Published online by Cambridge University Press: 02 December 2004
Antigenic variation of the intestinal protozoan parasite Giardia lamblia is caused by an exchange of the parasite's variant surface protein (VSP) coat. Many investigations on antigenic variation were performed with G. lamblia clone GS/M-83-H7 which produces surface antigen VSP H7. To generate novel information on giardial vsp gene transcription, vsp RNA levels were assessed by quantitative reverse transcription-(RT)-PCR in both axenic VSP H7-type trophozoites and subvariants obtained after negative selection of GS/M-83-H7 trophozoites by treatment with a cytotoxic, VSP H7-specific monoclonal antibody. Our investigation was not restricted to the assessment of the sense vsp transcript levels but also included an approach aimed at the detection of complementary antisense vsp transcripts within the two trophozoite populations. We found that sense vsp H7 RNA predominated in VSP H7-type trophozoites while sense RNA from only one (vsp IVg) of 8 subvariant vsp genes totally analysed predominated in subvariant-type trophozoites. Interestingly, the two trophozoite populations exhibited a similar relative distribution regarding the vsp H7 and vsp IVg antisense RNA molecules. An analogous sense versus antisense RNA pattern was also observed when the transcripts of gene cwp 1 (encoding cyst wall protein 1) were investigated. Here, both types of RNA molecules only appeared after cwp 1 had been induced through in vitro encystation of the parasite. These findings for the first time demonstrated that giardial antisense RNA production did not occur in a constitutive manner but was directly linked to complementary sense RNA production after activation of the respective gene systems.
Giardia lamblia (Giardia duodenalis, Giardia intestinalis) is a zoonotic protozoan parasite which resides in the small intestine of human and various mammalian hosts. In humans, G. lamblia is a common cause of endemic and epidemic diarrhoea throughout the whole world. Transmission of the parasite from one to another host individual occurs through per-oral ingestion of cysts which, following excystation in the small intestine, release 2 trophozoites each. The life-cycle is completed when cysts are formed through encystation of proliferating trophozoites and subsequently excreted in the feces. Both, en- and excystation can also be triggered in vitro by applying in vitro growth conditions which – to a certain extent – simulate the intestinal (for encystation) and gastric (for excystation) milieu of the mammalian hosts.
As extensively assessed in the in vitro cultivation system, stage conversion is associated with induction of various genes, some of which are structurally involved in cyst formation of the parasite (Hehl et al. 2000; Sun et al. 2002; Davis-Hayman et al. 2003; Lujan & Touz, 2003; Marti et al. 2003). Here, particularly the cwp genes encoding the cyst wall proteins are massively upregulated during encystation. Previous studies on cyst wall proteins (CWP) 1 (Hehl et al. 2000) and 2 (Davis-Hayman et al. 2003) indicated that control of the corresponding genes, cwp 1 and cwp 2, occurs on the transcriptional level and involves cis-acting elements in the 5′ flanking sequence of the gene. Transcriptional activation of encystation-inducible genes involves a nuclear protein which is related to the Myb family transcription factors and initiates transcription by interacting with a specific binding site in the promoter region (Sun et al. 2002). As demonstrated for gene cwp 1, steady-state levels of cellular mRNA are modulated by a cis-element in 3′ untranslated region which seems to be crucial for the processing of the transcripts during the late stage of the encystation (Hehl et al. 2000).
In the past decade, G. lamblia has been especially investigated in terms of the parasite's ability to continuously change its surface antigen coat (Müller & Gottstein, 1998; Nash, 2002). These studies have revealed that antigenic variation is associated with a unique family of surface antigens, named VSP (variant surface protein). By using trophozoites of G. lamblia clone GS/M-83-H7 (expressing VSP H7) and the neonatal mouse model for experimental infections, we recently quantitatively assessed the process of antigenic variation of the parasite on the transcriptional level (von Allmen et al. 2004). In this study, variant-specific regions identified on different GS/M-83-H7 vsp sequences served as targets for quantitative reverse transcription (RT)-PCR to monitor alterations in vsp mRNA levels during infection. Respective results demonstrated that antigen switching of both the duodenal trophozoite and the caecal cyst populations was associated with a strong reduction in vsp H7 mRNA levels. The same study also explored giardial variant-type formation and vsp mRNA levels after infection of mice with antigenically diversified (VSP H7-negative) GS/M-83-H7 cysts. This infection mode led to an antigenic reset of the parasite in that a VSP H7-negative inoculum ‘converted’ into a population of intestinal trophozoites that essentially consisted of the original VSP H7-type.
