Published online by Cambridge University Press: 08 November 2004
The human malarial parasite Plasmodium falciparum extensively modifies its host erythrocyte, and to this end, is faced with an interesting challenge. It must not only sort proteins to common organelles such as endoplasmic reticulum, Golgi and mitochondria, but also target proteins across the ‘extracellular’ cytosol of its host cell. Furthermore, as a member of the phylum Apicomplexa, the parasite has to sort proteins to novel organelles such as the apicoplast, micronemes and rhoptries. In order to overcome these difficulties, the parasite has created a novel secretory system, which has been characterized in ever-increasing detail in the past decade. Along with the ‘hardware’ for a secretory system, the parasite also needs to ‘program’ proteins to enable high fidelity sorting to their correct subcellular location. The nature of these sorting signals has remained until relatively recently, enigmatic. Experimental work has now begun to dissect the sorting signals responsible for correct subcellular targeting of parasite-encoded proteins. In this review we summarize the current understanding of such signals, and comment on their role in protein sorting in this organism, which may become a model for the study of novel protein trafficking mechanisms.
Beginning in the early 1970s, Günter Blobel and co-workers discovered that proteins exported to the plasma membrane have a signal sequence that directs them across the membrane of the endoplasmic reticulum (ER) via vesicles to the Golgi body and from there again by vesicles to the plasma membrane (Blobel & Dobberstein, 1975a,b; Blobel et al. 1979). During the next decades, scientists characterized in detail the molecular mechanisms underlying protein transport to the plasma membrane and other destinations within an eukaryotic cell, such as the nucleus, mitochondria and chloroplast (Gorlich & Mattaj, 1996; Truscott, Brandner & Pfanner, 2003; Soll & Schleiff, 2004). We now know that there are a variety of signals of different types responsible for targeting a given protein to its eventual localization, the route it takes to get there, and the mechanisms and factors involved in its transport. However, extrapolating the knowledge gained from the study of a few model organisms to malarial parasites quickly reveals limitations.
Plasmodia, such as Plasmodium falciparum the aetiological agent of tropical malaria, maintain a complex life-cycle that, apart from alternating between vertebrate and invertebrate hosts, includes invasion of host cells. A distinctive set of organelles has evolved in response to this life-cycle, some of which are specifically involved in host cell entry. These include apical organelles of invasive stages, called rhoptries, micronemes and dense granules, which contain adhesins, proteases and membrane-active substances involved in recognizing and modifying the host cell membrane and surface proteins (Blackman & Bannister, 2001). Further novel organelles include a large acidic food vacuole, the site of host cell haemoglobin digestion (Foley & Tilley, 1998a), and the apicoplast, a plastid remnant acquired as a result of a secondary endosymbiotic event, which allows the parasite to synthesize de novo various vitamins and aromatic precursor molecules which it cannot obtain from its host (Kohler et al. 1997; Roos et al. 2002; Waller et al. 2003). Thus, in addition to targeting proteins to the usual eukaryotic organelles, the parasite must sort proteins to a variety of novel subcellular compartments (Table 1).
Table 1. Localization and possible targeting signals of Plasmodium falciparum proteins (Signal peptide prediction was carried out by program signalP. Prediction of trans-membrane domains was taken from the literature, or PlasmoDB annotation. MC, Maurer's clefts; PV, parasitophorous vacuole; TVM, tubovesicular membrane network; ER, endoplasmic reticulum; iRBC, infected red blood cell.)

Still, these are not the only trafficking problems the parasite faces. The evolutionary decision to inhabit erythrocytes, highly specialized denucleated cells, places the parasite in a rather difficult situation, as it severs itself from the external nutrient supply essential for growth and reproduction. Moreover, by developing within erythrocytes the parasite becomes vulnerable to splenic clearance mechanisms that remove senescent and infected erythrocytes from circulation (Craig & Scherf, 2001). To circumvent these problems, the parasite is forced to modify its host cell by exporting proteins into the cytoplasm and plasma membrane of the erythrocyte, which create new permeation pathways for nutrient uptake or are involved in immune evasion mechanisms (Craig & Scherf, 2001; Kirk, 2004).
A prominent morphological alteration of infected erythrocytes is the formation of electron-dense structures, termed knobs, at the erythrocyte plasma membrane (Kilejian, 1979) (Fig. 1). Knobs play a crucial role in the pathophysiology of falciparum malaria. They are composed of the P. falciparum knob-associated histidine-rich protein (KAHRP) (Culvenor et al. 1987; Pologe et al. 1987) which serves to anchor parasite-encoded immuno-variant adhesins collectively called P. falciparum erythrocyte membrane protein 1 (PfEMP1) (Baruch et al. 1995; Smith et al. 1995; Su et al. 1995; Crabb et al. 1997) to the erythrocyte cytoskeleton (Waller et al. 1999, 2002; Magowan et al. 2000), thereby mediating their correct surface presentation (Crabb et al. 1997). PfEMP1 confers a broad range of cytoadhesive interactions, leading to the sequestration of P. falciparum infected erythrocytes in the deep vascular bed (Miller et al. 2002). While cytoadhesion to the vascular endothelial lining prevents passage of infected erythrocytes through the spleen, and hence removal from circulation, it also causes severe pathology to the patient, including localized hypoxia, inflammatory reactions and the syndromes associated with cerebral and maternal malaria (Miller et al. 2002).

