INTRODUCTION
Phosphoenolpyruvate carboxykinase (PEPCK, EC 4.1.1.32) is a CO2-fixing enzyme in the glycolytic pathway of parasitic helminths including Raillietina echinobothrida, a cestode parasite of domestic fowl (Behm and Bryant, Reference Behm and Bryant1975a; Rathaur et al. Reference Rathaur, Anwar, Saxena and Ghatak1982; Fukumoto, Reference Fukumoto1985; Smyth and McManus, Reference Smyth and McManus1989; Klein et al. Reference Klein, Winterrowd, Hatzenbuhler, Shea, Favreau, Nulf and Geary1992; Tandon and Das, Reference Tandon and Das2007), whereas in vertebrates its main role is in gluconeogenesis (Colombo et al. Reference Colombo, Carlson and Lardy1978; Nelson and Cox, Reference Nelson and Cox2008). The possible role of PEPCK in glycolysis of cestodes is depicted in Fig. 1 (excerpted from Smyth and McManus, Reference Smyth and McManus1989). Involvement of PEPCK in glycolysis has also been shown in the protoscolices of the horse and sheep strains of Echinococcus granulosus and the closely related E. multilocularis (McManus and Smyth, Reference McManus and Smyth1982). Also, in the adult liver fluke Fasciola hepatica and the nematode Dipetalonema viteae, PEPCK takes part in degradation of glucose rather than in gluconeogenesis (Christie et al. Reference Christie, Powell, Stables and Watt1987; Tielens et al. Reference Tielens, Van Den Heuvel and Van Den Bergh1987) although, in contrast, the involvement of PEPCK in glycolysis was not conclusive in the blood fluke, Schistosoma mansoni (Tielens et al. Reference Tielens, Van Der Meer, Van Den Heuvel and Van Den Bergh1991). PEPCK was first identified in the chicken liver where it catalyses the decarboxylation of oxaloacetate (Utter and Kurahashi, Reference Utter and Kurahashi1954), and since then the enzyme has been purified and characterized from various vertebrate sources (Ballard and Hanson, Reference Ballard and Hanson1969; Noce and Utter, Reference Noce and Utter1975; Hebda and Nowak, Reference Hebda and Nowak1982a).

Fig. 1. Schematic depiction showing the possible role of PEPCK (*) in glycolysis of cestodes (Smyth and McManus, Reference Smyth and McManus1989). HK, hexokinase; PEPCK, phosphoenolpyruvate carboxykinase; PK, pyruvate kinase; LDH, lactate dehydrogenase; cMDH, cytosolic malate dehydrogenase; cME, cytosolic malic enzyme; mME, mitochondrial malic enzyme; FUM, fumarase; PEP, phosphoenolpyruvate; OAA, oxaloacetate.
PEPCK plays a significant role in carbohydrate metabolism of parasitic helminths (Bryant, Reference Bryant1975); and the PEPCK/PK branch point is the first divergent step between metabolic pathways of helminth parasites and their vertebrate host, therefore, PEPCK has been considered an attractive anthelmintic drug target (Reynolds, Reference Reynolds1980). In view of the functional differences between the enzyme of the vertebrate and helminths, the PEPCK/PK branch point has been investigated in various helminths by several authors (Prichard, Reference Prichard1976; Moon et al. Reference Moon, Mustafa, Hulbert, Podesta and Metrrik1977; Hoffman et al. Reference Hoffmann, Mustafa and Jorgensen1979). However, PEPCK has been purified and characterized from only a few helminth species, such as: Moniezia expansa (partial purification), a cestode parasite of sheep; Hymenolepis diminuta, the rodent dwarf tapeworm; Fasciola hepatica; Ascaris suum; and Moniliformis dubius (Behm and Bryant, Reference Behm and Bryant1975b, Reference Behm and Bryant1982; Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Wilkes et al. Reference Wilkes, Cornish and Mettrick1981, Reference Wilkes, Cornish and Mettrick1982; Rohrer et al. Reference Rohrer, Saz and Nowak1986).
