfected mice reduced 34 and 30%, respectively, to QT and QTc intervals prolongation, compared with free-ATM. Although some bradycardia was observed for free-ATM and ATM-NC, there was no difference of HR. For uninfected mice treated with blank-NC, HR was about 308 ± 32, 360 ± 33 and 396 ± 31; for free-ATM, HR was 284 ± 37, 252 ± 29 and 285 ± 39; for ATM-NC, HR was 279 ± 17, 347 ± 29 and 335 ± 27, respectively, at 2, 6 and 24 h after treatment with 120 mg kg−1, all normal values for anaesthetized mice. For P. berghei-infected mice treated with free-ATM, QT and QTc intervals also increased significantly (Fig. 5G–L). QT interval (Fig. 5I) prolongation after 120 mg kg−1 was 53%, while QTc interval (Fig. 5L) increased 31%, both after 24 h. ATM-NC given to infected mice also reduced QT and QTc intervals prolongation, 28 and 27%, respectively, compared with free-ATM (Fig. 5G–L). At all doses, ATM-NC did not induce significant alterations of any ECG parameter, showing clearly the cardioprotection provided by NC, eliminating ATM toxicity.
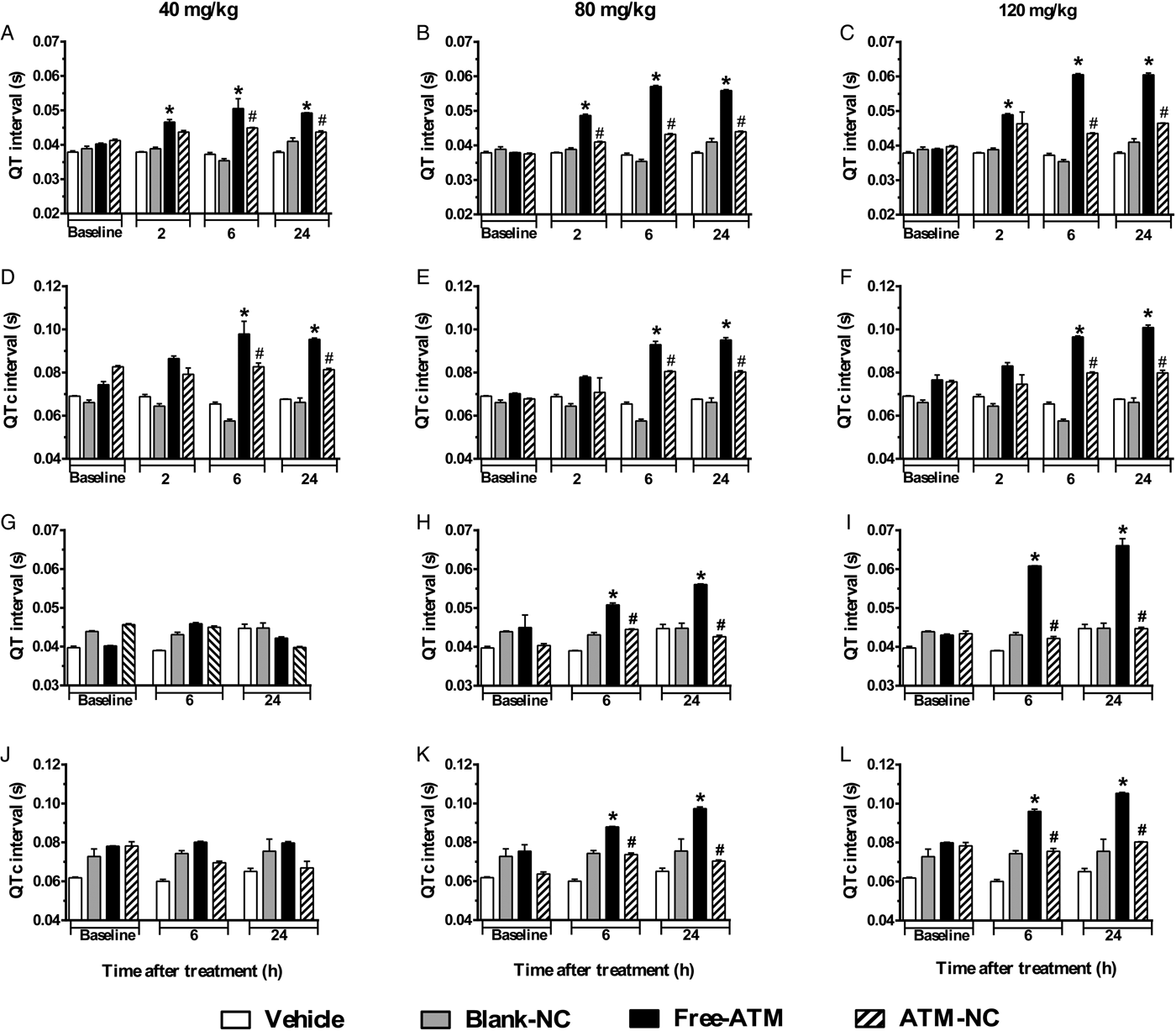
Fig. 5. QT and QTc intervals (mean ± s.e.m.) of ECG of uninfected and P. berghei-infected mice. Effect of ATM on QT and QTc intervals 2, 6 and 24 h for uninfected mice, 6 and 24 h for infected mice, after last oral dose treatment with free-ATM (40, 80 and 120 mg kg−1), ATM encapsulated in nanocapsules (ATM-NC; 40, 80 and 120 mg kg−1) or control solutions (vehicle or blank-NC). (A–C) QT interval for uninfected mice. (D–F) QTc interval for uninfected mice. (G–I) QT interval, for infected mice. (J–L) QTc interval for infected mice. *P < 0·05 compared with vehicle and blank-NC, #P < 0·05 compared with free-ATM. One-way ANOVA followed by Tukey's post-hoc test.
Discussion
The present work shows that ATM conveyed in nanocapsules (ATM-NC) administered orally can clearly reduce in vivo cardiotoxicity mainly by preventing QT interval prolongation, both in uninfected and P. berghei-infected mice. Even at the high doses used in this study, encapsulation completely prevented cardiotoxic effects of free-ATM. Additionally, the efficacy of ATM-NC against P. berghei was at least as good as free-ATM. In