Introduction
Mermithid nematodes (Nematoda: Mermithida: Mermithidae) are obligate endoparasites of invertebrates that are common in both aquatic and terrestrial insect hosts (Poinar, Reference Poinar1975). Mermithid parasitism is typically lethal to its host, as the exit of the fully developed nematode from the body of the host causes irreparable damage (Poinar, Reference Poinar1975). Although there are approximately 200 described mermithid species, the biology and distributions of only a few are well known (Poinar, Reference Poinar1975). Most mermithids infect aquatic hosts, although Mermis nigrescens specialize in terrestrial, herbivorous hosts, and those within Pheromermis have a paratenic life cycle, using aquatic or semiaquatic insects as intermediate, non-developmental hosts to gain access to their primary ant or wasp hosts through predation (Poinar, Reference Poinar1975; Poinar et al. Reference Poinar, Lane and Thomas1976). Of the handful of species that have been well researched, many appear to have wide geographic distributions. For example, Isomermis lairdi, a parasite of black fly (Diptera: Simuliidae) larvae, are found in different simuliid hosts throughout Africa and in Europe, and are morphologically most similar to I. benevolus, I. rossica and I. wisconsinensis, species known from Guatemala (North America), throughout Europe, and the USA (North America), respectively (Gradinarov, Reference Gradinarov2014). Similarly, M. nigrescens has been recorded from a variety of hosts across Europe, Asia, North America, South America, and, more recently, from Australia and New Zealand, where they were thought to have been anthropogenically introduced (Presswell et al. Reference Presswell, Evans, Poulin and Jorge2015). Efforts to expand our knowledge of the biology, host ranges and distributions of mermithids have been complicated by a troublesome taxonomic treatment of the group (Gradinarov, Reference Gradinarov2014).
On very few occasions, mermithid nematodes (Mermithida: Mermithidae) have been reported parasitizing bumble bees (Hymenoptera: Apidae: Bombus). Although reports have been rare, they have been observed on four continents, suggesting that mermithid parasitism is geographically widespread. Unfortunately, many reports of mermithid parasites of bumble bees are lacking details on the identity of both hosts and parasites, thus generalizations about the phenomenon, such as distributions, host ranges, frequency of occurrence, and pathology, are impossible to make. Here, we summarize the six known records of mermithid infections in bumble bees, double the known reports with six new records obtained in a single survey, and add genetic sequence data to begin to unravel this unusual phenomenon.
Of the six reports of mermithids parasitizing bumble bee hosts, none have been satisfactorily identified to species. An unidentified mermithid was recovered from an unidentified bumble bee in Indiana in the 1960s, making this North American occurrence the first known record of the phenomenon (MacLean, Reference MacLean1966). Mermithid parasitism was not reported again until the 1990s, in a study of 4366 bumble bees in Sweden that noted that mermithids were present in their samples, but neither the frequency of occurrence nor the host species were reported (Durrer and Schmid-Hempel, Reference Durrer and Schmid-Hempel1995). In South America, two unidentified mermithids were reported from a single Bombus bellicosus specimen (out of 403 bees examined) in Uruguay (Plischuk et al. Reference Plischuk, Salvarrey, Arbulo, Santos, Skevington, Kelso, Revainera, Maggi, Invernizzi and Lange2017). One immature mermithid matching the morphology of a Pheromermis sp. was found in a B. impatiens worker host (out of 68) in Massachusetts, but the specimen was not genetically characterized (Rao et al. Reference Rao, Poinar and Henley2017). There are two reports from Japan of mermithid nematodes in bumble bee hosts. Mermithids were reported from B. terrestris, an exotic species of European origin imported for pollination services (Kosaka et al. Reference Kosaka, Sayama, Kanzaki, Makino and Okabe2012). In another record from Japan, a single B. pseudobaicalensis queen was found to be harbouring three mermithids (Kubo et al. Reference Kubo, Ugajin and Ono2016). These were sequenced for regions of the 18S, small-subunit region of the genome and found to be more closely related to Ovomermis and other unidentified mermithid groups than to Pheromermis spp. (Kubo et al. Reference Kubo, Ugajin and Ono2016).