Antigenic variation and the VSPs have been extensively investigated on the molecular, biochemical and immunological level (Müller et al. 1998; Nash, 2002). Conversely, the genetic mechanisms controlling antigen switching are, as yet, poorly understood and only little information on this process is available in current literature. Nash et al. (2001) demonstrated that the shift of VSP expression coincides with transient appearence of few trophozoites which simultaneously express at least two VSPs. Furthermore, preliminary data suggested that VSP expression may be regulated at the transcriptional level (Nash & Mowatt, 1992). However, another study tackling the genome organization of a novel family of cysteine-rich proteins (CRP65, CRP136) in G. lamblia may allow the consideration of a gene rearrangement effect as a possible mechanism responsible for antigen switching of the parasite (Cheng, Upcroft & Upcroft, 1996; Upcroft et al. 1997). Since Elmendorf, Singer & Nash (2001) recently found that G. lamblia contains sterile antisense vsp RNA, reverse RNA transcription, and more specifically, RNA interference (RNAi) (Tijsterman & Plasterk, 2004) is currently also discussed as a potential mechanism that might be involved in vsp gene regulation (Ullu, Tschudi & Chkrabortx, 2004). In this context, the review of Ullu et al. (2004) refered to as yet unpublished findings from H. Lujan and colleagues, which suggested that RNAi controls expression of the vsp genes and that an RNA-dependent RNA polymerase is involved in restricting expression of the vsp gene repertoire to a single gene at one time. Interestingly, database mining provided clear evidence that the genome of G. lamblia encodes key functions of the RNAi pathway (Ullu et al. 2004). The functionality of this pathway in G. lamblia has already been experimentally proven in that transfection of the parasite with a vector carrying an antisense sequence of a target gene was demonstrated to downregulate corresponding gene expression (Touz et al. 2002). RNAi is now basically considered to be an ideal molecular biological tool to achieve targeted silencing of any gene function in various protozoan parasites, including G. lamblia (Ullu et al. 2004).
Elmendorf et al. (2001) found that G. lamblia trophozoites generate a substantial amount of antisense RNA molecules which correspond to approximately 20% of the total RNA content. Antisense RNA production in G. lamblia is highly diversified and involves vsp genes as well as many other constitutive and regulated genes, which e.g. are (possibly) relevant to developmental stage conversion, cell division, and gene transcription. The antisense transcripts are polyadenylated and they essentially consist of sterile RNA molecules that do not participate in protein synthesis of the parasite. The study described by Elmendorf et al. (2001) clearly demonstrated that antisense RNA production represents a striking molecular biological feature of G. lamblia. However, this investigation was not focused on the possible gene-regulatory function of antisense transcripts and a preliminary Northern blot analysis included in the respective experimentation was not able to reveal a participation of these RNA molecules in developmental gene regulation.
The major aim of the present study was to evaluate the possibility of a bidirectional vsp gene transcription in association with in vitro antigenic variation of G. lamblia clone GS/M-83-H7. For this purpose, VSP H7-type and subvariant-type GS/M-83-H7 trophozoite cultures were generated and subsequently analysed by quantitative reverse RT-PCR for their relative production of sense and antisense vsp H7 or subvariant vsp RNA, respectively. The quantitative RT-PCR approach was also applied for investigating the giardial sense and antisense transcription in the encystation-inducible gene system cwp 1 encoding the major cyst component CWP 1 of the parasite.