Fig. 1. Putative transport mechanisms in Plasmodium falciparum-infected erythrocytes. (1) Entry to the secretory pathway via the ER, mediated by an N-terminal signal peptide. The default pathway carries proteins through the secretory system, which may include a functional Golgi, via vesicles to the parasite plasma membrane. (2) At this point, proteins may travel through the parasitophorous vacuole in vesicles or as soluble, possibly chaperone bound, complexes. (3) Exit from the parasitophorous vacuole seems to be mediated by a ‘translocation’ domain, possibly involving putative protein transporters or coated vesicles. (4) Transport of coated vesicles to and from Maurer's clefts, which serve as an intermediate compartment where the cytoadhesion complex consisting of PfEMP1 and KAHRP appears to form. Maurer's clefts are membrane assemblies of parasite origin. (5) Chaperone-bound protein complexes may travel through the host cell cytosol before spontaneously inserting into or attaching to target membranes. (6) Integral membrane proteins may move by lateral diffusion from the PVM along a continuous intra-erythrocytic membrane network to the Maurer's clefts. This membrane network may involve the TVM or extensions of Maurer's clefts that are in contact with the PVM. Having reached the clefts, proteins such as PfEMP1 may travel to the surface of the infected red blood cell (7) by vesicular transport, or (8) at points of temporary and local fusion between Maurer's clefts and the erythrocyte plasma membrane. (9) Proteins may be transported by a translocon that bridges both the parasite plasma membrane and the PVM, similar to the major protein transport pathways across the outer membrane in certain bacteria (Nikaido, 2003). (10) Proteins trafficked to the food vacuole may be sorted into cytostomal vesicles which fuse with the membrane of the food vacuole. Import to the apicoplast (AP) and mitochondria (Mt) may involve translocases of TIC/TOC or TIM/TOM type (see other systems). ER, endoplasmic reticulum; N, nucleus; sERA, secondary ER of Apicomplexa; FV, food vacuole; PPM, parasite plasma membrane; PVM, parasitophorous vacuolar membrane; EM, erythrocyte plasma membrane; TVM, tubovesicular membrane network; Exp1, exported protein 1; MC, Maurer's cleft; PfEMP1, P. falciparum erythrocyte membrane protein 1; KAHRP, knob-associated histidine-rich protein; MESA, major erythrocyte surface antigen; RESA, ring erythrocyte surface antigen; STEVOR, subtelomeric variable open reading frame; Pfsec23, P. falciparum homologue of yeast sec23; PfSAR1p, P. falciparum homologue of yeast sar1p.
Given that the erythrocyte is lacking in even the basic machinery for protein transport, the parasite has evolved a protein trafficking machinery within its host cell. Trafficking proteins across two membrane systems (the parasite plasma and parasitophorous vacuolar membrane) and then through what is, correctly taken, an extracellular space (Fig. 1) poses an exceptional challenge in cell biology, which the parasite apparently mastered, testifying to its evolutionary flexibility. In this review, we summarize our current understanding of the signals required for the sorting of proteins to subcellular locations both within and outside the parasite's plasma membrane, and suggest possible mechanisms responsible for trafficking processes.
Immunolocalization studies have identified and mapped endoplasmic reticulum (ER) marker proteins such as the molecular chaperone PfBiP (Kumar et al. 1991), the calcium-binding protein PfERC (La Greca et al. 1997) and alpha and gamma subunits of the Pfsec61 translocon (Couffin et al. 1998) to the parasite cytoplasm or perinuclear compartments, suggested to be a plasmodial ER, or a subunit thereof. Coat protein (COP) I proteins, which in higher eukaryotes play a role in retrograde transport within the Golgi and also from the Golgi to the ER (Serafini et al. 1991; Ostermann et al. 1993), are present within the parasite cytoplasm, suggesting that vesicular transport between different subdomains of the secretory system takes place (Fig. 1, Table 1). However, at this time, there is only scattered evidence for the existence of a Golgi apparatus in P. falciparum. P. falciparum homologues of Golgi markers, such as PfERD2 and PfRab6 (cis- and trans-Golgi markers) can be shown to be segregated, and this has been suggested as being evidence of an unstacked Golgi in P. falciparum (Elmendorf & Haldar, 1993; de Castro et al. 1996; Van Wye et al. 1996).