Mermithids have invariably been found in adult bumble bees during host dissection and are therefore parasitic-stage juveniles. This stage is lacking morphological characters that allow for the identification of genera and species (Poinar, Reference Poinar1975; Rao et al. Reference Rao, Poinar and Henley2017). Genetic analyses can help to characterize these immature parasites, but currently, comparative data are lacking for most species. Here we report both the occurrence and genetic characterization of mermithid nematodes found during a large-scale survey of bumble bee parasites conducted across the USA in an effort to begin effectively documenting this rare parasitism by providing genetic data that may allow for more complete characterizations in the future.
Materials and methods
As part of a national survey, 3646 bumble bee samples were collected from 121 sites across the continental USA. Adult bumble bees were collected at field sites from flowers using aerial nets, held and transported in liquid nitrogen or on dry ice, to the laboratory, and then frozen at −80 °C until dissections were conducted. Each bee was dissected under a stereomicroscope (Wild M5, Heerbrugg, Switzerland) at 6–50× magnification and the presence of mermithids noted. When present, mermithids were removed from the body cavity, rinsed in ultra-pure water, and stored individually at −80 °C until DNA extraction. A subset of four of these was photographed, and the length and width were measured with ImageJ v.1.51j8 (Schneider et al. Reference Schneider, Rasband and Eliceiri2012). For two specimens, a 0.5–1 cm portion of the head and tail regions were removed and slide-mounted in a glycerine–lactic acid–fuschin stain for examination at 100–400× (BX51, Olympus Corporation, Center Valley, PA) prior to DNA extraction. DNA was extracted from the entire (N = 4) or remaining (N = 2) body of the nematode using a salting-out procedure with in-house reagents (Sambrook and Russell, Reference Sambrook and Russell2001). DNA extracts were stored at −20 °C until use in PCR.
Portions of the 18S locus were amplified in two reactions using the primer sets Kubo18SF (Kubo et al. Reference Kubo, Ugajin and Ono2016) and ApidaeR (Meeus et al. Reference Meeus, de Graaf, Jans and Smagghe2010) and 18S965 and 18S1573R (Mullin et al. Reference Mullin, Harris and Powers2005). Reactions consisted of 1 µL of DNA extract, 0.8 µ m of each primer, 1.3× buffer, 2 mm MgCl2, 0.8 mm total dNTPs and 1 unit of Taq polymerase, with molecular-grade water to bring the solution to a total volume of 25 µL. Thermal cycling consisted of an initial denaturation of 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 58 °C for 45 s, 72 °C for 45 s and a final elongation step of 72 °C for 10 min. A portion of the D2–D3 region of 28S was also amplified using the primers D2A and D3B (Courtright et al. Reference Courtright, Wall, Virginia, Frisse, Vida and Thomas2000), with the same thermal conditions, with the exception of decreasing the number of cycles to 35 and the annealing temperature to 55 °C. Amplification products were separated on 2% agarose gels, stained with 2.5× GelRed solution (Biotum, Fremont, CA), and visualized under UV light (Bio-Doc-It, UVP, Upland, CA). All successfully amplified products were enzymatically purified with ExoSap-It (Affymetrix, Sunnyvale, CA) and sent for sequencing in both directions to Eton Bioscience (San Diego, CA). All sequences generated in this study were deposited in GenBank (Accession numbers MG182363–MG182374). Additional sequences of a portion of the 28S region were generated for two specimens (accession numbers MG182376 and MG182377), but with so few reference sequences of this region available in GenBank, these were not analysed further.