From our earlier studies, it has been shown that phytochemicals isolated from the root-peel of Flemingia vestita and rhizome of Stephania glabra have vermifugal action against several intestinal cestodes and trematodes (Tandon et al. Reference Tandon, Pal, Roy, Rao and Reddy1997, Reference Tandon, Lyndem, Kar, Pal, Das and Rao2004). Further, it was shown that genistein, the phytoestrogen, present in the root-peel extract of F. vestita (Rao and Reddy, Reference Rao and Reddy1991), affects carbohydrate metabolism in the cestode, R. echinobothrida, when treated in vitro; the PEPCK activity, in particular, was found to be increased significantly (Das et al. Reference Das, Tandon and Saha2004a, Reference Das, Tandon and Sahab). With the aim of ascertaining the role of PEPCK as a likely target site of anthelmintic drug action, the intent of the present study was to purify and characterize the enzyme from the parasite. In this study, PEPCK from R. echinobothrida was purified to apparent homogeneity and characterized biochemically. In order to find out the possible modulators for the enzyme, ethanolic extract and phytochemicals derived from F. vestita and S. glabra were tested on the purified parasite PEPCK.
MATERIALS AND METHODS
Materials
Ammonium sulfate {(NH4)2SO4}, sodium dodecyl sulfate (SDS), acrylamide, bis-acrylamide, dithiothreitol (DTT), phenylmethanesulfonyl fluoride (PMSF), ethylenediaminetetraacetate (EDTA), 2-mercaptoethanol, phosphoenolpyruvate (PEP), hydroxyethyl piperazineethanesulfonic (HEPES) buffer, bromophenol blue, Coomassie Blue R 250, and genistein (G6649) were purchased from Sigma (St Louis, USA). DEAE-Sephacel (Code no. 17-0500-01), Blue Sepharose CL 6B affinity (Code no. 17-0830-01), and gel filtration calibration kit (Code no. 28-4038-42) were from GE Healthcare. All enzymes and co-enzymes were supplied by either Sigma (St Louis, USA) or Roche (Germany). Praziquantel (PZQ, Bayer, India), NaHCO3, divalent ions, nucleotides, and other general chemicals were of analytical grade and procured from local sources.
Isolation of phytochemicals
Genistein and other phytochemicals from the ethanolic crude root-peel extract of F. vestita were obtained as reported earlier by Tandon et al. (Reference Tandon, Pal, Roy, Rao and Reddy1997). Tetrahydropalmatine (THP) was also isolated from the ethanolic rhizome extract of S. glabra using flash chromatography (Persona Chromatograph, Jones Chromatography) at chloroform:ethanol/9:1 eluting solvent as described in detail by Das et al. (Reference Das, Tandon, Lyndem, Gray and Ferro2009). The ethanolic crude extract as well as the isolated phytochemicals was tested on purified R. echinobothrida PEPCK. To compare, synthetic genistein (Sigma) and PZQ, a common cestodicide, was also tested on the purified enzyme.
Purification of PEPCK from R. echinobothrida
PEPCK from the parasite was purified biochemically following the modified procedure as described by Brinkworth et al. (Reference Brinkworth, Hanson, Fullin and Schramm1981). All steps in the purification procedure were carried out at 4 °C.
Preparation of supernatant and ultracentrifugation
Live cestode parasites were collected from the intestines of freshly slaughtered domestic fowl (Gallus domesticus) and washed in 0·9% phosphate-buffered saline (PBS, pH 7·2). R. echinobothrida Megnin 1881 was identified on the basis of the scolex morphology (presence of a heavily armed rostellum with 2 rows of hooklets and 4 circular suckers) (Soulsby, Reference Soulsby1982), and immediately frozen in liquid N2. Within 24 h, a 20% (w/v) homogenate was made from the frozen parasites (∼5 g) in Tris-HCl homogenate buffer (50 mM, pH 7·4) containing 0·25 M sucrose, 2 mM EDTA, 1 mM DTT and 0·01 mM PMSF. The crude homogenate was first sonicated for 10 cycles (30 s pulse at 25W with 30 s interval) at 4 °C using an Ultrasonic processor (Heat System Inc., Model-XL-2020) and then spun at 3000 rpm for 10 min and 10 000 rpm for 30 min to remove the macromolecules. Subsequently, the homogenate was subcellularly fractionated at 40 000 rpm for 60 min to collect the soluble fraction using a Beckman Optima LE 80 K.
