Introduction
Parasite-mediated host mortality is generally assumed to be a consequence of direct harm caused by damage to host tissue, leeching of micronutrients and/or perturbations to host energy budgets (Poulin and Morand, Reference Poulin and Morand2000; Robar et al. Reference Robar, Burness and Murray2010; Adelman and Hawley, Reference Adelman and Hawley2017). However, direct effects during infection or upon contact are not the only effects parasites have on their host populations. Indirect effects that arise during proximity to infective stages of parasites can have potentially significant consequences for host ecology and evolution. These include the costs of behavioural defences, parasite avoidance, maintaining immunity and compensatory physiological changes (Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000; Hart, Reference Hart2011). The indirect effects of parasitism on potential hosts are analogous to the non-consumptive effects observed in predator–prey systems, in which exposure to predators alters traits among prey species even if the prey is not eaten (Peacor and Werner, Reference Peacor and Werner2008). Non-consumptive effects are trait-mediated as they depend on the presence of predators and the effect their presence has on the traits (physiological and behavioural) of their prey (Peacor and Werner, Reference Peacor and Werner2008). Changes in the prey include behavioural avoidance of predators and/or risky habitats, elevated stress responses, not exploiting resources fully, altered competitive ability and physiological changes (Preisser and Bolnick, Reference Preisser and Bolnick2008). Peacor and Werner (Reference Peacor and Werner2008) distinguished non-consumptive effects from the indirect effects of predators on a third species, which they call trait-mediated indirect effects: e.g. a species benefits from a predator preferentially consuming its competitor. Non-consumptive effects are known to reduce the fitness of prey species (Peckarsky et al. Reference Peckarsky, Cowan, Penton and Anderson1993; Buchanan et al. Reference Buchanan, Hermann, Lund and Szendrei2017). An early experiment by Peckarsky et al. (Reference Peckarsky, Cowan, Penton and Anderson1993) showed that mayfly larva exposed to predators with glued mouthparts had 21–25% slower growth rates and produced fewer eggs later in life than control larva. This area of predator–prey research is often called the ‘ecology of fear’ due to the physiological stress and neuro-behavioural responses predators cause in prey (Preisser and Bolnick, Reference Preisser and Bolnick2008; Clinchy et al. Reference Clinchy, Schulkin, Zanette, Sheriff, McGowan and Boonstra2011). Our objective is to extend the ecology of fear and test if parasites suffer a trait-mediated reduction in fitness from the presence of parasites.
Current attempts to integrate parasitology and community ecology may underestimate the effects parasites have on host populations by ignoring the effects of parasites on host populations when contact does not take place. The direct effects of infection with macroparasites is generally dependent on the intensity of infection (Wilber et al. Reference Wilber, Weinstein and Briggs2016), while indirect changes in host physiology and behaviour in response to parasites are typically trait-mediated (Raffel et al. Reference Raffel, Martin and Rohr2008). Recent work has shown that initial contact with parasites has physiological and fitness effects on the host even when infection does not proceed (Rohr et al. Reference Rohr, Raffel, Halstead, McMahon, Johnson, Boughton and Martin2013; Sears et al. Reference Sears, Snyder and Rohr2015). Contact with the infectious stages of trematodes significantly impacted tadpole fitness, even when infection failed to establish (Rohr et al. Reference Rohr, Raffel and Hall2010). Comparable results are seen in fungal diseases (Rohr et al. Reference Rohr, Raffel, Halstead, McMahon, Johnson, Boughton and Martin2013), suggesting adverse effects from parasite contact occur with a wide range of parasites. Furthermore, the mere presence of parasites may adversely affect hosts; e.g. tadpoles increase their activity in response to the parasite-derived chemical cues and avoid the areas containing those cues (Rohr et al. Reference Rohr, Swan, Raffel and Hudson2009). Their study showed that parasites have trait-mediated impacts on their potential hosts, similar to those predators have on potential prey (Raffel et al. Reference Raffel, Martin and Rohr2008; Rohr et al. Reference Rohr, Swan, Raffel and Hudson2009). Analogous to non-consumptive effects, we refer to these indirect effects of parasitism as ‘non-infective’ effects. We aim to determine if non-infective effects are sufficient to reduce host fitness without direct contact. On initial consideration, the cost of infection may appear low relative to predation. This relatively low cost may minimize the amount of investment in parasite defences relative to predation defences, and by extension reduce potential non-infective costs (Raffel et al. Reference Raffel, Martin and Rohr2008). However, the frequency of parasite exposures for many free-living organisms is greater than the number of potential predation events (Raffel et al. Reference Raffel, Martin and Rohr2008). As such, hosts should invest more heavily in parasite defences than initially anticipated.