Bayesian analysis was used to examine potential relationships among nematodes and aid taxonomic classification. Sequences were aligned and trimmed in Geneious v.6.1.8 (Biomatters, Auckland, NZ) to match other nematode sequences available on GenBank, separately for each region (aligned lengths: V2–V6 region of 18S: 930 bp, V5–V9 region of 18S: 608 bp). Alignments were analysed in jModelTest v.2.1.4 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012) to determine the most appropriate substitution models to use in phylogenetic construction using corrected Akaike Information Criterion (GTR + I + G for both loci; V2–V6: I = 0.449, γ = 0.698; V5–V9: I = 0.546, γ = 0.755). Relationships among sequences were estimated with Bayesian inference using the Mr. Bayes plug-in (Huelsenbeck and Ronquist, Reference Huelsenbeck and Ronquist2001) within Geneious using model parameters determined in jModelTest and a subsampling frequency of 1 × 103 over 1.1 × 107 generations, after discarding the first 1 × 105 generations. Clade support was determined by posterior probabilities.
Results
Six bumble bee specimens (0.2%) each harboured a single mermithid. The nematodes were uniformly cream-coloured, rounded at the anterior, and tapered at the posterior end. Each one was coiled several times within the metasomal cavity of its host, occupying most of the haemocoel. The length and mid-body width of four specimens were measured. Their lengths ranged from 66.5 to 130 mm (average: 88.6 ± 28.3 s.d. mm), and their mid-body widths ranged from 0.26 to 0.34 mm (average: 0.29 ± 0.0416 s.d. mm). The anterior portion of the specimen from Delaware is illustrated in Fig. 3, showing the labial papillae, cephalic papillae and stoma.