Studies on the protozoan parasite Entamoeba histolytica have also failed to identify a morphologically recognizable Golgi apparatus (Dacks & Doolittle, 2001; Hehl & Marti, 2004). However, transport of a subset of exported proteins could be stopped by treatment with brefeldin A (BFA), a fungal metabolite which blocks the secretion of polypeptides by redistributing cis-Golgi components to the ER, suggesting the existence of a functional Golgi (Manning-Cela et al. 2003). Giardia intestinalis also lacks a classical Golgi apparatus, but manages to sort proteins to a whole variety of intracellular organelles (Hehl & Marti, 2004). These data suggest that a classical Golgi apparatus, as defined by morphological criteria, is not a strict requirement for the functioning of secretory pathways in some eukaryotes.
In the case of Plasmodia, studies on the rodent malarial parasite P. chabaudi suggest that secreted proteins in BFA-treated cells accumulate in a compartment that is distinct from the parasite ER, as defined by localization of the ER chaperone BiP (Wiser et al. 1997). Wiser et al. (1997) proposed naming this compartment the secondary ER of Apicomplexa (sERA) and hypothesized that it may represent a specialist organelle for trafficking of proteins to sites beyond the parasite plasma membrane (Wiser et al. 1997). Other studies suggested, however, that the contracted compartment observed within the cytoplasm of BFA-treated parasites represents an early stage in the elaboration of the ER, rather than a secondary ER (Wickham et al. 2001).
In higher eukaryotes, a large proportion of proteins are routed to their final location by vesicle-mediated transport, which is controlled by classes of cytosolic proteins that are recruited by the surface of vesicles to form a characteristic coat structure. Three types of coats have been identified in higher eukaryotes and yeast (Serafini et al. 1991; Ostermann et al. 1993; Kirchhausen, 2000). COP I vesicles mediate retrograde transport within the Golgi and from the Golgi back to the endoplasmic reticulum (ER) (Serafini et al. 1991; Ostermann et al. 1993; Letourneur et al. 1994; Orci et al. 1997), although some vesicles moving from the ER to the Golgi stack may also be COP I-coated (Pepperkok et al. 1993). COP I proteins may also play a role in transport along the endocytic pathway (Whitney et al. 1995; Aniento et al. 1996; Gu et al. 1997). The COP I coat consists of a heptamer plus the regulatory GTPase ARF (Waters, Serafini & Rothman, 1991). COP II-coated vesicles mediate anterograde transport between the ER and the Golgi (Kirchhausen, 2000). The COP II coat consists of Sec13/31 and Sec23/24 protein complexes and requires the activity of a small GTPase, referred to as the Sar1-activating complex (Kirchhausen, 2000). The third class of vesicles is the clathrin-coated vesicles, which form at the plasma membrane to mediate transport of molecules to endosomes and lysosomes (Robinson, 1994).
Homologues of P. falciparum COP I and COP II proteins have been identified and partially characterized (Albano et al. 1999; Adisa et al. 2001; Wickert et al. 2003a). All the putative COP I proteins found thus far have been mapped to the parasite cytoplasm. In comparison, COP II proteins have been mapped to membranous structures within the parasite body and within the erythrocyte cytoplasm (Table 1), suggesting that vesicular transport occurs both within the parasite and the host erythrocyte (Albano et al. 1999; Adisa et al. 2001). How the erythrocytic vesicular pathway is organized and where vesicular transport exactly takes place is still a matter of debate. There is, however, growing consensus that Maurer's clefts, membranous structures of parasite origin within the host erythrocyte cytoplasm play a pivotal role in transport processes across the host erythrocyte cytosol (Przyborski et al. 2003b). The putative COP II protein PfSec23, and a regulatory GTPase PfSar1p, both homologues of yeast proteins involved in the budding of vesicles from ER exit sites, have been localized to the Maurer's clefts (Albano et al. 1999; Wickert et al. 2003a) (Table 1). PfSec31, another putative COP II protein, also partly co-localized at the Maurer's clefts as does PfNSF (N-ethylmaleimide-sensitive factor) (Adisa et al. 2001; Hayashi et al. 2001) (Table 1). NSF, as shown in other systems, is part of a larger complex consisting of soluble NSF attachment protein (SNAP) and receptors for SNARE (SNAP receptor) and is involved in the docking/fusion of secretory vesicles with their target membrane (Mayer & Wickner, 1997; Kirchhausen, 2000).