Ammonium sulfate fractionation
The soluble fraction from ultracentrifugation was subjected to ammonium sulfate precipitation; the precipitate (40–70%) which contained most of the PEPCK activity was collected and dissolved in a minimal volume (∼2 ml) of Tris-HCl buffer (50 mM, pH 7·4) containing 0·1 mM EDTA and 0·01 mM DTT. The resultant precipitate was dialysed overnight against 2 changes of 4 litres of Tris-HCl buffer (50 mM, pH 7·4) containing 2 mM EDTA and 1 mM DTT.
DEAE-Sephacel ion-exchange chromatography
The dialysed fraction (40–70%) from the ammonium sulfate precipitation was applied on to a DEAE-Sephacel (GE Healthcare, Code no. 17-0500-01) column (9·5 × 0·85 cm) that had been previously packed and equilibrated with Tris-HCl buffer (50 mM, pH 7·4) containing 0·1 mM EDTA and 0·01 mM DTT. After loading on to the top of the column, the latter was washed with the equilibrating buffer. Then, the enzyme was eluted with an elution buffer containing Tris-HCl (50 mM, pH 7·4), 2 mM EDTA, 1 mM DTT and a gradient of NaCl (0–200 mM) at a flow rate of 1 ml/min using ÄKTA Prime Chromatography system (UV detector and recorder (REC 112); GE Healthcare). The fractions containing more than 15 U of PEPCK activity were pooled and concentrated to about 2 ml by reverse osmosis using sucrose. The pool was dialysed overnight as described in the previous section.
Blue Sepharose CL 6B affinity chromatography
The dialysed fraction from the DEAE-Sephacel chromatography step was applied on to a Blue Sepharose CL 6B affinity column (Amersham Biosciences, Code no. 17-0830-01, 7·5 × 0·7 cm), which had previously been packed and equilibrated with Tris-HCl buffer (50 mM, pH 7·4) containing 0·1 mM EDTA and 0·01 mM DTT. The column was washed with 50 ml of the same buffer, and then the enzyme was eluted with an elution buffer (described previously) containing a gradient of NaCl (0–200 mM) at a flow rate of 1 ml/min. The fractions containing PEPCK activity were pooled together and characterized.
Analysis of purified fractions and determination of subunit molecular weight of PEPCK by SDS-PAGE
For analysis of different purified fractions and determination of subunit molecular weight, protein samples (30–40 μg) were resolved on 10% (w/v) SDS-PAGE as described by Sambrook and Russell (Reference Sambrook and Russell2001). Gels were stained using 0·25% Coomassie Blue R 250, destained and captured using a UVP GelDoc-It 310.
Determination of native molecular weight of PEPCK by high pressure liquid chromatography (HPLC)
To determine the native molecular weight of the purified PEPCK, the HPLC experiment was carried out using a gel filtration column (Agilent Zorbax Bio Series GF 250, 4·6 mm × 250 mm), which was calibrated with 20 mM Tris-acetate buffer (pH 8·0) that contained 100 mM Na2SO4. 5 μl of the standard proteins mix, followed by 50 μl of purified PEPCK, was run for 30 min each at a flow rate of 1 ml/min. Standard proteins (ovalbumin, 43 kDa; conalbumin, 75 kDa; aldolase, 158 kDa; ferritin, 440 kDa; thyroglobin, 669 kDa; and blue dextran 2000 kDa) in the gel filtration calibration kit (GE Healthcare) were used to determine their retention times. The data were analysed using Empower2 software loaded to the system. The retention time of the purified PEPCK was extrapolated on the calibration curve between the retention time and log molecular weight of standard proteins in order to find out the native molecular weight of the purified enzyme.