In this study, we experimentally test if exposure to parasites is sufficient, per se, to reduce host fitness (i.e. exposure without contact). Our study extends previous research on parasite exposure, by testing if hosts suffer deleterious fitness effects from the presence of parasites even when direct contact does not occur. Macrocheles subbadius (Acari: Macrochelidae) is a naturally occurring facultative parasite of D. nigrospiracula (Diptera: Drosophilidae) that feeds on fly haemolymph and uses flies for dispersal (Polak, Reference Polak1996). Previous studies have found that infection with mites reduces host longevity and fecundity (Polak, Reference Polak1996). Fruit fly hosts typically respond to approaching mites with behavioural defences that are energetically demanding, including bursts of movement and intense grooming behaviour (Polak, Reference Polak1996; Luong et al. Reference Luong, Horn and Brophy2017). Previously, we showed that when flies are exposed to mites (sequestered behind a mesh wall), their energy consumption increases, suggestive of increased stress and/or activity linked to the defensive behaviours (Luong et al. Reference Luong, Horn and Brophy2017). In the present study, we examined whether chronic exposure to mites adversely affects the fitness of D. nigrospiracula. The long-term energetic costs of exposure to parasites likely diverts essential resources away from somatic maintenance and reproduction (Auld et al. Reference Auld, Penczykowski, Ochs, Grippi, Hall and Duffy2013; Lu et al. Reference Lu, Wang, Zhu, Yu, Guo and Wan2014). We therefore hypothesize that chronic exposure to the infective stages of a parasite will reduce host fitness. Reproduction may impose additional demands on host resources that cause parasites to impact host physiology in ways that would otherwise be undetectable (Odiere et al. Reference Odiere, Koski, Weiler and Scott2010). For example, sham infections induce more energetically demanding immune responses in pregnant mice relative to non-pregnant females (Odiere et al. Reference Odiere, Koski, Weiler and Scott2010). Specifically, we predict that exposure to restrained mites will decrease the longevity and fecundity of female flies, and that the effect on longevity will be exasperated for mated flies. We experimentally manipulated exposure to mites by housing fruit flies, from the time of eclosion to death, with caged mites to assess the impact of parasite proximity on host fitness. To our knowledge, this is the first study to experimentally demonstrate a loss in host fitness due to the indirect effects of exposure sans parasite contact.
Materials and methods
Fly and mite cultures
Drosophila nigrospiracaula Patterson and Wheeler were cultured from flies (120 adults of each sex) collected in the Sonoran desert (Phoenix, Arizona) from necrotic cacti (Carnegiea gigantea). Larval stages were kept on a medium consisting of instant potato flakes, Drosophila medium (Formula 4–24 Instant Drosophila Medium, Carolina Biological Supply Company, Burlington, NC, USA), nutritional yeast and autoclaved necrotic cactus. Newly eclosed adult flies were transferred to vials with agar medium within 48 h of emergence. Fly cultures were kept in incubators at 24 °C and 50% relative humidity with a 12 h day–night cycle (Percival Scientific, Perry, IA, USA).
Macrocheles subbadius Berlese (200–300 adult females) were collected from naturally infected wild flies and used to initiate laboratory cultures. Cultured mites were reared on 2:1 wheat bran to wood chips media. Free-living bacteriophagic nematodes (Rhabditida) were co-cultured with the mites as a food source. Mite cultures were maintained at 26 °C and 70% relative humidity and a 12 h light cycle. Mites used in the experiments below were collected from the stock culture using a Berlese funnel (Smith, Reference Smith1980).