The origin, axenization and cloning of G. lamblia clone GS/M-83-H7 has been described by Aggarwal et al. (1989). This clone expresses a major 72 kDa antigen (VSP H7) on its surface which is recognized by MAb G10/4. Trophozoites from G. lamblia clone GS/M-83-H7 were cultivated in modified TYI-S-33 medium with antibiotics as previously described (Keister, 1983). In vitro growth conditions for induction of encystation of the parasite were described by Kane et al. (1991).
Expression of the major surface antigen VSP H7 in in vitro cultivated GS/M-83-H7 trophozoites was tested by immunofluorescence using VSP H7-specific MAb G10/4 as described (Gottstein et al. 1990). CWP 1 synthesis in encysting trophozoites was detected by incubation of cells with 1[ratio ]20 Texas Red-conjugated mouse MAb A300-TR (Waterborne, New Orleans, LA, USA), an anti-CWP 1 antibody. For staining of nuclei, trophozoites were incubated for 3 min in the presence of 1[ratio ]300-diluted double strand (ds) DNA-specific fluorescent dye Hoechst 33258 (Sigma, Steinheim, Germany) stock solution (1 mg/ml in distilled H2O) and subsequently washed twice in PBS and once in distilled H2O.
Specimens were inspected on a Nikon Eclipse E800 digital confocal fluorescence microscope at a 600-fold magnification. Processing of images was performed using the Openlab 3.11 software (Improvision, Heidelberg, Germany). Percentages of VSP H7- or CWP 1-positive parasites were determined by inspection of approximately 400 parasites.
The procedure used for cytotoxic (VSP H7-specific) MAb G10/4-mediated antigen switching of in vitro cultivated G. lamblia clone GS/M-83-H7 trophozoites from a VSP H7-positive to a – negative (subvariant-type) parasite population was previously described (Bienz et al. 2001). After confirming by immunofluorescence that the resulting MAb G10/4-resistant parasite population was essentially VSP H7-negative (>99%), about 106 of these negatively-selected variant trophozoites were resuspended in 100 μl of lysis buffer-β-ME mixture from the StrataPrep™ Total RNA Microprep Kit (Stratagene, La Jolla, CA, USA). In parallel, an analogous cellular lysate was prepared from about 106 VSP H7-type trophozoites that had not been treated with MAb G10/4. Both cellular lysates were then further processed for total RNA extraction as described above. Finally, total RNA preparations were solubilized in 30 μl of elution buffer and stored at −80 °C until further used.
Complementary DNA (cDNA) was synthesized by reverse transcription from total RNA, prepared from VSP H7-type and subvariant-type trophozoites by using 17·5 μM of degenerate forward vsp primer 1 (5′-ACIAAYGGIGTITGYACIGC-3; positions I=deoxyinosine, Y=C,T; Invitrogen, Basle, Switzerland) (reverse transcription of antisense vsp RNA) or reverse vsp primer (5′-GAACCACCAGCAGAGGAA-3′) (reverse transcription of sense vsp RNA) (see Fig. 1) as well as M-MLV reverse transcriptase (Promega, Madison, WI, USA) and other components as instructed by the manufacturer of the reverse transcriptase. The forward vsp primer 1 covers a relatively conserved vsp region located between nucleotides (nt) 1147 and 1166 of gene vsp H7. The reverse vsp primer is complementary to nt 1636 to 1653 of the vsp H7 encoding part of the highly conserved transmembrane domain of the surface protein. The reverse transcription reaction for generating cwp 1 cDNA included forward cwp 1 primer 1 (5′-CACCTGGACTGCAACCAGCTG-3′) (reverse transcription of antisense cwp 1 RNA) and reverse cwp 1 primer (5′-AGTACTCTCCGCAGTCCGGATC-3′) (reverse transcription of sense cwp 1 RNA). The forward cwp 1 primer 1 corresponds to nt positions 6 to 26 and the reverse cwp 1 primer to nt positions 207 to 228 of a 3′ terminal GS/M-83-H7 cwp1 gene segment (GeneBank™ Accession number: AY676465). Reverse transcription of sense RNA from gene gdh (encoding glutamate dehydrogenase) was performed as previously described (von Allmen et al. 2004).