PEPCK enzyme assay and kinetics
PEPCK activity was assayed following the modified method of Mommsen et al. (Reference Mommsen, Walsh and Moon1985) using a spectrophotometer (Carry 50, Varian, USA) at 340 nm. In brief, the enzymatic activity of PEPCK was assayed using coupled reactions in which the oxaloacetate produced in the first reaction by the carboxylation of PEP gets reduced to malate by the oxidation of NADH in the presence of malate dehydrogenase (MDH). One ml of the reaction mixture contained Tris-HCl (50 mM, pH 7·4), 5 mM PEP, 0·15 mM NADH, 0·6 mM GDP, 20 mM NaHCO3, 4 mM MnCl2, 15 units of MDH and various fractions as the enzyme source. One unit of enzyme activity is defined as the amount of the enzyme that catalyses the oxidation of 1 μmole of NADH per min under standard reaction conditions. The background and negative controls were also maintained without NADH and the enzyme source, respectively. Various buffers were also used to find out the maximal PEPCK activity. The optimal pH for PEPCK activity was measured by using Tris-HCl buffer (50 mM) of pH range 6·6–9·6. Apparent Michaelis constants (Km) or inhibitor constants (Ki) values for the substrate or co-factors were determined by plotting the reaction rates at varying concentrations of the substrate or co-factors (Lineweaver-Burk and Michaelis–Menten plots). The effect of various phytochemicals was studied by pre-incubating the enzyme with the ligand for 5 min under standard reaction conditions.
Protein estimation
Protein in the crude homogenate, supernatant and fractions was determined by Bradford's method (Bradford, Reference Bradford1976) using Coomassie Blue G 250. Bovine serum albumin was used as the standard.
Statistical analysis
Data are represented as the mean±s.e.m. (n = 4) and probability values less than 0·05 were taken to be statistically significant. Statistical analysis was performed using the Student's t-test; comparisons of the paired mean values were calculated between treatments and their respective controls. Multiple regression was calculated using Origin 6.0 software and the coefficient for linear lines was not less than 0.99 (R2 > 0·99).
RESULTS
Purification and characterization of purified PEPCK from R. echinobothrida
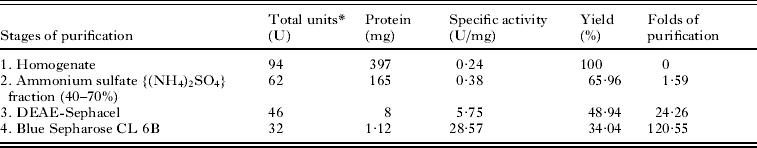
PEPCK was purified biochemically from the parasite following the modified procedure as described by Brinkworth et al. (Reference Brinkworth, Hanson, Fullin and Schramm1981). The purification procedure (outlined in Table 1) lead to apparently the pure enzyme from the Blue Sepharose CL 6B affinity chromatography stage at 100 mM NaCl. The fold of purification was about 121 times and yields about 34%, whereas the specific activity of the purified PEPCK was about 29 U/mg. The purified PEPCK was found to be an apparently pure enzyme as seen from the 10% (w/v) SDS-PAGE (Fig. 2A; Lane 4). An average subunit molecular weight of the parasite PEPCK (65 kDa) was determined when relative mobility values of standard proteins of known molecular weight were plotted as a function of their log molecular weight (Fig. 2B and C). To determine the native molecular weight of the purified PEPCK, gel filtration on HPLC was carried out using a gel filtration column calibrated with a standard protein mix (GE Healthcare) (Fig. 3A). The protein peak with the retention time of 6·88 min was found to be PEPCK (Fig. 3B). The native molecular weight of PEPCK was determined by plotting the retention time of standard proteins as a function of their log molecular weight (Fig. 3C), and found to be 69 kDa; thereby suggesting that the purified PEPCK is a monomer.

Fig. 2. PEPCK purified from Raillietina echinobothrida: SDS-PAGE analysis of purification steps and determination of the subunit molecular weight of the enzyme. (A) Different fractions from the purification steps analysed. Lane 1: ultracentrifugation (40 000 rpm), Lane 2: 40–70% {(NH4)2SO4} fraction, Lane 3: DEAE-Sephacel fraction, and Lane 4: Blue Sepharose CL 6B column fraction. Arrow indicates the purified PEPCK. (B) Determination of subunit molecular weight. Lane 1: marker proteins and Lane 2: purified PEPCK from Blue Shepharose CL 6B column. Arrow indicates the parasite PEPCK. (C) A plot of the log molecular weight of the marker proteins versus relative mobility (Rm) as calculated from Fig. 2 (B); (●) molecular weight of marker proteins; (♦) purified R. echinobothrida PEPCK.