Longevity-exposure experiment
This experiment was conducted to determine if chronic exposure to mites, without contact, affects fly longevity. Two primary factors were considered, parasite exposure and fly reproductive status. Female flies were therefore assigned randomly to one of four groups: reproducing and exposed to mites, reproducing without mites, virgin and exposed to mites or virgin without mites. Adult female flies were moved to agar vials upon emergence and housed with or without restrained mites and mates present depending on the experimental condition. Exposed groups were housed with mites without a realized risk of infection. This was achieved by creating a small divot (~0.5 cm deep and 1.5 cm across) in the underside of the foam plug in the fly vials. Five adult female mites were placed into the divot, which was then covered (using Elmer's super glue) by a small piece of polyester mesh, sequestering the mites. The mesh allowed chemical and visual cues to pass through, but prevented physical contact between flies and mites. With the mites restrained behind the mesh screen, the plug was lowered into the vial, leaving a 2.5 cm space between the agar and the base of the plug (13.3 cm3), maximizing the proximity and likelihood the flies could detect the mites. Control flies had plugs prepared the same way, but without mites, and had identical living spaces.
Fly reproductive status was established by rearing flies with either a male or female companion. Mated females were housed with a male fly and virgin flies were reared with another female companion to control for population density effects. Companion flies had the tip of a wing clipped (~0.5 mm) with micro-scissors to distinguish them from the experimental fly. The plug of each vial and the mites within were replaced biweekly, alternating 3 and 4 days between changes (average 3.5 days). Macrocheles subbadius can survive 4–5 days without food if kept in a humid environment (unpublished data), and the majority of mites survived until replacement. Agar vials were changed simultaneously with a plug replacement once a week. In order to make sure the reproducing flies had a constant source of sperm, the companion male was replaced at the same time as the vial change. Since the novelty of new companions may affect fly longevity, we replaced both the male and female companions during the vial change. New companions were all virgins pulled from the stock cultures at random.
Flies in the longevity experiment were kept in an incubator at 26 °C and 70% RH. Survival was checked every 24 h until the day of death (i.e. days alive). Following death, flies were frozen at −20 °C allowing thorax length to be measured post-mortem as a potential cofactor of longevity. Thoraxes were measured from the most anterior part of the thorax to the scutellum tip (Bergland et al. Reference Bergland, Genissel, Nuzhdin and Tatar2008). Measurements were made using a Leica M120HD camera mounted on a Leica M80 dissecting microscope and processed with the LAS EZ software (Leica Microsystems, Wetzlar, Germany). Two blocks of the experiment were conducted in an identical manner. Both replicate blocks consisted of 40 experimental flies (20 control and 20 exposed flies).
Fecundity-exposure experiment
This experiment measured the lifetime fecundity of flies exposed to mites as compared with unexposed flies. We defined fecundity as the number of offspring to survive to the adult life stage (i.e. offspring that survive to eclosion). Flies were housed in vials with foam plugs containing mites restrained behind a mesh screen or plugs without mites (described above). The experiment commenced 3 days post-eclosion to avoid female flies dying before reproductive maturity (Markow, Reference Markow1996). Since D. nigrospiracula offspring fail to develop on agar medium, flies in this experiment were maintained on an 18:5 mix of instant potato and fly media supplemented with autoclaved cactus. Incidentally, this medium is not optimal for the survival of adult D. nigrospiracula, and as such flies in this experiment survived for less time than flies in the longevity-exposure experiment (see results). To avoid the risk of flies drowning in the relatively wet media, occupancy space was increased to 18.6 cm3. Experimental females were maintained in an incubator at 26 °C and 70% relative humidity. Female flies were housed with a single male companion. Experimental flies were transferred to fresh vials every 3–4 days, at which time the male was replaced with a mature virgin male. Survival was checked every 24 h until death. Post-mortem, thorax length was measured (as above). Previously occupied vials were maintained in the incubator until F1 emergence. Vials were monitored for 2 weeks following the removal of the adult flies, newly eclosed adults were harvested and counted within 48 h of emergence.