Three of the mermithids were found in bumble bee samples collected throughout the Northeastern region of the USA, in Delaware, Pennsylvania and Vermont. The hosts were workers and represented three species, Bombus bimaculatus, B. impatiens and B. vagans, all members of the subgenus (Pyrobombus). In both 18S regions, sequences from these organisms formed a well-supported, monophyletic clade with GenBank sequences of M. nigrescens (Figs 1, 2). Sequence similarity within this clade was between 99.3 and 100%, with the three sequences obtained in this study a 100% match to one another and a M. nigrescens from an earwig host sampled in New Zealand (KF583882) in both gene regions (Figs 1, 2).
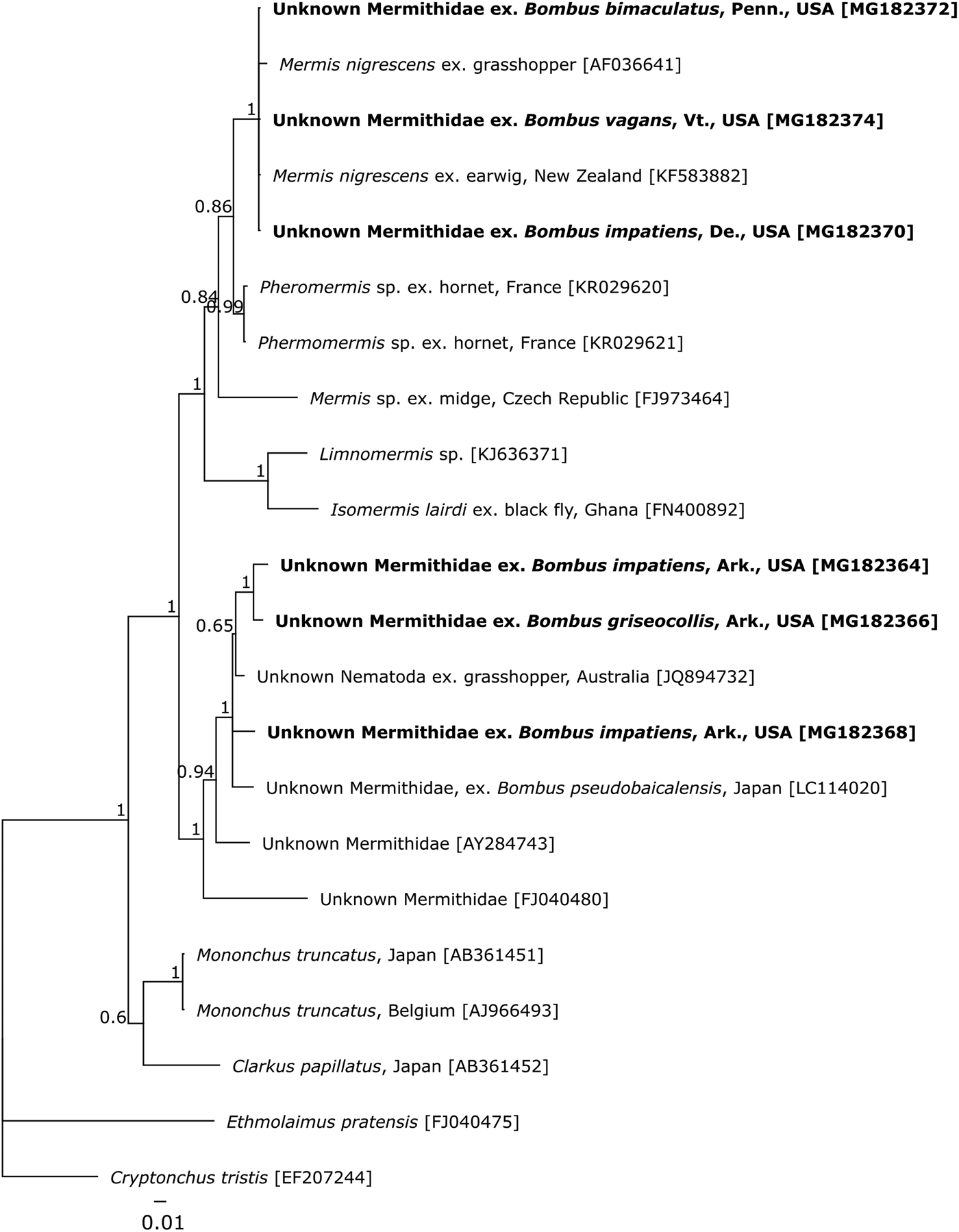
Fig. 1. Bayesian inference showing hypothesized relationships among mermithid sequences across the V2–V6 region of 18S. As far as data are available, tips are labelled with the lowest taxonomic name assigned, location collected, and the host, followed by GenBank accession numbers in brackets. Tips in bold indicate specimens sequenced in this work. Nodes indicate posterior probabilities. The scale bar shows the number of nucleotide substitutions per site.
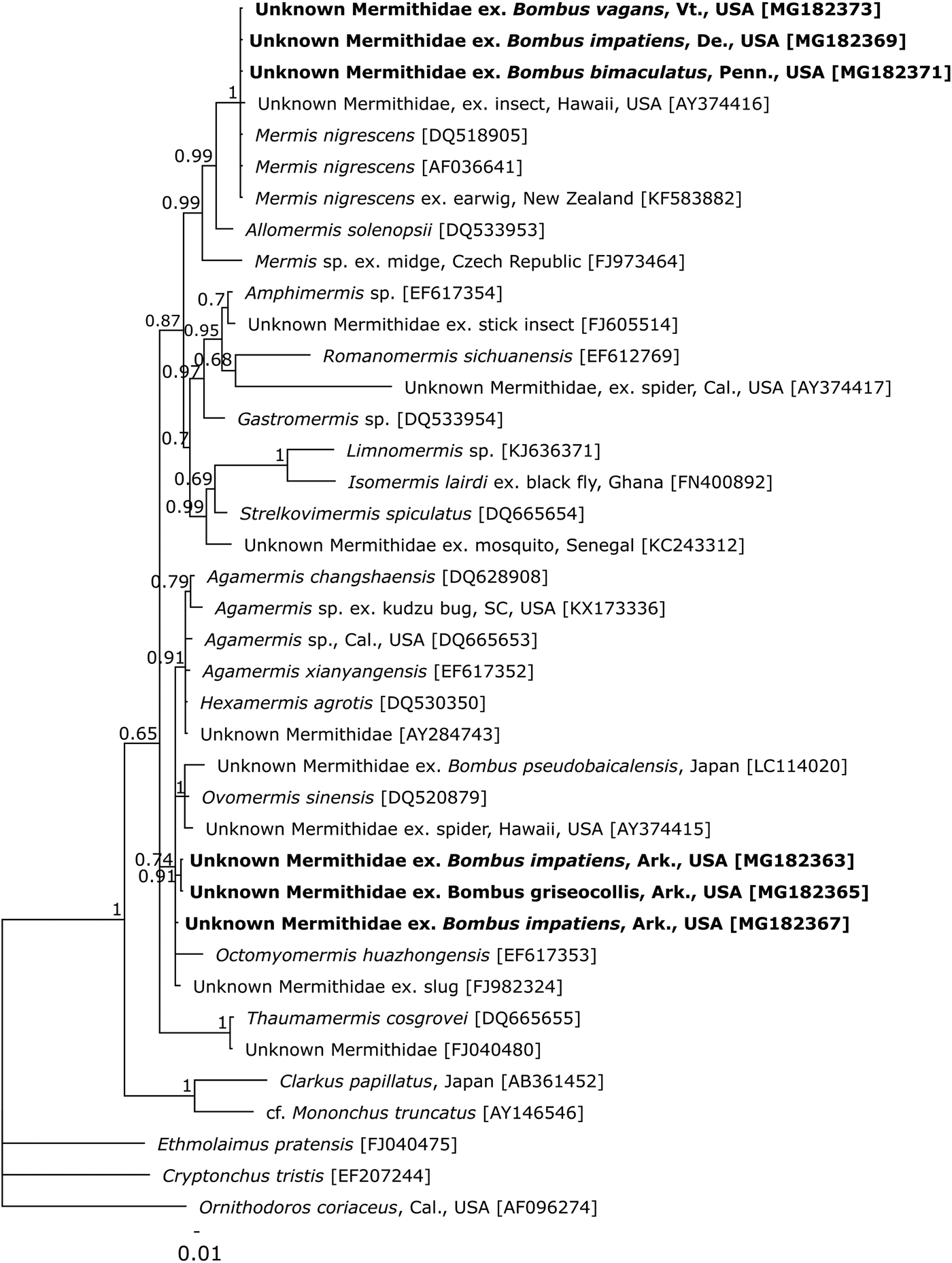
Fig. 2. Bayesian inference showing hypothesized relationships among mermithid sequences across the V5–V9 region of 18S. As far as data are available, tips are labelled with the lowest taxonomic name assigned, location collected, and the host, followed by GenBank accession numbers in brackets. Tips in bold indicate specimens sequenced in this work. Nodes indicate posterior probabilities. The scale bar shows the number of nucleotide substitutions per site.