PfEMP1 transiently associates with the Maurer's clefts on its way to the cell surface (Wickham et al. 2001; Kriek et al. 2003; Wickert et al. 2003b) (Fig. 1). At the Maurer's clefts, the cytoadherance complex composed of PfEMP1 and KAHRP is assembled, which then travels to the surface of the infected erythrocyte (Wickham et al. 2001). How the integral membrane protein PfEMP1 accesses the Maurer's clefts and how it leaves it is hotly debated in the literature. One school of thought suggests that Maurer's clefts receive and deliver cargo by vesicles (Wickham et al. 2001) (Fig. 1). Vesicles budding off from the parasitophorous vacuolar membrane (PVM) would carry PfEMP1 and possibly other trans-membrane proteins to the Maurer's clefts, which, according to this model, are considered as large anchored vesicles below the erythrocyte surface (Etzion & Perkins, 1989; Stenzel & Kara, 1989; Hibbs & Saul, 1994; Trelka et al. 2000; Taraschi et al. 2001, 2003; Wickham et al. 2001). The vesicular pathway would then continue at the trans-side of the Maurer's clefts, carrying the formed cytoadhesion complexes to the erythrocyte plasma membrane.
Another model suggests that Maurer's clefts are part of a large membranous network encompassing membranous profiles previously referred to as tubovesicular network, whirls and rings, originating from the PVM and extending to the periphery of the erythrocyte (Elford, Cowan & Ferguson, 1995; Lauer et al. 1997; Haldar et al. 2001; Wickert et al. 2003b) (Fig. 1). Once inserted into the network, integral membrane proteins such as PfEMP1 are suggested to move by lateral diffusion to the periphery of the erythrocyte until they reach a site where the network approaches the inner leaflet of the erythrocyte plasma membrane (Wickert et al. 2003b). At these sites the membrane network may temporarily fuse with the erythrocyte plasma membrane to deliver integral membrane proteins, or alternatively vesicles may bridge the remaining gap (Wickert et al. 2003b). An alternative model may be that PfEMP1 is first secreted as a soluble protein into the host erythrocyte cytoplasm before it spontaneously inserts into the Maurer's clefts (Fig. 1). The pros and cons of the various models have recently been reviewed in depth and will not be dealt with in this review due to space constraints (Przyborski et al. 2003b).
In higher eukaryotes, the first sorting point in protein trafficking takes place on free ribosomes within the cell cytoplasm, where proteins destined for entry to the secretory (bulk flow) pathway are recognized on the basis of an N-terminal signal sequence, which mediates co-translational translocation into either the lumen or membrane of the endoplasmic reticulum and hence entry to the secretory pathway (Kalies & Hartmann, 1998). It has been recognized for some time that some, but by far not all, P. falciparum proteins exported into the host erythrocyte contain canonical signal sequences at their N-termini (Lingelbach, 1993, 1997; Foley & Tilley, 1998a; Albano, Foley & Tilley, 1999; Kirk, Tilley & Ginsburg, 1999; Nacer et al. 2001) (Table 1). However, a detailed investigation of signals required for protein targeting has thus far been conducted on only three parasite-encoded proteins: exported protein-1 (Exp-1), the knob-associated histidine rich protein (KAHRP, HRPI) and the histidine rich protein II (HRPII).
Exp-1 is an integral membrane protein (Table 1), which is transported from the parasite to the PVM and to membranous structures within the host erythrocyte (Taylor et al. 1987; Panton et al. 1989; Behari & Haldar, 1994). Fusing Exp-1 fragments to luciferase revealed that the first 35 N-terminal amino acids of Exp-1, containing a canonical signal sequence, are sufficient to direct the chimera to the host cell cytosol (Burghaus & Lingelbach, 2001). The PfExp1 signal sequence was also sufficient to cause translocation across membranes in a reconstituted microsome system (Gunther et al. 1991; Lingelbach, 1993; Mattei et al. 1999). On the basis of these data Burghaus & Lingelbach (2001) suggested that a canonical signal sequence allows proteins to translocate across both the parasite plasma membrane and the PVM and access the erythrocyte cytosol, which was declared to be the default secretory pathway in P. falciparum (Burghaus & Lingelbach, 2001). However, a subsequent study presented contrasting data. Adisa et al. (2003) could show that the same first 35 amino acids of Exp-1 fused to green fluorescent protein (GFP) traffic the chimeric protein only to the lumen of the parasitophorous vacuole, and no further (Adisa et al. 2003). Control constructs encoding GFP alone are retained within the cytoplasm of the parasite (Przyborski, unpublished observations). The difference between the two studies are not yet resolved and may require further investigation. One possible explanation may be that luciferase carries an intrinsic SKL sequence at its C-terminus that, as shown in other eukaryotes, functions as a peroxisomal import sequence (Miura et al. 1992). Thus it is possible that this motif or other cryptic motifs within the luciferase are responsible for translocation of the chimera into the erythrocyte cytosol (Adisa et al. 2003).