Statistical analyses
All statistical analyses were performed in R studio (R studio team, 2013, Version 0.98.932). Linear models for analysis were made using the GLM function (R, Stats package). We analysed the longevity-exposure experiment using two different methods: generalized linear models with stepwise backwards deletion and a Kaplan–Meier technique. During model reduction, data were normalized using a Box–Cox transformation, λ = 0.38 (R, MASS Package). The full model included parasite exposure, mating status, thorax length and block as independent factors. Starting with the least significant variable, factors were removed sequentially. The new model was compared with the previous model with an analysis of variance (ANOVA), and a variable was only retained if there was a significant difference between models (χ 2 test, α = 0.05). We compared the survivorship curves for each treatment group using the Survdiff function (R, Survival Package). None of the flies were censored in the longevity-exposure experiment. Since the longevity-exposure experiment was carried out in two replicate batches, block was included as a cofactor.
The fecundity-exposure data were also analysed with backwards model comparison. The glm function was used with a Poisson distribution (R, Stats package), and models were compared with an ANOVA (χ 2 test, α = 0.05). Fecundity was the response variable in this experiment; longevity, thorax length and parasite exposure were treated as independent variables. One fly in the control group survived beyond the 28-day experimental period and was censored from the longevity analysis, but not fecundity analysis.
Results
Longevity-exposure experiment