Fig. 3. Raillietina echinobothrida PEPCK: determination of native molecular mass. (A) Retention time (in min) of standard proteins. (B) Retention time of the purified parasite PEPCK. (C) A plot of log molecular weight of standard proteins versus retention time was calculated from Fig. 3 (A and B); (●) molecular weight of standard proteins; (♦) purified R. echinobothrida PEPCK.
Table 1. Purification of PEPCK from the cestode, Raillietina echinobothrida
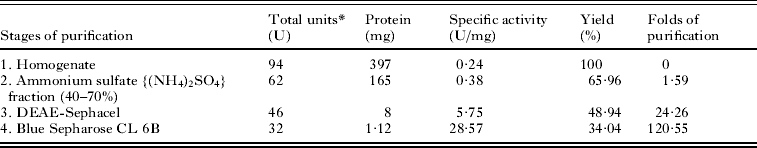
* One unit of enzyme activity is the amount of enzyme that catalyses 1 μmole of NADH oxidation per min under standard reaction conditions as described in the Materials and Methods section.
Effect of buffers and pH on PEPCK activity
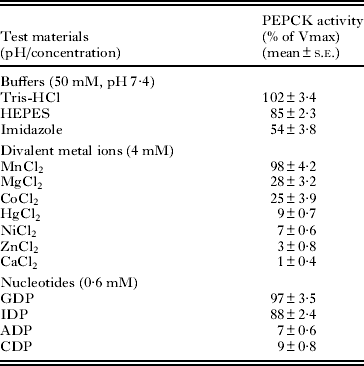
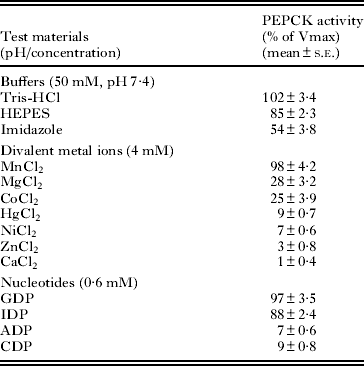
Several buffers were used to determine the optimal PEPCK activity. Tris-HCl (50 mM) was found to be a more suitable buffer in comparison to HEPES and imidazole (Table 2); whereas other buffers (potassium phosphate, sodium acetate and sodium glycine) were found to have a partial inhibitory action on the enzyme activity (results not shown). Different pH conditions were also tried out to find the optimal pH for the PEPCK activity. As seen from Fig. 4, the purified PEPCK showed an optimal activity at pH 7·4. There was a steep rise in the enzyme activity (about 2-fold) between the pH 6·6 and pH 7·4, although a sharp decline was observed within a narrow range (pH 7·4–pH 8·0).

Fig. 4. Effect of pH on Raillietina echinobothrida PEPCK activity. 100% PEPCK activity is equivalent to 29 μmol/min/mg under standard reaction conditions as described in the Materials and Methods section. Values are taken from 2 separate purifications and expressed as mean±s.e.m.
Table 2. Effect of the various test materials on the purified Raillietina echinobothrida PEPCK activity
Effect of divalent metal ions and nucleoside diphosphates on PEPCK activity
Several divalent metals ions (Mn2+, Mg2+, Co2+, Hg2+, Ni2+, Zn2+ and Ca2+) were tested with an intention to find out the effective co-factor for PEPCK activity (Table 2). Mn2+ (4 mM) was found to be the best co-factor for the parasite PEPCK activity, even though a 28% and 25% activity was observed when Mg2+ and Co2+, respectively, were used as a replacement for Mn2+ at the same concentration. However, in the presence of other divalent metal ions, no PEPCK activity was observed under the standard assay conditions. Of several nucleoside diphosphates tested on the parasite PEPCK activity (Table 2), GDP or IDP (0·6 mM) were found to be the most appropriate for the carboxylation reaction; however, there was no enzyme activity observed when ADP or CDP was used, even at 4 mM (results not shown).