At a single site at the University of Arkansas Experimental Farm, in Fayetteville, Arkansas, three bumble bee specimens (out of a total of 94; 3.2%) were found harbouring mermithid parasites. All three hosts were workers, but of two species: B. impatiens (N = 2) and B. griseocollis, which is in the subgenus (Cullumanobombus). None of the sequences of mermithids from the Arkansas samples matched sequences for any of the organisms on GenBank in the V2–V6 region of 18S. Furthermore, these three sequences showed 2.6–3.0% divergence from one another, and collectively, they clustered with the only known mermithid sequence to have been derived from an unidentified mermithid inhabiting a bumble bee host (3.3–4% divergence, LC114020), as well as an unidentified mermithid extracted from a grasshopper host (2.2–2.8% divergence, JQ894732) (Fig. 1). Unfortunately, none of the other sequences within this clade have originated from organisms with reported identities. The divergence between these sequences and a Phermomermis sp. from a hornet host (KR029621) was 7.2–7.5%. In the V5–V9 alignment, there was 0–2.0% divergence among these three sequences, with the closest matches to Ovomermis sinensis (97.7–99.0% similarity, DQ520879) and an unknown mermithid from a slug host (98.5–98.8% similarity, FJ982324). Within the Bayesian analysis, these sequences clustered with a more diverse set of mermithids, including members of four genera from a wide range of host organisms (Fig. 2). The lack of monophyly at the genus level suggests that this region is less informative for classification purposes.