Fig. 1. Schematic illustration of the RT-PCR-based quantification of sense and antisense vsp RNA. First, sense and antisense RNA (within total RNA preparations) from VSP H7-type and subvariant-type G. lamblia clone GS/M-83-H7 trophozoites were reverse transcribed into cDNA using reverse vsp primer (synthesis of cDNA from sense vsp RNA) or forward vsp primer 1 (synthesis of cDNA from antisense RNA). The cDNAs were taken as template for quantitative PCRs which allowed specific amplification of different vsp cDNA molecules. Amplification of vsp cDNA was achieved by performing the PCR with forward vsp primers listed in Table 1 and the reverse vsp primer as indicated.
Quantitative RT-PCR was carried out on a LightCycler™ Instrument (Roche Diagnostics, Rotkreuz, Switzerland) by using SYBR™ Green I as a dsDNA-specific fluorescent dye and continuous fluorescence monitoring as described (Wittwer et al. 1997). Amplification reaction mixes for the vsp PCRs included forward primers specific for individual vsp genes (indicated in Fig. 1 as forward vsp primer 2 and listed in Table 1), and reverse vsp primer. For the cwp 1 PCRs, forward cwp 1 primer 2 (5′-GTCCCAGTTGGCCTTATGACTCT-3′, corresponding to nt positions 36 to 58 of 3′ terminal GS/M-83-H7 cwp 1 gene segment) and reverse cwp 1 primer (see above) were applied. Quantitative PCR was done with 4 μl of 1[ratio ]100-diluted cDNA using the Quanti Tect™ SYBR Green PCR Kit (Qiagen) in a 10 μl standard reaction containing a 0·5 μM concentation of forward and reverse primers (Invitrogen). All PCRs containing cDNA were performed in triplicates. Furthermore, a control PCR included RNA equivalents from samples that had not been reverse transcribed into cDNA (not shown) to confirm that no DNA was amplified from any residual genomic DNA that might have resisted DNase I digestion (see above). PCR was started by initiating the ‘Hot-Start’ Taq DNA-polymerase reaction at 95 °C (15 min). Subsequent DNA amplification was done in 50 cycles including denaturation (94 °C, 15 s), annealing (48 °C, 30 s), and extension (72 °C, 30 s); temperature transition rates in all cycle steps were 20 °C/s. Fluorescence was measured at 82 °C during the temperature shift after each annealing phase in the ‘single’ mode with the channel setting F1. Fluorescence signals from the amplification products were quantitatively assessed by applying the standard software (version 3.5.3) of the LightCycler™ Instrument. Quantification of PCR products was performed during the log phase of the reaction and was achieved by using the secondary derivative maximum mode for plotting of the fluorescence signals versus the cycle numbers. As external standards, serial 10-fold dilutions (4 μl aliquots) of previously generated amplification products from the different target sequences were included in the quantitative PCR analyses. The standard curves from the different assays (vsp- and cwp 1-PCRs) were run in duplicates and contained 4 log units within a linear range that essentially covered the maximal and minimal concentrations of the vsp- and cwp1-cDNA sequences within the different samples. Linearity among the standard reactions was reflected by the correlation coefficient which was calculated by the computer program to be extremely high (between 0·99 and 1·0) for all PCR assays applied.

Lack of PCR-inhibitory effects and overall comparability of the different standard and sample reactions were evidenced by the quasi-identity of the slopes from the amplification plots (monitoring amplification rates).