KAHRP lacks a classical N-terminally located signal peptide (Table 1), and it is not translocated across membranes in the microsomal system (Mattei et al. 1999). Despite conflicting reports, the general consensus is that KAHRP transport is BFA sensitive, suggesting that it traffics via the ER/Golgi secretory system of the parasite (Mattei et al. 1999; Wickham et al. 2001; Lopez-Estrano et al. 2003). By fusing KAHRP fragments N-terminally to GFP Wickham et al. (2001) have shown that amino acids 1 to 60 of KAHRP (containing a hydrophobic region) are necessary to allow export of a reporter protein from the parasite into the lumen of the parasitophorous vacuole (Wickham et al. 2001). To reach the final destination of KAHRP within the host cell cytosol, another 60 amino acids were necessary for passage across the PVM (Wickham et al. 2001). This additional recessed trafficking signal contained a histidine-rich domain with interspersed stretches of non-histidine residues, and it was suggested that these non-histidine residues might be responsible for trafficking across the PVM (Wickham et al. 2001). However a later, more detailed study presented evidence that the histidines themselves are a feature necessary for translocation across the PVM (Lopez-Estrano et al. 2003) (see below).
The histidine rich protein II HRP II is a heme binding protein exported into the host cell cytosol (Taylor et al. 1987; Panton et al. 1989; Papalexis et al. 2001). Lopez-Estrano et al. (2003) presented evidence suggesting that HRP II traffics along the lumen of the inter-erythrocytic membrane network, before exiting at the Maurer's clefts, where it associates with the external face of the membrane system (Lopez-Estrano et al. 2003). By generating chimeric proteins of HRP II fragments with GFP it was found that the first 124 amino acids are sufficient to allow transport to the host cell cytoplasm. Delineation of this sequence revealed a canonical signal sequence of 27 amino acids (Table 1), an asparagine-rich domain of 29 amino acids and a histidine-rich domain of 68 amino acids. The signal sequence alone allows access to the secretory pathway and hence lumen of the parasitophorous vacuole. The histidine-rich domain, which can be replaced by a KAHRP minimal histidine-rich domain, mediates transport along the lumen of an intra-erythrocytic membrane system to the internal face of the Maurer's clefts, where the asparagine-rich domain appears then to be responsible for translocation across the Maurer's clefts membrane into the erythrocyte cytosol (Lopez-Estrano et al. 2003). Whilst these studies may show that histidine rich regions can function to target soluble proteins through the lumen of the intra-erythrocytic membrane system, the fact remains that most exported parasite proteins, including many exported to the Maurer's clefts, do not contain such histidine rich domains, suggesting that KAHRP and HRP II may represent special cases, rather than the general rule.
The export of PfEMP1 is an interesting quandary. PfEMP1 contains neither a classical, nor an internal signal sequence (Table 1), but manages to transverse both the parasite plasma and PVM on its way to the surface of the host cell (Albano, Foley & Tilley, 1999; Kriek et al. 2003; Wickert et al. 2003b). PfEMP1 transport is BFA sensitive, suggesting that (at least at some stage during the transport process) it passes through the endo-membrane system; however, the signals mediating its translocation into the ER membrane remain unknown. Whether insertion of PfEMP1 into the ER occurs co-, post-translationally or spontaneously remains to be shown. A model of C-terminal post-translational insertion may be difficult to reconcile with evidence regarding the membrane topology of PfEMP1 in the Maurer's clefts or on the surface of the infected erythrocyte (Kriek et al. 2003). Several studies have shown that the acidic terminal sequence of PfEMP1 is presented to the cytoplasm during its transport (Waterkeyn et al. 2000; Wickham et al. 2001; Kriek et al. 2003; Wickert et al. 2003b). Another possibility is that PfEMP1 is post-translationally co-translocated (hitchhiker mechanism) into the ER membrane by association with another protein which contains the necessary targeting sequence. Such co-translocation has been described in bacteria (Rodrigue et al. 1999).
A recent publication has identified a new family of resident Maurer's clefts proteins termed PfMC-2TM (Sam-Yellowe et al. 2004). PfMC-2TM, STEVOR (another integral trans-membrane protein located at Maurer's clefts) and RIFIN (immuno-variant antigens located on the host erythrocyte surface) share structural similarities in that they all contain a canonical N-terminal sequence (Table 1) followed by a domain enriched in asparagines and basic amino acids (usually lysine) and two trans-membrane domains. It has been suggested that the asparagine/lysine rich domain may represent a translocation signal for transport across the parasitophorous vacuole, albeit experimental proof is lacking (Sam-Yellowe et al. 2004).
Taken together, the available body of evidence suggests that transport from the parasite to the host erythrocyte is a multi-step process, requiring at least a bi-partite signal. The first N-terminal sequence, which may or may not be a canonical signal sequence, appears to mediate entry into the secretory pathway and transport into the parasitophorous vacuolar lumen. A recessed trafficking signal then allows proteins to pass the PVM and enter the erythrocyte cytosol. These data are consistent with a model proposed by Lingelbach almost a decade ago, in which protein transport to the host cell cytoplasm takes place in two steps, with the parasitophorous vacuole acting as an intermediate compartment (Ansorge et al. 1996). As stated above these conclusions stem from fusions of the protein of interest with reporter proteins. Using reverse genetics on the full length, original protein to unequivocally demonstrate functionality of the signal sequences is difficult in malarial parasites and has not yet been successfully attempted. Plasmodium ssp. have a haploid genome and mutation of essential genes/proteins frequently results in a lethal phenotype.