We measured the life span of mated and unmated flies that were either exposed to mites, or not exposed and found only exposure to be a significant predictor of longevity. Exposed flies (N = 40) survived 15.3 ± 1.76 (mean ± s.e.) days post-emergence, a 38% difference compared with unexposed flies (N = 40) that lived 22.4 ± 1.83 days [Δresidual sum of squares (RSS) = 5.37, P = 0.003]. Overall, virgin flies (N = 40) lived on average 18.7 ± 1.82 days and mated flies (N = 40) lived 19.2 ± 1.97 days; reproductive status was not a significant predictor of longevity (ΔRSS = 0.85, P = 0.85). The interaction between parasite exposure and mating status was not significant (ΔRSS = 0.064, P = 0.75). Thorax lengths of six flies were not measured due to poor specimen preservation. The mean thorax length in the control group was 1.11 ± 0.007 mm (N = 39) and 1.16 ± 0.016 s.e. mm (N = 35) in the exposed group; thorax length was not a significant predictor of longevity (ΔRSS = 0.835, P = 0.25). Block was also not a significant factor (ΔRSS = 0.982, P = 0.21). Parasite exposure was the only significant predictor of longevity among female flies.
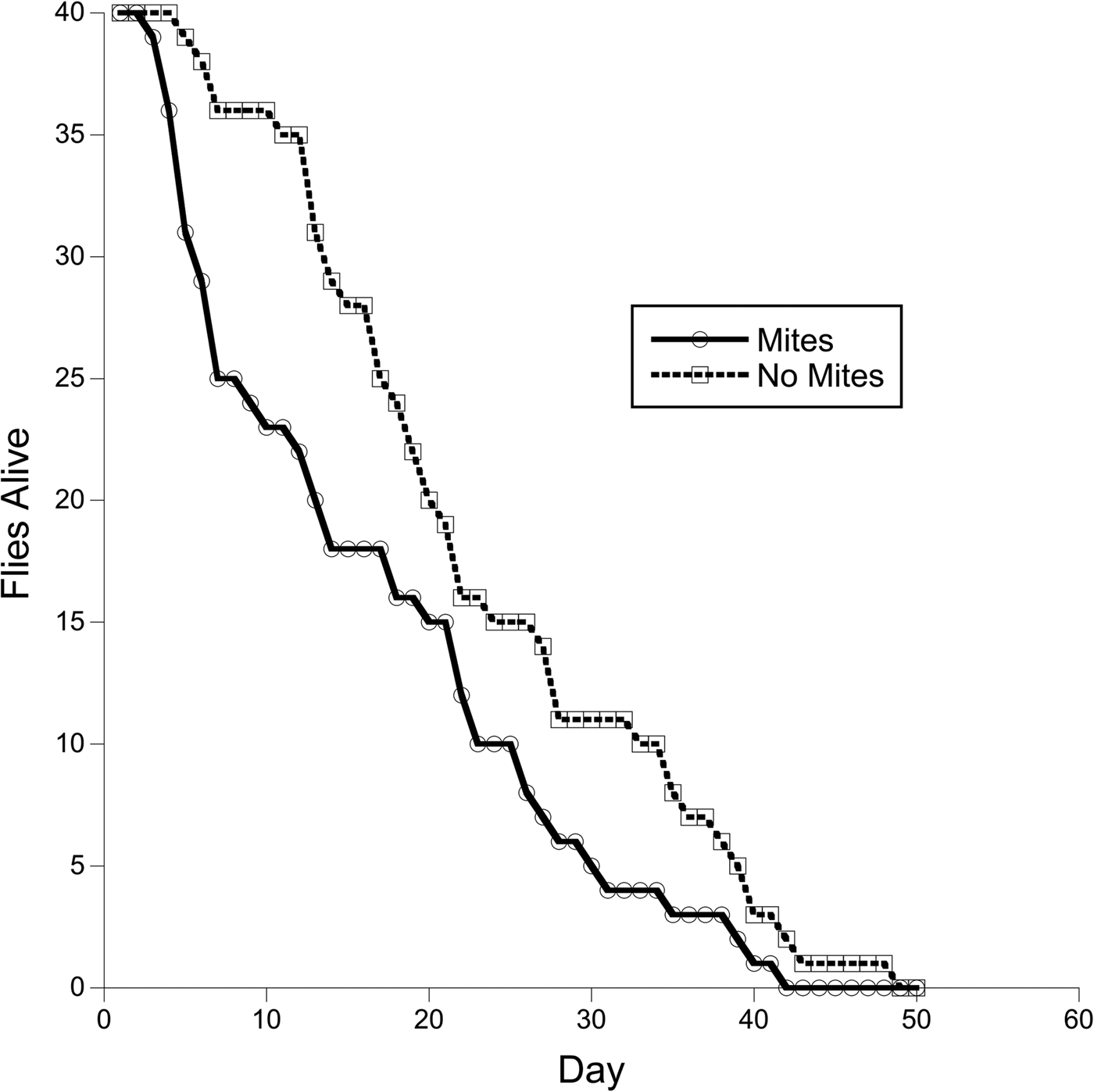
The survival curves of the exposed and unexposed groups were compared using the Survdiff function (Fig. 1). Flies exposed to mites experienced greater early die off, and although this trend slowed with time the two groups did not achieve parity. The survivorship curves for the exposed group and unexposed group were significantly different (Survdiff, χ 2 = 5.1, P = 0.018).
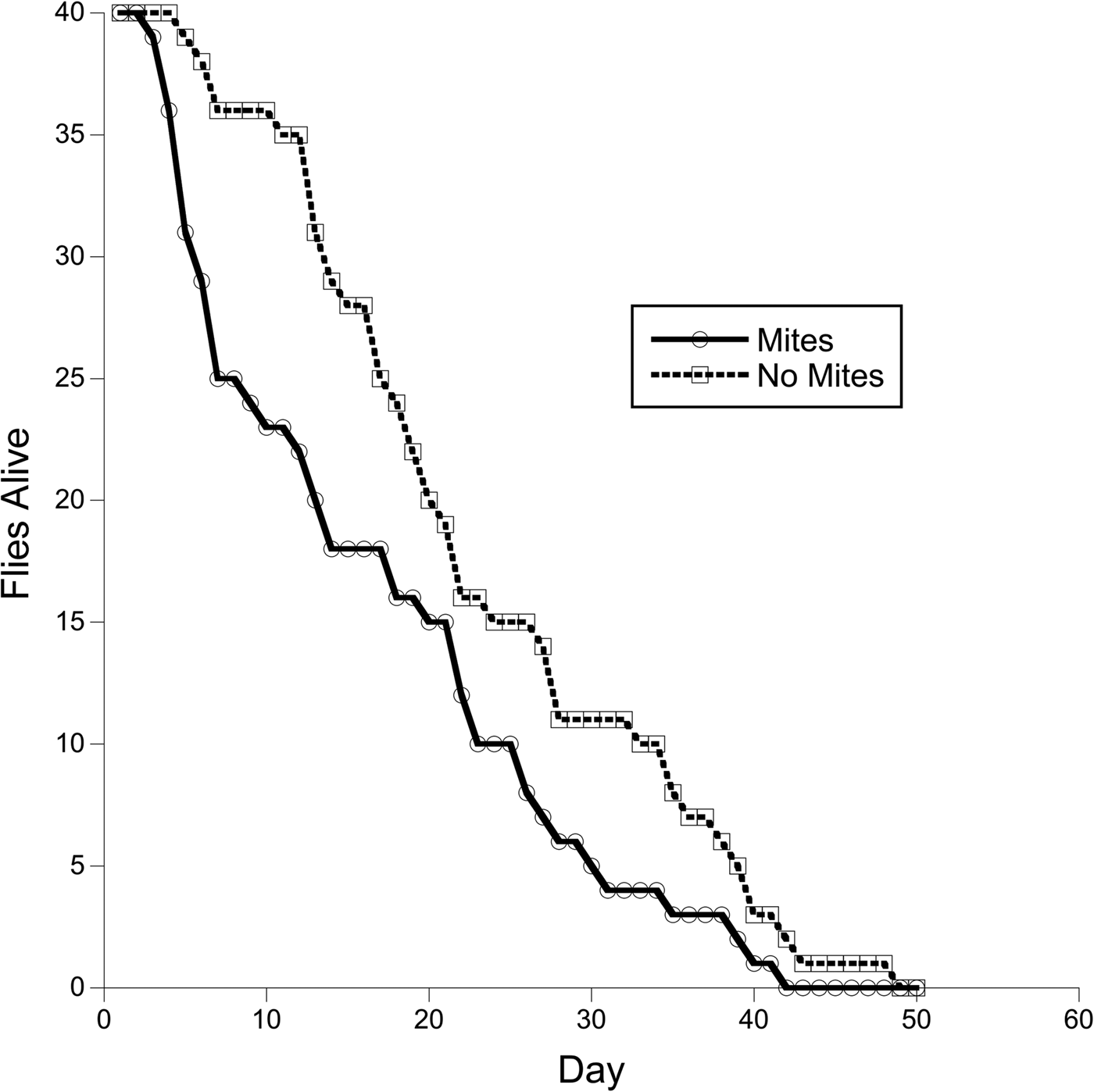
Fig. 1. Survivorship curves for flies that were either exposed to restrained mites (N = 40, solid line) or not exposed at all (N = 40, dashed line). The survivorship curves are significantly different (Survdiff, χ 2 = 5.1, P = 0.018).
Fecundity-exposure experiment
There was a 13% difference in fecundity between exposed flies and control flies; the unexposed group had a mean fecundity of 36.4 ± 8.0 offspring (N = 20), while the exposed group produced 32.1 ± 6.7 offspring (N = 20). Exposed flies lived on average 10.0 ± 0.87 days (N = 20), a 23.2% difference from the control flies, which