Control experiments for identification of PCR products included a DNA melting point analysis (Ririe, Rasmussen & Wittwer, 1997) (not shown). The DNA melting profile assay was run after the final PCR cycle by gradually increasing the temperature to 95 °C at a transition rate of 0·1 °C/s with continuous acquisition (determination of the melting profile by measuring loss of fluorescence). Data from the DNA melting profile assay were processed by using the standard software (version 3.5.3). In all PCR tests performed, identical melting temperatures of amplicons from samples and respective standards indicated identical and specific amplification reactions without unwanted primer-dimer formation (not shown). This overall identity and specificity of reactions was confirmed by subsequent agarose gel electrophoresis (2% gels) (Sambrook & Russel, 2001) which monitored the PCR products as single DNA bands of expected sizes (not shown). In the cases of the PCRs amplifying segments of vsp H7, vsp IVg, and cwp 1, nucleotide sequence authenticity of the amplification products was confirmed by automated sequencing through a commercial sequencing service (Microsynth, Balgach, Switzerland).
In order to compensate for variations in input, RNA amounts and efficiencies of reverse transcription, sense RNA of the ‘housekeeping’ gene gdh was quantitated as recently described (von Allmen et al. 2004). Respective mean values from triplicate determinations were taken for the calculation of the relative sense and antisense vsp RNA levels (vsp RNA level/gdh RNA level) or sense and antisense cwp 1 RNA levels (cwp 1 RNA level/gdh RNA level), respectively.
Alignment of the vsp nucleotide sequences derived from a previous study (Bienz et al. 2001) and accessible in GenBank™ (Accession numbers, see Table 1), was done using the MultAlin™ and the ESPript1.9™ computer software, available at the ExPASy™ Molecular Biology Server.
To select for new variants within G. lamblia clone GS/M-83-H7 originally consisting of approximately 95% VSP H7-type (MAb G10/4-positive) trophozoites (not shown), cultivated parasites were treated twice with VSP H7-specific, cytotoxic MAb G10/4. The efficacy of this selection was assessed by immunofluorescence which demonstrated that the treated culture contained more than 99% VSP H7-negative (MAb G10/4-negative) trophozoites (not shown).
For RT-PCR-based quantitative assessment of sense and antisense vsp RNA levels in VSP H7-type and subvariant-type GS/M-83-H7 trophozoites, total RNA was prepared and then used as a templete for synthesis of cDNA representing either sense or antisense vsp RNA (Fig. 1). The primers for reverse transcription were designed on the basis of previously generated GS/M-83-H7 vsp gene sequence information and allowed synthesis of cDNAs from sense and antisense RNA templates covering the 3′ terminal region of the individual vsp genes (Fig. 1). For specific reverse transcription of sense RNA, a primer (reverse vsp primer) was used that was complementary to nts 1636 to 1653 close to the 3′ terminus of the vsp H7 encoding sequence. This sequence encodes part of the transmembrane domain of the surface protein and had previously been revealed to be highly conserved within the vsp genes of clone GS/M-83-H7 and other G. lamblia isolates (Bienz et al. 2001). For specific reverse transcription of antisense vsp RNA, we applied a previously described degenerate primer (forward vsp primer 1) corresponding to nt positions 1147 to 1166 of vsp H7 and covering a relatively conserved region of GS/M-83-H7 vsp genes (von Allmen et al. 2004).
To establish a PCR system for quantitative and differential amplification of cDNA synthesized from sense and antisense vsp RNA, a nucleotide sequence alignment was performed with 3′ regions of gene vsp H7 and subvariant vsp genes that had previously been identified in VSP H7-negative trophozoite cultures of G. lamblia clone GS/M-83-H7. This comparative sequence analysis led to the identification of a relatively variable vsp region which allowed to design forward primers (forward vsp primer 2) that were specific for individual vsp genes (Table 1). Each of these forward vsp primers in combination with a reverse primer (reverse vsp primer) targeted to a conserved vsp stretch close to the 3′ terminus (Fig. 1) were used for PCR-based specific quantification of vsp cDNA molecules generated from VSP H7-type and subvariant-type trophozoite cultures outlined above.