order to increase the efficacy of antimalarial drugs and reduce their adverse effects, several studies have shown the use of nanocarriers as a valorous strategy for malaria therapy improvement (Aditya et al. Reference Aditya, Patankar, Madhusudhan, Murthy and Souto2010; Prabhu et al. Reference Prabhu, Suryavanshi, Pathak, Sharma and Patravale2016). Polymeric NC present some advantages, as easy one-step production, biocompatibility and biodegradability (Legrand et al. Reference Legrand, Barratt, Mosqueira, Fessi and Devissaguet1999), high ability to encapsulate ATM (Vidal-Diniz, Reference Vidal-Diniz2014), low-size polydispersity and physicochemical stability (Legrand et al. Reference Legrand, Barratt, Mosqueira, Fessi and Devissaguet1999). Our results of ATM are in accordance with encapsulation of a lipophilic sesquiterpene lactone, lychnopholide, which was also encapsulated with EE higher than 95% with good stability (Branquinho et al. Reference Branquinho, Pound-Lana, Marques, Saúde-Guimarães, Vilela, Spangler, de Lana and Mosqueira2017a).
ATM-NC, at all doses used in our study, increased survival of infected mice and significantly reduced parasite number. It was observed complete parasite clearance as expected with such high doses. This effect could be attributed to ATM bioavailability changes. Our results are in accordance with previous findings that showed similar polymeric NC able to alter the pharmacokinetics of lipophilic molecules, to improve drug body exposure and increase efficacy (Mosqueira et al. Reference Mosqueira, Loiseau, Bories, Legrand, Devissaguet and Barratt2004; Branquinho et al. Reference Branquinho, Pound-Lana, Marques, Saúde-Guimarães, Vilela, Spangler, de Lana and Mosqueira2017a). Free-ATM has prompt but incomplete absorption by the oral route, with only 40% of bioavailability (Karbwang et al. Reference Karbwang, Na-Bangchang, Congpuong, Molunto and Thanavibul1997). An important aspect to highlight is the ATM-NC efficacy by oral route as liquid dosage form, since this route is the most convenient and feasible to use in low income countries, mainly when we consider that severe malaria reaches particularly children (WHO, 2016b).