At present there is no information available regarding the factors that interact with trafficking signals in P. falciparum. The little conservation amongst trafficking signals of different exported P. falciparum proteins suggests that there may be alternative secretory pathways. Energy appears to be an important mediator in the translocation of proteins across the PVM (Ansorge et al. 1996). Removing ATP from the cytosol of the host erythrocyte ablated transport processes, which has been interpreted as being evidence of a protein carrier that transports proteins across the PVM in an energy-dependent fashion (Ansorge et al. 1996) (Fig. 1). Despite the identification of putative ABC transporters in membranous compartments within the host erythrocyte cytosol (Bozdech et al. 1996), whether they play a role in such transport processes requires further experimentation. Alternatively, energy may be consumed by molecular chaperones present within the parasitophorous vacuolar lumen, which would unfold or prevent folding of proteins that have translocated across the parasite plasma membrane into the parasitophorous vacuolar lumen, thereby keeping them in a translocation competent state before their insertion into, or across the PVM (Fig. 1). In other systems, such as that of mitochondrial protein import, molecular chaperones are present on the receiving side of the membrane, helping proteins thread through the membrane, and preventing their back-translocation (Lill & Neupert, 1996). A recent study has presented evidence suggesting that parasite encoded chaparones remain in the parasite's body and do not participate in protein transport through the infected erythrocyte cytosplasm (Banumathy, Singh & Tatu, 2002). Interestingly, host chaperones and co-chaperones were recruited in the knobs, suggesting that host cell chaperones may play a role in export of knob-associated proteins (Banumathy et al. 2002).
Provided export into the parasitophorous vacuolar lumen represents the default secretory pathway in P. falciparum, the parasite needs, like any other eukaryotic cell, a mechanism to retain ER and Golgi resident proteins. In other systems, several specific signals have been identified for the retention/retrieval of ER proteins (Nilsson, Jackson & Peterson, 1989; Nilsson & Warren, 1994). Many soluble ER resident proteins contain a tetra-amino acid sequence KDEL (HDEL in yeast), which by binding to a specific receptor, ERD2, facilitates their retrograde transport from the cis-Golgi back to the ER (Lewis & Pelham, 1990, 1992; Lewis, Sweet & Pelham, 1990). The retrieval signals for ER trans-membrane proteins include a C-terminal di-lysine (KKXX) or an N-terminal di-arginine motif (XXRR) (Nilsson et al. 1989; Jackson, Nilsson & Peterson, 1990, 1993). Experimental evidence from studies on the related parasite Toxoplasma gondii (Hager et al. 1999; Roos et al. 1999) would suggest that similar mechanisms may exist in Apicomplexa including P. falciparum. Several P. falciparum homologues of ER marker proteins contain the classical luminal –XDEL-COOH signal. PfERC for example contains –IDEL-COOH (La Greca et al. 1997) and a BiP homologue contains –SDEL-COOH (Peterson et al. 1988). Retrieval of the membrane bound – XDEL receptor PfERD2 may be mediated by a C-terminal KKXX motif.
Mitochondria and the chloroplast of plants represent one class of organelles to which proteins are sorted independent of the ER. Over a billion years of evolution, genes originally encoded in the endosymbiont from which these organelles originated, have been transferred to the nucleus. The corresponding proteins, therefore, need to be post-translationally imported. P. falciparum contains mitochondria and several studies have successfully targeted GFP chimera to this organelle. In the case of PfHSP60, recent studies have identified the import signal(s) within a region of 68 amino acids (Sato, Rangachari & Wilson, 2003; Sato et al. 2004; Sato & Wilson, 2004) (Table 1). In higher eukaryotes, the signals required for import into mitochondria contain N-terminal pre-sequences, termed transfer peptides, of 25 to 125 amino acids in length, which are rich in hydroxylated amino acids, but sparse of acidic residues. Plant mitochondrial transfer peptides frequently form amphipathic α-helices, with positive charges clustered on one side. To enter the mitochondrium the transfer peptides associate with a protein translocase complex in the outer membrane, termed TOM (translocase of outer mitochondria membrane) (Hoogenraad, Ward & Ryan, 2002; Neupert & Brunner, 2002). A recent report has described P. falciparum orthologues of several subunits of the TOM complex (Macasev et al. 2004) and proteins eligibly targeted to the parasite's mitochondria appear to possess N-terminal sequences with homologies to mitochondrial transfer peptides (Bender et al. 2003).