Discussion
This study examined 3646 bumble bees of 27 species and observed only six instances of mermithid parasitism. Although this phenomenon is indeed rare, with the total reports raised to 12 worldwide with this work, mermithids have been observed parasitizing bumble bee hosts across four continents. Without allowing the nematodes to finish their development and emerge from their hosts, it is not clear if bumble bees are targeted hosts of mermithids or not, but the size of the specimens recovered here indicate that the nematodes were capable of growth and development while inside bumble bees. If these follow the growth patterns observed in M. nigrescens parasites of earwigs, the mermithid juveniles observed here are likely to be at least 18–22 days old (Baylis, Reference Baylis1947). Parasitized bumble bees did not show any external indication of parasitism or abnormal morphology as often seen in mermithid-parasitized ants (e.g. Borowiec and Salata, Reference Borowiec and Salata2015), but given the life history of mermithids, there is no doubt that the nematode is detrimental to its bumble bee host.
The three mermithid specimens obtained from the northeastern USA are most likely M. nigrescens. The morphology of the anterior portion (Fig. 3) was consistent with the morphology of juvenile M. nigrescens (as M. subnigrescens) illustrated by Cobb (Reference Cobb1929) and photographed by Mongkolkiti and Hosford (Reference Mongkolkiti and Hosford1971). Genetically, 18S sequences from these three specimens matched known representatives of M. nigrescens with <1% divergence. The discovery of M. nigrescens parasitizing bumble bees over a large geographic range was surprising. Mermis nigrescens is primarily a parasite of the Orthoptera, with additional records from the Dermaptera (Baylis, Reference Baylis1947; Presswell et al. Reference Presswell, Evans, Poulin and Jorge2015). However, experimental work has shown that M. nigrescens will develop in Lepidopteran larvae, with some adult mermithids recovered from post-pupation moths (Poinar, Reference Poinar1975; Capinera, Reference Capinera1987). This suggests that the host range of this nematode may be quite large, and much broader than the current recorded host range. Indeed, honey bees have been reported harbouring infections of M. nigrescens, as well as Agamomermis sp. and Hexamermis (as Mermis) albicans (Poinar, Reference Poinar1975; Bailey and Ball, Reference Bailey and Ball1991). The life cycle of M. nigrescens is unusual for the family, in that females distribute their eggs on vegetation surfaces, and infection is achieved through hosts inadvertently feeding on eggs while they consume plant tissue, rather than through active penetration of the host's cuticle by the infective juvenile (Poinar, Reference Poinar1975). This unusual mode of dispersal provides a convenient means by which the nematode can infect herbivore hosts, such as the grasshoppers and earwigs they are best known for parasitizing. Bumble bees are not typical herbivores, feeding on nectar and pollen, rather than other plant tissues, but bumble bees could ingest the eggs of M. nigrescens while consuming nectar and pollen from flowers.