Michaelis constants of PEPCK
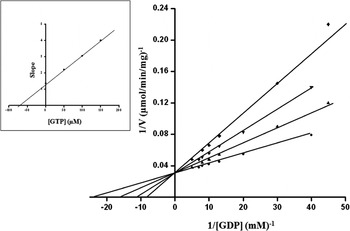
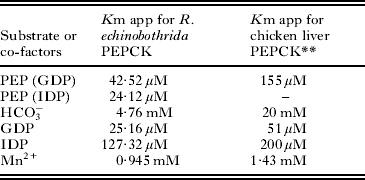
For the purpose of characterizing the purified parasite PEPCK, Km app values were determined for the substrate and co-factors. The Km app for the substrate was found to be 42·52 μM (Fig. 5); whereas, 25·16 μM, 127·32 μM, 4·76 mM and 0·945 mM for GDP, IDP, HCO3, and Mn2+, respectively (Table 3). Several competitive inhibitors (GTP, GMP, ITP, and IMP) for the carboxylation reaction were tested and their K i values calculated at 3 different inhibitor concentrations. The effect of GTP on R. echinobothrida PEPCK activity is shown in Fig. 6; the inset is a re-plot of the slopes from the double reciprocal plot versus GTP concentration (R2 = 0·99908). Ki for GTP was determined and found to be 76·42 μM; Ki for other competitive inhibitors was also determined (Table 4). A comparision of properties of purified PEPCK from various helminths hitherto is given in Table 5.

Fig. 5. Lineweaver-Burk and Michaelis-Menten (inset) plots to determine Km for PEP. The plots were calculated from assays of at least 8 different concentrations of PEP (25 μM to 250 μM). The intercept on the X-axis is ™1/Km (R2 = 0·99871).

Fig. 6. Lineweaver-Burk plot showing inhibition of Raillietina echinobothrida PEPCK activity by GTP at variable GDP concentrations. The plot was calculated from assays at 6 different concentrations of GDP in the presence of 3 different concentrations of GTP {(●) control, (▲) 50 μM, (▼) 100 μM, and (▪) 150 μM GTP}. The re-plot (inset) of the slopes of the lines in the Lineweaver-Burk plot versus GTP concentration gives a straight line (R2 = 0·99908).
Table 3. Kinetic characteristics of Raillietina echinobothrida PEPCK
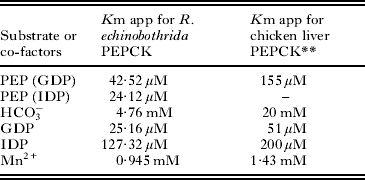
* Lineweaver-Burk plot was used for the determination of Km app of PEPCK.
** Hebda and Nowak (Reference Hebda and Nowak1982a).
Table 4. Inhibitor constants (Ki)* for various competitive inhibitors of Raillietina echinobothrida PEPCK

* Lineweaver-Burk plot was used for the determination of Ki of PEPCK.
Table 5. Comparative properties of purified PEPCK from helminths

Effect of phytochemicals on PEPCK activity
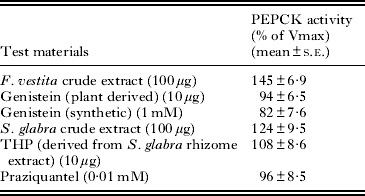
In order to identify the possible modulators from plant sources for the parasite PEPCK, the ethanolic crude extract and phytochemicals isolated from F. vestita and S. glabra were tested on the parasite PEPCK activity. As shown in Table 6, the activity of the purified PEPCK increased significantly by 45% (P < 0·01) and 24% (P < 0·05) when incubated with the crude extract of F. vestita and S. glabra, respectively, in comparison to their respective controls. However, the PEPCK activity was found to decrease non-significantly when the enzyme was incubated with the purified fraction containing genistein. In the case of PZQ, a broad-spectrum anthelmintic, and THP (derived from the rhizome crude extract of S. glabra), the purified PEPCK activity remained unchanged in comparison to their respective controls.