Malaria parasites have an evolutionary remnant of a plant chloroplast, termed apicoplast, resulting from secondary endosymbiosis of a red alga (Kohler et al. 1997; Foth & McFadden, 2003). The apicoplast is surrounded by four membrane systems, and hence represents a more complex system for protein import than chloroplasts that are surrounded by only two membranes (Foth et al. 2003). Accordingly, studies on P. falciparum and its apicomplexan cousin T. gondii, have shown that targeting to the apicoplast differs from targeting to the chloroplast (Waller et al. 1998, 2000; Field, Ali & Field, 1999; Roos et al. 1999; van Dooren et al. 2002; Foth et al. 2003). The major difference is that apicomplexan proteins require an N-terminal signal sequence in addition to a recessed transit peptide (Table 1). This finding was first revealed by the analysis of the acyl-carrier protein (ACP). GFP constructs containing only the ACP N-terminal signal sequences were targeted to the lumen of the parasitophorous vacuole. Constructs that, in addition to the signal sequences, contained the recessed transit peptide were imported into the apicoplast (Waller et al. 2000).
Comparative sequence analysis of a large data set of P. falciparum proteins predicted certain requirements for plasmodial transit peptides, which was subsequently verified using an elegant mutagenesis approach (Foth et al. 2003). According to these studies, the apicoplast transit peptides are highly enriched in lysine and asparagines and depleted in acidic glutamic and aspartic acid residues, especially in the first 20 amino acids. This results in an overall positive charge, a feature shared with plant chloroplast transit peptides. In plant transit peptides, however, this positive charge is mostly provided by arginine (Bruce, 2001). The apicoplast transit peptide contains a putative HSP70 binding site (Foth et al. 2003). Binding of HSP70 may hold the transit peptide in the primary conformation, thereby presenting the charge patch to the import machinery.
On the basis of these findings, the following model for protein trafficking into the apicoplast was formulated (Foth et al. 2003). The N-terminal signal sequence mediates co-translational import to the ER, and is cleaved upon access to the ER lumen (Waller et al. 2000). The transfer peptide then diverts the protein away from the default secretory pathway and into the apicoplast. A consequence of this model is that all proteins entering the secretory pathway wash past the apicoplast and only those possessing an appropriate transit peptide are fished out by the apicoplast import machinery. The fact that the addition of the -XDEL ER retention signal does not affect trafficking of proteins to the apicoplast provides further support for this model (Roos et al. 1999). Apparently, apicoplast proteins exit the secretory pathway before reaching the cis-Golgi. Along the same lines, BFA has no effect on protein transport to the apicoplast (Foth & McFadden, 2003), suggesting that vesicular transport steps are not required for targeting to the apicoplast. A simple explanation for these findings is that the apicoplast resides within the ER, which would not be without precedent. In cryptomonads, the apicoplast is surrounded by the rough ER (Gilson & McFadden, 2002), and the outer membrane of the heterokont chloroplast is continuous with the rough ER (Gilson & McFadden, 2002).
Another class of proteins that are trafficked independently of the ER are the nuclear proteins. Analysis of the nuclear localization signals (NLS) of many eukaryotic proteins has revealed four main classes (Gorlich & Mattaj, 1996): PKKKRKV as exemplified by SV40 large T antigen (Lanford, Kanda & Kennedy, 1986); KKPAATKKAGQAKKKK, a bipartite signal consisting of two clusters of basic residues separated by a 10-14 spacer (Robbins et al. 1991); PAAKRVKLD, as found in the c-Myc NLS (Dang & Lee, 1988); and various other NLSs, such as the ones associated with ribosomal proteins and hnRNPS (Weighardt, Biamonti & Riva, 1995). Some nuclear proteins do not appear to have a NLS and it seems that they enter the nucleus via co-transport with a protein that has one (Gorlich & Mattaj, 1996).
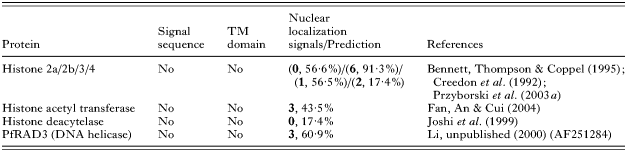
At this time, there are no publications concerned with the import of P. falciparum proteins to the nucleus, however a PSORTII analysis of various nuclear located proteins in P. falciparum has revealed that some, but not all, nuclear proteins contain one or more NLS (Table 2). For example, histone H4 contains 2 NLSs (Przyborski et al. 2003a). P. falciparum homologues of karyopherin alpha (importin alpha) and karyopherin beta (transportin 1, importin beta) (Xu et al. 2002; Mohmmed et al. 2003), responsible for import of proteins into the nucleus, also carry classical NLS. In comparison, histone deacetylase appears to lack a NLS and it may be co-imported by a member of the MEF2 family of transcription factors, which provides the NLS (Borghi et al. 2001). The non-classical M9 nuclear localization signal has previously been described in P. falciparum. This signal has been shown to function both as a nuclear import as well as export signal in higher eukaryotes (Bogerd et al. 1999), suggesting that P. falciparum may engage in nucleo-cytoplasmic shuttling of importins and exportins.