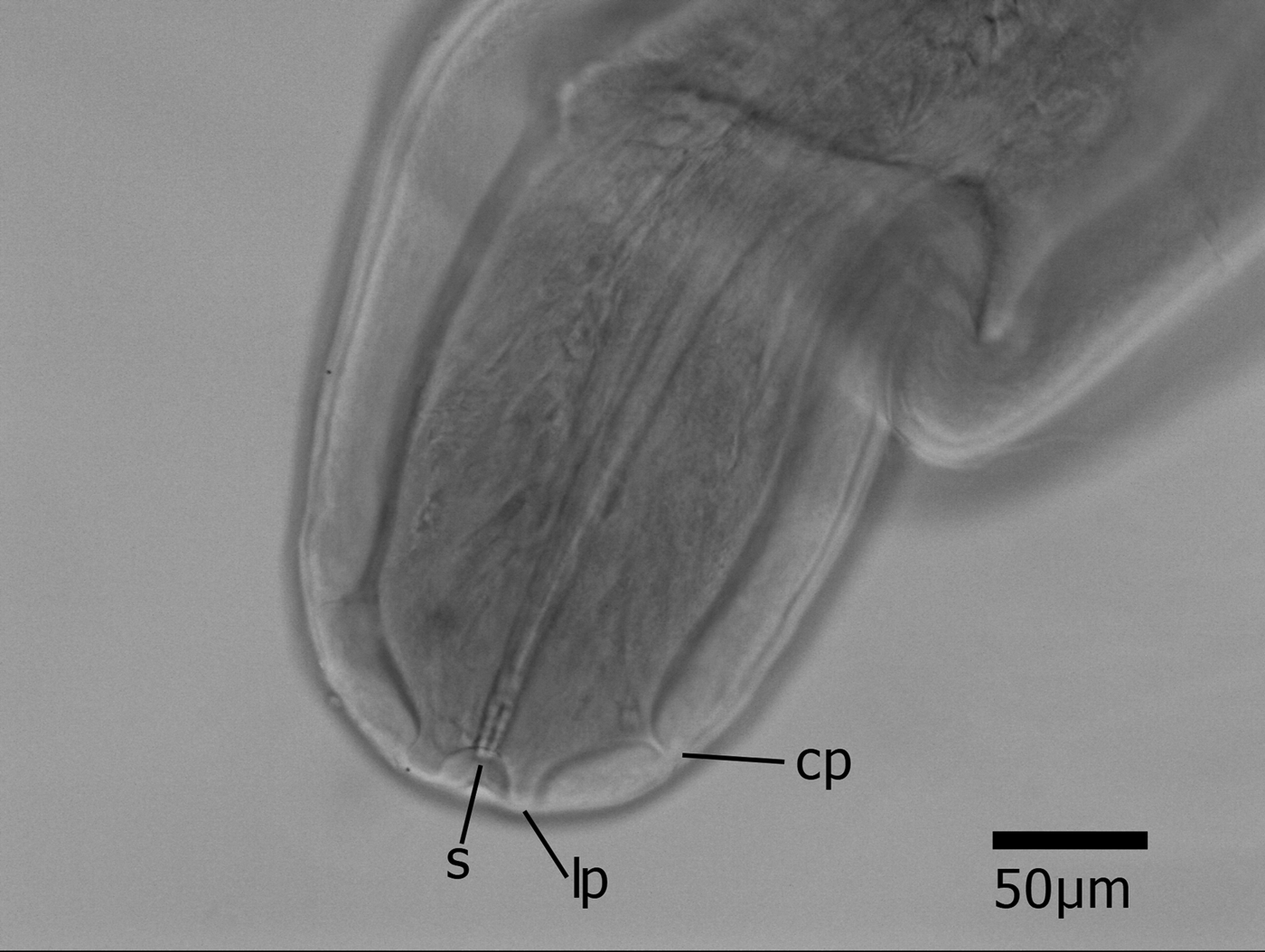
Fig. 3. Anterior portion of a juvenile mermithid dissected out of a Bombus impatiens worker collected in Hockessin, Delaware, USA, with labelled features. s = stoma, lp = labial papilla, cp = cephalic papilla, terminology after Presswell et al. Reference Presswell, Evans, Poulin and Jorge2015. Scale bar = 50 µm.