Table 6. Effect of ethanolic crude extract and the phytochemcials from Flemingia vestita and Stephania glabra and other test materials on purified Raillietina echinobothrida PEPCK activity

DISCUSSION
Purification of PEPCK from the cestode, R. echinobothrida, was successfully accomplished by using the mentioned procedure (Brinkworth et al. Reference Brinkworth, Hanson, Fullin and Schramm1981), which resulted in apparently pure monomeric enzyme with a good yield. So far, R. echinobothrida is the second cestode from which PEPCK has been purified and characterized, H. diminuta being the first one (Wilkes et al. Reference Wilkes, Cornish and Mettrick1981). The specific activity of the parasite PEPCK was found to be roughly 29 U/mg protein, which is similar to the specific activity of H. diminuta PEPCK. The enzyme activity remained stable for more than 1 year at −80 °C. The molecular weight of the cestode PEPCK is well within the range (70–80 kDa) reported in other helminth parasites (Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Behm and Bryant, Reference Behm and Bryant1982; Wilkes et al. Reference Wilkes, Cornish and Mettrick1982; Rohrer et al. Reference Rohrer, Saz and Nowak1986, Klein et al. Reference Klein, Winterrowd, Hatzenbuhler, Shea, Favreau, Nulf and Geary1992) and is very much close to that of PEPCK purified from H. diminuta (Wilkes et al. Reference Wilkes, Cornish and Mettrick1981).
In the present study, Tris-HCl (50 mM) was found to be the ideal buffer for the purified R. echinobothrida PEPCK; and the optimum pH was 7·4 under the standard assay conditions. There was a steep rise (about 2-fold) in PEPCK activity between pH 6·6 and 7·4, indicating that the activity may be quite responsive to a slight change in the physiological pH. The kinetic parameters of the purified enzyme were determined using the optimum pH 7·4. However, the optimum pH for the carboxylation reaction is within an acidic range of pH 5·0 to pH 6·0 in most helminths characterized hitherto (Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Behm and Bryant, Reference Behm and Bryant1982; Wilkes et al. Reference Wilkes, Cornish and Mettrick1981, Reference Wilkes, Cornish and Mettrick1982), except for A. suum, for which the optimum pH is 7·1 (Rohrer et al. Reference Rohrer, Saz and Nowak1986).
Among the various metal ions tested to investigate the effect of divalent ions on PEPCK activity, maximal enzyme activity was observed in the case of Mn2+ (which forms a complex with GDP and PEP). As revealed by earlier studies, the concentration of Mg2+ in R. echinobothrida tissue is about 1400 μg/g dry tissue weight while Mn2+ is below the level of detection (Das et al. Reference Das, Tandon and Saha2006). Although the concentration of Mg2+ is much higher than that of Mn2+ in the tissue of M. dubius and H. diminuta, there was no activity observed when Mg2+ was used (Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Wilkes et al. Reference Wilkes, Cornish and Mettrick1981). Only a slight activity was observable when Mg2+ was used at a higher concentration (4·0 mM) in place of Mn2+ (in this study; Rohrer et al. Reference Rohrer, Saz and Nowak1986). Thus Mn2+ is suggested to be the preferred metal ion with regards to the PEPCK activity, not only in parasites but also in higher vertebrates (Lee et al. Reference Lee, Hebda and Nowak1981; Hebda and Nowak, Reference Hebda and Nowak1982b). In the present study, about 25% PEPCK activity was also observed when Co2+ was used as the metal ion, although no activity was recorded when other metal ions were used. In A. suum muscle too, Co2+ (4 mM) activates PEPCK activity, which is half of the maximal at equimolar concentration of Mn2+ (Rohrer et al. Reference Rohrer, Saz and Nowak1986).
In the present study, GDP or IDP (not ADP or CDP) was found to be the nucleoside diphosphate for the parasite PEPCK activity. These observations conform with those in M. dubius and H. diminuta (Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Wilkes et al. Reference Wilkes, Cornish and Mettrick1981); however, in the crude tissue extract of these cestodes, PEPCK activity was observed in the presence of ADP (Korting and Fairbairn, Reference Korting and Fairbairn1972; Moon et al. Reference Moon, Mustafa, Hulbert, Podesta and Metrrik1977).