survived on average 12.6 ± 1.33 days (N = 19). Thorax length was not a significant predictor of fecundity (deviance = 0.34, P = 0.56). While exposure status had a significant effect on fecundity (deviance = 18.0, P < 0.001), survival time (deviance>100, P < 0.001) was a more important factor in predicting fecundity. In other words, exposure to mites strongly impacted longevity, which in turn affected lifetime fecundity (Fig. 2).

Fig. 2. Reproductive output (measured in adult offspring) of female flies vs. age at death. Flies (N = 39) were raised on a fly medium-instant potato mix supplemented with necrotic cactus. Survival was positively and significantly correlated with fecundity (ΔRSS>100, P < 0.001).
Discussion
We tested the hypothesis that chronic proximity to parasites incurs non-infective effects that adversely affect host fitness. As predicted, female flies exposed to mites suffered reduced survival and lifetime fecundity relative to unexposed females. Not surprisingly, flies that lived longer produced more offspring. As such, the reduction in fecundity among flies exposed to mites was likely driven by strong effects on longevity. Indeed, longevity was a stronger predictor of fecundity than exposure status. Thus, the effect of exposure on life-time fecundity was primarily longevity mediated, though the fact remains that exposed flies produced fewer offspring. Previous research showed that female D. nigrospiracula infected with mites produced 102% fewer eggs than uninfected flies (Polak, Reference Polak1996). In that study, the decreased egg output was driven by a 59% reduction in life span among infected flies compared with uninfected flies (Polak, Reference Polak1996). Not surprisingly, we observed a smaller effect size as infection likely has a stronger biological effect than exposure alone. Still, the relatively smaller effect size can potentially incur an accumulated cost at the population level.