Although there are not enough data to identify the three mermithids found at the Arkansas site to genus or species, their similarity to another bumble-bee inhabiting mermithid from Japan is of interest. Based on both our data (Figs 1, 2) and that of Kubo et al. (Reference Kubo, Ugajin and Ono2016), these mermithids are clearly distinct from M. nigrescens and Phermermis sp., the only mermithids identified from bumble bee hosts thus far (this work; Rao et al. Reference Rao, Poinar and Henley2017). The striking genetic similarity among mermithid sequences from both bumble bee and grasshopper hosts collected in Australia, Japan, and the USA (Fig. 1) warrants some speculation on the geographic origin of these parasites and their true host range. The nematodes found in the Arkansas bumble bees could either be native to the region and previously unrecorded as parasites of native bumble bees, or they could have been transported into Arkansas where they encountered a new, viable host in the native bumble bee fauna there. Mermithids have been recorded invading new areas via agricultural trade, as seen with the introduction of M. nigrescens along with their European earwig hosts into Tasmania and New Zealand (Presswell et al. Reference Presswell, Evans, Poulin and Jorge2015). In this case, however, both natural hosts and their mermithid parasites were introduced together. Bombus terrestris has been exported from Europe to both Japan and Tasmania as a commercial pollinator, but not to the USA. As there has not been any formal trade in bumble bees between the USA and Japan or Australia, these mermithids were not likely to have been introduced to or from North American bumble bees. Trade in other bees, particularly honey bees, has occurred with the USA importing bees from Australia as recently as 2010 (Code of Federal Regulations (CFR) 7, Subpart B§322.4) however a 2007 federal order halted that exchange. The mermithid could, theoretically have originated in another exotic host and jumped to bumble bees as a novel host group. Without more data on the identity, distribution and host range of these parasites, any consideration of these scenarios is pure speculation. Whether or not these Arkansan and Japanese mermithids represent a distinct group of bumble-bee-infesting nematodes or another case of an extended host range as seen in M. nigrescens remains to be seen.
Mermithids can be effective regulators of host population densities and have often been targeted as biocontrol organisms for pestiferous hosts (Welch, Reference Welch1965; Mongkolkiti and Hosford, Reference Mongkolkiti and Hosford1971). Unlike pests, bumble bees are important pollinators of both natural and agricultural systems, and parasites that might influence population numbers can be of great concern. With so few records, it is difficult to imagine that mermithid parasitism could heavily impact bumble bee populations; however, the concentration of specimens found at the Arkansas site (3% of bees examined) does suggest that under some circumstances, mermithid parasitism might impact bumble bee populations at a local level. Interestingly, there is a single record of another unidentified nematode infecting an unusual host [armyworm, Lepidoptera: Noctuidae: Mythimna unipuncta (reported as Pseudoletia unipuncta)] from another agricultural site near (<50 km) our sampling site (Steinkraus et al. Reference Steinkraus, Mueller and Humber1993). Here, five of 37 sampled caterpillars were killed by a mermithid resembling M. nigrescens upon exit, but the authors were unable to identify the nematode. Gravid mermithids require moist environments to oviposit, and at least one study has seen an increased prevalence of mermithid parasitism in irrigated agricultural systems. Capinera (Reference Capinera1987) found that 50% of 204 grasshoppers were infected at irrigated sites, compared with 0% of 301 grasshoppers collected from non-irrigated natural areas. It is possible that the higher rate of parasitism observed at the Arkansas site was driven by the greater availability of water due to irrigation at this site. However, during this study 1399 bumble bees were collected at 18 other irrigated sites that did not exhibit mermithid parasitism, so this is unlikely to be a generalized pattern.
In a single study and with only six records, we have doubled the number of known occurrences of mermithid parasitism of bumble bee hosts. Many questions remain about the nature of this rare phenomenon: Who are the mermithids found parasitizing bees in Arkansas and Japan? Are there undescribed mermithids that target bumble bee hosts? How is infection achieved? The lack of distinguishing morphological features in the juveniles found during host dissection complicates our understanding of the natural history of mermithid infections in bees, and with so few occurrences and no exterior indication of parasitism, the likelihood of encountering adult mermithids emerging from their bumble bee hosts is very low. Luckily, the availability of genetic analysis offers insight into these rare occurrences, which will improve as data accumulate.
Acknowledgements
The authors wish to thank Clinton Trammell and Allen Szalanski for sampling Arkansas bumble bees and an anonymous reviewer for helpful suggestions that improved this manuscript.
Financial support
This work was supported by an award from the USDA-APHIS-PPQ Farm Bill (PN 1S.0521.00).