ATM doses used in the present work were based on a previous study (Beckman et al. Reference Beckman, Youreneff and Butt2013), that reported neurotoxicity in juvenile rats by different protocols using doses from 20 to 200 mg kg−1 day−1 for 7 days, by the oral route. They showed that doses higher than 30 mg kg−1 day−1 increased mortality, renal necrosis and brain haemorrhage. Thus, the doses 40, 80 and 120 mg kg−1 of ATM were chosen for evaluation of cardiotoxicity and efficacy.
Other studies reported about another nanocarrier that allows a considerable bioavailability increase. ATM in nanoemulsion drug delivery system was able to increase oral bioavailability in 2·6-fold (Laxmi et al. Reference Laxmi, Bhardwaj, Mehta and Mehta2015). Oral administration of beta-ATM in microemulsifying drug delivery system (Mandawgadea et al. Reference Mandawgadea, Sharma, Pathak and Patravale2008) and liposomal formulation (Chimanuka et al. Reference Chimanuka, Gabriëls, Detaevernier and Plaizier-Vercammen2002) were effective in reducing the number of P. berghei and P. chabaudi, respectively.
We hypothesized that NC could reduce free-ATM induced cardiotoxicity, in healthy and malaria mice models. ECG is an important tool used both for diagnosis of cardiac diseases (Surawicz et al. Reference Surawicz, Childers, Deal, Gettes, Bailey and Gorgels2007) and for detection of cardiotoxic effects caused by drugs (Salvi et al. Reference Salvi, Karnad, Panicker and Kothari2010; Farraj et al. Reference Farraj, Hazari and Cascio2011). QT interval prolongation is a major alteration of ECG indicative of drug toxicity (Crumb and Cavero, Reference Crumb and Cavero1999; Redfern et al. Reference Redfern, Carlsson, Davis, Lynch, MacKenzie, Palethorpe, Siegl, Strang, Sullivan, Wallis, Camm and Hammond2003). It is associated with malignant ventricular tachyarrhythmia and susceptibility to TdP, which may lead to sudden death (Redfern et al. Reference Redfern, Carlsson, Davis, Lynch, MacKenzie, Palethorpe, Siegl, Strang, Sullivan, Wallis, Camm and Hammond2003). Male mice model were used for cardiovascular studies because, according to Patten (Reference Patten2007), female animals are less sensitive to cardiovascular alterations, since ovarian hormones may be important in reducing the risk of vascular disease. ATM-NC was able to prevent QT interval prolongation observed with free-ATM in both uninfected and P. berghei-infected mice, reducing cardiac risk. Infection itself caused QRS enlargement, indicating cardiac depolarization abnormalities (Bassareo and Mercuro, Reference Bassareo and Mercuro2013), an effect reported for malaria model for the first time here. No previous work reported a reduction of cardiotoxicity through association with nanocarriers, as we observed herein.
Only for infected mice, PR interval increased at 24 h after treatment with a higher dose of free-ATM, but not with ATM-NC. PR interval prolongation refers to atrial alterations, a possible onset of heart failure and atrial fibrillation (Holmqvist et al. Reference Holmqvist, Thomas, Broderick, Ersbøll, Singh, Chiswell, Shaw, Hegland, Velazquez and Daubert2015). Since infected mice treatment with vehicle and uninfected mice treatment with free-ATM did not induce PR interval alteration, we should hypothesize that combination of malaria effects and ATM toxicity could induce this cardiac disturbance.