As can be seen in Fig. 2, sense vsp H7 RNA predominated in VSP H7-type trophozoites. Subvariant-type trophozoites, however, did not produce significant amounts of sense vsp H7 RNA but exhibited predominance of subvariant sense vsp IVg RNA. The low, but clearly detectable, amount of sense vsp IVg RNA in the VSP H7-posititive culture was indicative for the existence of a minor subvariant-type population representing approximately 5% of the total trophozoite population (see above). Taken together, these finding were in concordance with results from the immunofluoresence analysis (not shown) indicating that in vitro antigen switching ‘converted’ an essentially VSP H7-positive into a VSP H7-negative (subvariant-type) trophozoite culture. Furthermore, data evidenced at least on the transcriptional level that the in vitro-switched trophozoite culture represented a relatively homogeneous subvariant-type population which preferentially expressed vsp IVg.

Fig. 2. Quantitative RT-PCR-based assessment of relative sense (black bars) and antisense (grey bars) vsp RNA levels (vsp RNA level/gdh RNA level) in VSP H7-type (A) and subvariant-type (B) trophozoite cultures from Giardia lamblia clone GS/M-83-H7. The relative levels of mRNA from vsp genes H7 and IVa-IVh (see Table 1) represent mean values (plus standard deviations) from triplicate determinations.
RT-PCR-based assessment of the antisense vsp RNA contents in both VSP H7-type and subvariant-type trophozoites provided analogous results although cellular concentrations of these molecules were lower than those of corresponding sense RNAs. Antisense vsp H7 RNA predominated in VSP H7-positive trophozoites and antisense vsp IVg RNA in -negative trophozoites. Sequence analysis revealed that sense and antisense RNAs from these two genes were fully complementary, and the corresponding PCR products exhibited the expected sizes of 377 base pairs (bp) (sense and antisense vsp H7 RNA) and 361 bp (sense and antisense vsp IVg RNA), respectively.
To test whether the simultaneous production of sense and complementary antisense RNA is unique to the vsp genes or rather a more general phenomenon of giardial gene transcription, we tested an encystation-inducible gene system, cwp 1, regarding its sense versus antisense transcription profile. Gene cwp 1 encodes cyst wall protein (CWP) 1 which represents a major component of the giardial cyst (Lujan et al. 1995; Mowatt et al. 1995). As assessed by immunofluorescence assay using an antibody against CWP 1 for immunostaining of VSP H7-positive GS/M-83-H7 trophozoites, in vitro stage conversion resulted in approximately 12% CWP 1-positive parasites after 24 h growth in encystation medium (not shown). For RT-PCR-based quantitative determination of sense and antisense cwp 1 RNA levels in trophozoites, corresponding cDNA was generated from parasites sampled at 0, 6, 12 and 24 h of the encystation period. The RT-PCR approach monitoring relative sense and antisense cwp 1 levels in encysting trophozoites was designed in analogy to the procedure applied for the assessment of sense and antisense vsp RNA (see above). For specific reverse transcription of sense RNA, reverse cwp 1 primer was used that was complementary to nts 207 to 228 of a 3′ terminal GS/M-83-H7 cwp 1 gene segment. For specific reverse transcription of antisense vsp RNA, we applied forward cwp 1 primer 2 corresponding to nt positions 6 to 26 of the same gene segment.
RT-PCR-based quantification of cwp 1 RNA revealed that in vitro encystation of clone GS/M-83-H7 induced simultaneous synthesis of sense and, to a lower extent, antisense cwp 1 RNA. While sense cwp 1 RNA levels increased for at least 12 h during encystation, antisense cwp 1 RNA already reached its maximal level at 6 h. At 24 h, a strong reduction in the cellular contents of both sense and antisense cwp 1 RNA was detected. Sequence analysis demonstrated full complementarity between the sense and antisense cwp 1 RNAs, and sizes of the cwp 1 amplicons were determined to be 192 bp (not shown).