Previous studies have shown that initial contact with parasites can negatively affect hosts, even if that contact does not lead to a sustained infection (Rohr et al. Reference Rohr, Raffel and Hall2010; Sears et al. Reference Sears, Snyder and Rohr2015). Tadpoles that experienced epidermal damage typical of trematode attack, but did not develop lasting infections, exhibited reduced longevity compared with control tadpoles (Rohr et al. Reference Rohr, Raffel and Hall2010). Sears et al. (Reference Sears, Snyder and Rohr2015) showed the fitness cost of coming into contact with parasites depends on the host's relative investment in resisting or tolerating parasites. We extend these findings and show that direct contact between the host and parasite is not necessary for exposure to have a negative effect on host fitness. It is possible that host resistance may influence the extremity of the non-infective effects, and could be investigated in future research.
The presence of mites and ostensibly cues they produce are sufficient to decrease fly fitness. Since the mites in our study were restrained behind mesh, we can conclude that the reduction in fly longevity and fecundity was due to the costs associated with the proximity of mites and/or their cues. Currently, we do not know which cue(s) D. nigrospiracula use to detect M. subbadius, though it is clear that these cues can pass through translucent mesh (Luong et al. Reference Luong, Horn and Brophy2017). Experimental manipulations of fly vision and olfaction are needed to understand how flies detect mites (Larsson et al. Reference Larsson, Domingos, Jones, Chiappe, Amrein and Vosshall2004; Gaudry et al. Reference Gaudry, Nagel and Wilson2012). Larsson et al. (Reference Larsson, Domingos, Jones, Chiappe, Amrein and Vosshall2004) identified Or83b as a gene necessary for olfaction in Drosophila, and advances in rapid gene manipulation makes manipulation of fly olfaction viable for future studies (Koutroumpa et al. Reference Koutroumpa, Monsempes, Francois, de Cian, Royer, Concordet and Jacquin-Joly2016).
We also predicted that mating status would exacerbate non-infective reductions in longevity because of potential trade-offs between coping with parasite exposure and reproduction. However, we did not find a relationship between mating status and loss of fitness from proximity to parasites. Although in some cases reproduction can make previously undetected parasite effects worse (Careau et al. Reference Careau, Thomas and Humphries2010; Odiere et al. Reference Odiere, Koski, Weiler and Scott2010), parasite exposure alone was sufficient to reduce host longevity in our study. It is possible that the impact of the non-infective effect was large enough that it masked any interaction between exposure and mating status.
Among the indirect effects of parasites on host fitness, the costs of immune activation are the most well studied; for instance, an immune response against heat-inactivated bacteria reduces the survival of calorically restricted bees (Moret and Schmid-Hempel, Reference Moret and Schmid-Hempel2000). Insects can also experience autoimmune tissue damage (Sadd and Siva-Jothy, Reference Sadd and Siva-Jothy2006), so both energetic costs and self-damage from immune activation could mediate reductions in life span from immunity. The ecological consequences of immunity and defence have become the purview of ecological immunology (Sadd and Schmid-Hempel, Reference Sadd and Schmid-Hempel2009; Schulenburg et al. Reference Schulenburg, Kurtz, Moret and Siva-Jothy2009). In their review, Schulenburg et al. (Reference Schulenburg, Kurtz, Moret and Siva-Jothy2009) categorized costs of host defences into three primary groups: genetic (fixed costs), usage (costs at activation) and immunopathology (self-damage from immune processes). Behavioural defences by Drosophila spp. against approaching mites have high energetic costs at activation (Luong et al. Reference Luong, Horn and Brophy2017), and thus energetic trade-offs likely contribute to the decrease in fitness observed in the present study. Interestingly, some insects also express an uptick in respiration upon predator exposure (Slos and Stoks, Reference Slos and Stoks2008), suggesting that similar mechanisms may drive non-consumptive and non-infective effects alike.
Other resource-intensive methods of resistance involve the production of costly defensive features. The production of chitin by arthropods is plastic and increases in many arthropods in response to threats from both predators and parasites (Beckerman et al. Reference Beckerman, de Roij, Dennis and Little2013). Similarly, in insects the hardening of the cuticle via melanization has been shown to either kill or fend off several parasites and pathogens (Nakhleh et al. Reference Nakhleh, Moussawi, Osta and Ligoxygakis2017). The energetic and material costs of producing defensive structures and compounds divert resources away from somatic and reproductive activities. Future research should examine if long-term exposure to parasites upregulates the expression of fly genes associated with defensive elements.