The Km app values for PEP, GDP, IDP, HCO3, and Mn2+ in R. echinobothrida were found to be matching with the range reported in other helminths (Cornish et al. Reference Cornish, Wilkes and Mettrick1981; Behm and Bryant, Reference Behm and Bryant1982; Wilkes et al. Reference Wilkes, Cornish and Mettrick1981, Reference Wilkes, Cornish and Mettrick1982; Rohrer et al. Reference Rohrer, Saz and Nowak1986). In this study, the Km app for PEP in the presence of GDP was found to be slightly higher than that observed in H. diminuta (Wilkes et al. Reference Wilkes, Cornish and Mettrick1981) and about 3 times lower than that of its avian host (Hebda and Nowak, Reference Hebda and Nowak1982a). The concentration of PEP in R. echinobothrida is not known, though in helminths it varies from 0·06 to 0·35 mM (Behm and Bryant, Reference Behm and Bryant1982). As observed in H. diminuta (Wilkes et al. Reference Wilkes, Cornish and Mettrick1981), in the present study also the Km app for PEP in the presence of IDP is lower than that when GDP was used. However, the Km app for GDP is lower than that for IDP for R. echinobothrida PEPCK; hence, it is difficult to predict which nucleoside diphosphate is favoured in vivo for the carboxylation reaction. In general, IDP is present in very negligible amounts in comparison to GDP in most organisms (Rohrer et al. Reference Rohrer, Saz and Nowak1986); hence, GDP may be the preferred nucleoside diphosphates for PEPCK activity. The Km app for HCO3− for PEPCK in R. echinobothrida is very low in comparison to published data for its avian host (Hebda and Nowak, Reference Hebda and Nowak1982b), but is almost similar to the values in other helminths. Hence, it may be speculated that the bicarbonate (with much lower Km for PEP) would facilitate carbon fixation in the intestinal milieu of the host (Moon et al. Reference Moon, Mustafa, Hulbert, Podesta and Metrrik1977; Rohrer et al. Reference Rohrer, Saz and Nowak1986; Davila et al. Reference Dávila, Malagón, Valero, Benítez and Adroher2006).
In determining the effectiveness of GTP and ITP as putative competitive inhibitors for PEPCK, both these nucleoside triphosphates were found to be strong competitive inhibitors for GDP or IDP in the carboxylation reaction. A similar observation was also reported for H. diminuta PEPCK (Wilkes et al. Reference Wilkes, Cornish and Mettrick1981). The Ki value for GTP was approximately 3 times higher than the Km app of GDP; therefore, both GDP and GTP concentrations in R. echinobothrida need to be determined before concluding that GTP would act as a regulator of PEPCK activity. In the present study, Ki values for GMP and IMP were higher than those for GTP and ITP. Since physiological levels of GMP and IMP are reportedly below detectable levels in helminths (Senft et al. Reference Senft, Miech, Brown and Senft1972; Barrett, Reference Barrett1973), hence, these molecules, with their high Ki values and low physiological concentrations, are not the likely candidates for PEPCK regulation in vivo.
Only a small number of modulators from plant sources are known for PEPCK (Witters et al. Reference Witters2001; Kim et al. Reference Kim, Park, Lee, Park, Kim, Nedumaran, Jang, Cho, Ha, Lee, Lee and Choi2008; Foretz et al. Reference Foretz, Hébrard, Leclerc, Zarrinpashneh, Soty, Mithieux, Sakamoto, Andreelli and Viollet2010; Xia et al. Reference Xia, Yan, Shen, Tang, Yin, Zhang, Yang, Liang, Ye and Weng2011; Haridas et al. Reference Haridas, Xu, Kitchen, Jiang, Michels and Gutterman2011). In our exploration for modulators from medicinal plants, the phytochemicals isolated from F. vestita and S. glabra were tested on the purified PEPCK; however, these phytochemicals did not show any inhibitory effect on the parasite PEPCK even at higher concentrations.
In conclusion, with regard to the molecular weight and other kinetic properties, the purified PEPCK from R. echinobothrida appears to be similar to the enzyme purified from other helminths. In the avian host of the parasite, and also in mammals, the enzyme is involved in gluconeogenesis and its physiochemical properties differ from those of the parasite PEPCK. Because of these differences in the primary function as well as other properties of PEPCK of the parasite and its host, this enzyme might be exploited as a potential target site for anthelmintic action.
ACKNOWLEDGEMENTS
The Department of Science and Technology, GOI, provided financial assistance to B.D. through the ‘Fast Track Scheme’ (SR/FT/L-107/2004 dated 4 May 2005). Thanks are also due to The British Council, UK, for providing a travel grant to B.D. under UKIERI programme. The authors also thank the Director, CDRI, Lucknow; Head, Department of Zoology; and Coordinator, Bio-informatics Centre, NEHU, Shillong, for allowing access to instrumental facilities, infrastructure and on-line facilities, respectively.