The discovery of giardial antisense vsp transcription has implications for the current thinking about the as yet poorly understood genetic mechanism controlling surface antigen alterations in G. lamblia (Ullu et al. 2004). Considering still unpublished data presented by H. Lujan and colleagues at the 2002 Molecular Parasitology Meeting in Woods Hole (MA, USA), particularly RNA interference (RNAi) (Tijsterman & Plasterk, 2004) has to be discussed as a potential mechanism regulating giardial vsp gene activity. Such a mechanism could favour transcription of a primary vsp gene in that all other genes from the vsp repertoire are silenced by overproduction of complementary antisense vsp RNA molecules, and small (20–26 nucleotide pairs) interfering RNA (siRNA) (Tijsterman & Plasterk, 2004), respectively. According to this scenario, antigen switching could occur through a spontaneous, or controlled, mechanism which up-regulates antisense RNA from the primary vsp gene and down-regulates antisense RNA from a secondary vsp gene. In the present study, we demonstrated for the first time that in vitro antigen switching of G. lamblia is associated with alterations in both sense and antisense vsp RNA production. In our analysis, we found that sense and complementary antisense vsp RNA production in both VSP H7-type and subvariant-type trophozoites occurred in a simultaneous, and not a reciprocal, manner. This finding may be taken as an argument against the abovementioned speculation suggesting that RNAi could act as a mechanism involved in regulation of vsp gene expression during antigen switching of the parasite. The solution of this problem relies on the determination of the relative vsp H7 versus vsp IVg RNA composition (see above) on the level of the extremely fragmented siRNA sequences. Respective experimentation will be rather difficult because the standard RNA isolation protocols were not designed to efficiently recover small RNA pieces. Furthermore, methods for detection of small RNA molecules are supposed to be relatively insensitive.
As outlined in the Materials and Methods section, the individual vsp primers used for the quantitative RT-PCRs target to a sequence stretch that is located in the 3′ terminal region of the corresponding vsp genes. In this region, the vsp H7 sequence is indistinguishable from the sequence of another vsp gene (vsp H7-1) previously identified in G. lamblia clone GS/M-83-H7 (Nash, Conrad & Mowatt, 1995). Accordingly, our present RT-PCR approach was not suitable to discriminate between transcripts from these two closely related genes. However, since vsp H7-1 had previously been shown to be silent in clone GS/M-83-H7 (Nash et al. 1995), we concluded that our approach must have exclusively monitored transcripts that originated from vsp H7.
By analysing cwp 1 transcription of G. lamblia clone GS/M-83-H7, we detected maximal levels of sense and antisense RNA between 6 h (antisense RNA) and 12 h (sense RNA) after encystation had been induced (see Fig. 3). Interestingly, the cwp 1 gene activation resulted in sense/antisense co-expression patterns that resembled those observed for transcription of the vsp genes. Based on this observation, a participation of RNAi in control of the cwp 1 gene activity can still not be excluded but seems to be rather unlikely (see also above). This assumption is compatible with data from previous studies which demonstrated that the up-shift of the cwp 1 mRNA levels during early encystation is the consequence of the induction of the transcriptional activity of a classical promoter (Hehl et al. 2000; Sun et al. 2002).

Fig. 3. Quantitative RT-PCR-based assessment of relative sense (black bars) and antisense (grey bars) cwp 1 RNA levels (cwp 1 RNA level/gdh RNA level) in Giardia lamblia clone GS/M-83-H7 trophozoites sampled at 0, 6, 12, and 24 h after induction of encystation. The relative levels of mRNA from cwp 1 gene represent mean values (plus standard deviations) from triplicate determinations.
In agreement with previous data (Hehl et al. 2000), we found that late-stage encystation was associated with a dramatic decrease of the intracellular cwp 1 mRNA concentration. In addition, we observed that the antisense cwp 1 RNA levels started to decrease earlier (before 12 h) during encystation than complementary sense RNA levels (between 12 and 24 h). According to data from Hehl et al. (2000), the reduction in the sense cwp 1 RNA concentration may be due to a post-transcriptional RNA degradation process which is modulated by a cis-acting element in the 3′ untranslated region of the RNA molecules. Since antisense cwp 1 RNA is supposed to lack an equivalent regulatory element, it may even undergo faster degradation than sense cwp 1 RNA. Such a differential RNA degradation process may have caused the observed bias in the relative sense and antisense cwp 1 RNA levels at advanced encystation stages of the parasite.