Reduction or absence of electrocardiographic alterations after oral administration of ATM-NC was also probably due to the ability of nanocarriers to modify the distribution of entrapped drug into body, as discussed before. It was very likely that there was a slow release of ATM into the bloodstream, thus reducing the availability of ATM to get into cardiac tissue as recently observed with another sesquiterpene lactone (Branquinho et al. Reference Branquinho, Pound-Lana, Marques, Saúde-Guimarães, Vilela, Spangler, de Lana and Mosqueira2017a, Reference Branquinho, Roy, Farah, Garcia, Aimond, Le Guennec, Saude-Guimarães, Grabe-Guimaraes, Mosqueira, Lana and Richardb). In addition, less ATM is available to be metabolized to dihydroartemisinin, which is potentially cardiotoxic as proposed before (Aditya et al. Reference Aditya, Patankar, Madhusudhan, Murthy and Souto2010; Manning et al. Reference Manning, Vanachayangkul, Lon, Spring, So, Sea, Se, Somethy, Phann, Chann, Sriwichai, Buathong, Kuntawunginn, Mitprasat, Siripokasupkul, Teja-Isavadharm, Soh, Timmermans, Lanteri, Kaewkungwal, Auayporn, Tang, Chour, Prom, Haigney, Cantilena and Saunders2014). In vitro study (Borsini et al. Reference Borsini, Crumb, Pace, Ubben, Wible, Yan and Funck-Brentano2012) showed that dihydroartemisinin in different concentrations, and associated with piperaquine, can cause a significant hERG-channel block. The hERG gene codes a subunit of the potassium channel that controls the repolarizing current IKr (rapid delayed rectifier potassium current). Its drug-induced blockade is related to QT interval prolongation, increasing cardiac risk to sudden death (Fermini and Fossa, Reference Fermini and Fossa2003).
The nanometric size of NC is an important characteristic for oral administration and uptake by intestinal cells (McClean et al. Reference McClean, Prosser, Meehan, O'Malley, Clarke, Ramtoola and Brayden1998). In this context, two events are possible: (1) NC entrapped in the villi network may be retained in the intestine longer than macroscopic oral dosage forms (e.g. capsules or tablets); or (2) NC might be internalized by the intestinal cells, which allows drug pharmacokinetics modification and efficient delivery at target sites in the body. It was demonstrated that orally absorbed NC altered the elimination and distribution of docetaxel, as shown in the organ biodistribution rat study, due to their reinforced coating, while transiting through the enterocytes by surface adsorption of apolipoproteins and phospholipids (Attili-Qadri et al. Reference Attili-Qadri, Karra, Nemirovski, Schwob, Talmon, Nassar and Benita2013). Oral administration of docetaxel NC modifies the pharmacokinetic profile of docetaxel and improved anticancer activity when compared with free form intravenous administration. In addition, De Mello et al. (Reference De Mello, Branquinho, Oliveira, Milagre, Saúde-Guimarães, Mosqueira and Lana2016) showed that orally administration of sensitive sesquiterpene lactones NC was more effective in combating parasitic disease than free-molecule, probably by protecting the drug from degradation. For different drugs having a short half-life, such as ATM, nanoencapsulation and oral administration have advantages such as protection from ATM degradation in the gastrointestinal tract (GIT) and upon absorption of NC provides changes in plasma profile, increasing bioavailability (Galindo-Rodriguez et al. Reference Galindo-Rodriguez, Allemann, Fessi and Doelker2005). ATM is not a stable molecule in the GIT. However, our results showed that ATM-NC exhibit efficacy after oral administration, indicating that NC uptake is probable. NC is supposed to be absorbed gradually and release the free form into the blood at a slow rate, enough to kill the parasites but, at the same time, reducing ATM exposure to the heart tissue.
Thus experimental data showed here provide strong results of ATM-NC ability to reduce cardiotoxicity and give efficacy improvement against P. beghei in monotherapy. One more option for therapeutic improvement of ATM against severe malaria was provided, since ATM-NC administered orally showed to be effective and non-toxic against experimental malaria model in vivo.
Acknowledgements
The authors would like to tank also Ministry of Health (Programa Nacional de Controle da Malária-Brazil) for a donation of Paluther® ampoules. VCF Mosqueira is a research fellow of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil. A.C.M. Souza thanks her scholarship grant from CAPES.
Financial Support
This work was supported by the Bilateral Research Collaboration CAPES-COFECUB between Brazil and France (no. 768/13). This work was also supported by the NANOBIOMG-Network (#0007-14 and #40/11), APQ 01510-14 and PPM grants (# 00432-13; #CDS-PPM-00481-13) from FAPEMIG, Minas Gerais, Brazil.
Conflict of Interest Statement
The authors declare that there is no conflict of interests regarding the publication of this paper.