Other possible mechanisms underlying the observed loss of fitness include the detrimental effects of vigilance and chronic stress. Maintaining vigilance against impending infection may reduce a fly's ability to forage and/or exploit resources. In predator–prey systems, the need to remain vigilant can reduce prey species fitness relative to competitors and reduce the efficiency of resource exploitation (Peacor and Werner, Reference Peacor and Werner2008). In their meta-analysis of non-consumptive effects in arthropods, Buchanan et al. (Reference Buchanan, Hermann, Lund and Szendrei2017) found that predator presence has a significant effect on the feeding behaviour of arthropod prey. Rohr et al. (Reference Rohr, Swan, Raffel and Hudson2009) showed that tadpoles change their behaviour and location in response to parasite-derived cues, but did not measure changes in fitness resulting from these behavioural changes. The changes in tadpole behaviour were similar with parasite exposure and predator exposure (Rohr et al. Reference Rohr, Swan, Raffel and Hudson2009), suggesting that the former may induce changes in hosts similar in extent to predator exposure (Raffel et al. Reference Raffel, Martin and Rohr2008). Changes in feeding behaviour and foraging ecology may explain the decrease in fly fitness observed in our study.
The risk of infection may also have implications for fly dispersal, which is known to be influenced by threats of predation (Geraldi and Macreadie, Reference Geraldi and Macreadie2013). If the mere presence of mites imposes a fitness decrease on flies, it may influence the conditions under which flies will leave a resource patch (Peacor and Werner, Reference Peacor and Werner2008). Dispersal in turn may also limit the impact of non-infective effects endured by hosts with implications for fly population structures and dispersal patterns (Geraldi and Macreadie, Reference Geraldi and Macreadie2013). Future studies should integrate parasites into the ecology of fear hypothesis, and examine the indirect effects of parasitism on host population structures outside of infection.
Chronic stress from parasite exposure may impact host fitness. Many prey insects undergo hormonal changes following predator exposure that can affect growth, metamorphosis and immune function (Adamo et al. Reference Adamo, Easy, Kovalko, MacDonald, McKeen, Swanburg, Turnbull and Reeve2017; Kulkarni and Gramapurohit, Reference Kulkarni and Gramapurohit2017). Slos and Stoks (Reference Slos and Stoks2008) found that increases in the stress proteins of larval damselflies following predator exposure is linked with decreases in antioxidative catalase activity. In Drosophila, several stress-associated hormones are known to reduce long-term survival (Ekengren et al. Reference Ekengren, Tryselius, Dushay, Liu, Steiner and Hultmark2001; Kubrak et al. Reference Kubrak, Lushchak, Zandawala and Nassel2016), and if expressed following parasite exposure may explain the decrease in longevity observed here. Threat-induced stress in insects can both increase life-shortening traits and reduce life-sustaining processes. However, there is a paucity of research investigating the link between Drosophila stress hormones and the risk of parasitism. Based on general trends in the ecology of fear, we expect that related hormones may be produced in response to both predation and infection risk.
In conclusion, we investigated the fitness costs of chronic exposure to parasites independent of contact or infection with the parasite itself. To our knowledge, no other studies have experimentally shown a decrease in host fitness in the absence of direct contact between hosts and parasites. Ultimately, our results suggest another important mechanism by which parasites impact host populations, and that the costs of living in an infectious world extend beyond the direct effects of infection itself. Our work fits into the growing body of literature expanding the roles of parasites in a community ecology context. Studies may underestimate the effects of parasites on communities by neglecting the non-infective effects of parasites. Our findings also demonstrate the fruitfulness of testing hypotheses derived from predator–prey models in parasite–host systems and potential unities within natural enemy ecology.
Acknowledgements
Flies and mites used in this experiment came from cultures maintained by Keiran Mahden and Taylor Brophy. Cynthia Paszkowski assisted in statistical analysis. Carolyn McKinnon reviewed the manuscript for clarity and copy edited. Two anonymous reviewers provided constructive comments.
Financial support
Luong is supported by an Natural Sciences and Engineering Research Council discovery grant (#435245).