As assessed by determination of the nucleotide sequences of RT-PCR amplification products, sense and antisense RNAs from vsp H7, vsp IVg, and cwp 1 exhibited complete sequence complementarity. This suggested that the individual sense/antisense RNA pairs had been transcribed in cis from opposing DNA strands at the same genetic locus. A significant amount of antisense RNA molecules from many different genes including vsp genes had already been detected in G. lamblia clones GS/M-83-H7 and WB/1267, respectively (Elmendorf et al. 2001). In this previous investigation, the overall content of sense RNA was estimated to be approximately four times higher than the content of antisense RNA. Based on this finding, the authors concluded that this comparably low synthesis of antisense RNA was eventually driven by frequently occurring AT-rich sequences acting as cryptic promoters on the antisense DNA strand of the respective gene loci. This conclusion is compatible with our present data demonstrating that giardial antisense RNA contents were substantial, but unambigously lower than those of simultaneously produced sense RNA molecules.
It is certainly feasible that antisense RNA production in G. lamblia is only a tribute to the simplicity of this organism which perhaps cannot control ‘unspecific’ antisense gene transcription eventually occurring as a side-effect of DNA unwinding during regular gene transcription. Nevertheless, it has to be kept in mind that the parasite has evidently maintained abundant antisense RNA production during evolution although a high energy metabolism is needed for running this process. Accordingly, further studies will have to address the question whether antisense RNA production in G. lamblia is biologically significant in that it is relevant for the cell biology or even pathogenicity of the parasite.
We acknowledge A. Hehl, M. Marti (Institute of Parasitology, Zürich, Switzerland), N. Keller (Institute of Parasitology, Berne, Switzerland) and M. E. Weiland (Karolinska Institute, Stockholm, Sweden) for technical support, and T. E. Nash (NIH, Bethesda, Maryland, USA) for his gift of MAb G10/4 and G. lamblia clone GS/M-83-H7. This work was financed through a grant obtained from the Swiss National Science Foundation (No. 31-066795.01).
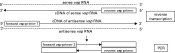
Fig. 1. Schematic illustration of the RT-PCR-based quantification of sense and antisense vsp RNA. First, sense and antisense RNA (within total RNA preparations) from VSP H7-type and subvariant-type G. lamblia clone GS/M-83-H7 trophozoites were reverse transcribed into cDNA using reverse vsp primer (synthesis of cDNA from sense vsp RNA) or forward vsp primer 1 (synthesis of cDNA from antisense RNA). The cDNAs were taken as template for quantitative PCRs which allowed specific amplification of different vsp cDNA molecules. Amplification of vsp cDNA was achieved by performing the PCR with forward vsp primers listed in Table 1 and the reverse vsp primer as indicated.

Table 1. Forward vsp primers used for quantification of Giardia lamblia GS/M-83-H7 vsp transcripts by RT-PCR

Fig. 2. Quantitative RT-PCR-based assessment of relative sense (black bars) and antisense (grey bars) vsp RNA levels (vsp RNA level/gdh RNA level) in VSP H7-type (A) and subvariant-type (B) trophozoite cultures from Giardia lamblia clone GS/M-83-H7. The relative levels of mRNA from vsp genes H7 and IVa-IVh (see Table 1) represent mean values (plus standard deviations) from triplicate determinations.
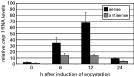
Fig. 3. Quantitative RT-PCR-based assessment of relative sense (black bars) and antisense (grey bars) cwp 1 RNA levels (cwp 1 RNA level/gdh RNA level) in Giardia lamblia clone GS/M-83-H7 trophozoites sampled at 0, 6, 12, and 24 h after induction of encystation. The relative levels of mRNA from cwp 1 gene represent mean values (plus standard deviations) from triplicate determinations.