Table 2. Nuclear localization signals (NLS) of nuclear targeted proteins of Plasmodium falciparum (Numbers in bold refer to number of predicted NLS, percentage refers to % chance of nuclear localization, as determined by program PSORTII Nakai & Horton (1999).)
The food vacuole of P. falciparum is the site of haemoglobin degradation and heme detoxification (Foley & Tilley, 1998b). During intra-erythrocytic development P. falciparum digests the erythrocyte haemoglobin to meet is nutrient requirements and to compensate for osmotic imbalances resulting from its own anabolic activities (Lew, Tiffert & Ginsburg, 2003). Heme released during this process is believed to be detoxified in the food vacuole through mechanisms involving peroxidative decomposition and crystallization to inert hemozoin (Ginsburg et al. 1998; Loria et al. 1999; Pagola et al. 2000). Further, the food vacuole is a dynamic acidic calcium store (Biagini et al. 2003), and it contains in its membrane several transporters implicated in various mechanisms of anti-malarial drug resistance (Winstanley, 2001; Warhurst, Craig & Adagu, 2002). Enzymes responsible for metabolism of haemoglobin need to be targeted to this organelle; however, the pathway responsible for their trafficking remains poorly characterized. The P. falciparum aspartic proteinase plasmepsin II is made as a pre-protein (pro-plasmepsin), containing an N-terminal trans-membrane domain that is suggested to play a role in transport to its site of action, the lumen of the food vacuole (Klemba et al. 2004) (Table 1). A recent publication suggested that pro-plasmepsin is transported in membrane bound vesicles from the parasite ER/Golgi to cytostomic evaginations of the parasite plasma/vacuolar membrane, where it appears to insert into the outer of the two membranes enclosing the cytostomic vesicle (Klemba et al. 2004) (Fig. 1). These vesicles would then travel to, and fuse with the food vacuole (Klemba et al. 2004).
The invasion of apicomplexan parasites into host cells involves proteins located in specialized exocytic organelles (micronemes, rhoptries, and granula) (Aikawa et al. 1978; Dubremetz & Schwartzman, 1993; Carruthers & Sibley, 1997). Trafficking of rhoptry proteins has been studied in detail in T. gondii, and may provide some clues as to mechanisms in P. falciparum.
Rhoptry proteins of both P. falciparum and T. gondii seem to have an N-terminal signal sequence (Lustigman et al. 1988; Peterson et al. 1989; Howard & Reese, 1990; Saul et al. 1992; Saul, Yeganeh & Howard, 1992) (Table 1). It is proposed that the signal sequence directs these proteins to the secretory pathway, where a secondary targeting signal then directs them away from the bulk flow. Data obtained in T. gondii seem to support this model, showing that this secondary transport step is mediated by specific amino acid motifs, or interaction with an escorter protein (Baldi et al. 2000; Di Cristina et al. 2000; Hoppe & Joiner, 2000; Hoppe et al. 2000; Cerede et al. 2002; Ngo et al. 2003). Sorting and trafficking of T. gondii membrane proteins to the micronemes and rhoptries appears to require essential tyrosine-based and acidic amino acid motifs (Di Cristina et al. 2000; Hoppe & Joiner, 2000; Reiss et al. 2001).
So far, only two studies have attempted to relate these data to P. falciparum, both failing to implicate such C-terminal motifs as essential trafficking signals (Baldi et al. 2000; Gilberger et al. 2003). A C-terminal truncation mutant of the rhoptry-associated protein 1 (Rap1) was correctly targeted to the rhoptries, suggesting that rhoptry sorting signals are not conserved between Apicomplexa. Another study investigating trafficking of the erythrocyte-binding antigen 175 (EBA175), a protein targeted to micronemes, supports this conclusion (Gilberger et al. 2003). The 50 amino acid long C-terminal cytoplasmic domain of EBA175 contains both acidic, and tyrosine motifs which could be expected to be responsible for its trafficking to the micronemes of the apical complex. A C-terminal truncation mutant of EBA175, missing these motifs, is however, still trafficked correctly (Gilberger et al. 2003).
Interestingly, analysis of the transgenic Rap1 parasites revealed that deletion of the C-terminal domain of Rap1 ablated the transport of a second rhoptry protein, Rap2 (Baldi et al. 2000). It appears that, in the transgenic parasites, Rap2 is retained in a compartment related to the lumen of the ER. This finding suggests that the C-terminal domain of Rap1 is necessary for proper targeting of Rap2, possibly by affecting the correct folding of Rap2 (Baldi et al. 2000).
Like protein transport to apical organelles in T. gondii, Rap1, EBA175 and other proteins may be transported in concert with an escorter protein containing essential targeting motifs or P. falciparum may utilize further, as yet uncharacterized, targeting motifs. It has also been suggested that signals may be unnecessary to target proteins to the rhoptries, and that the time-point of expression alone may allow proteins to be trafficked to this specialized organelle (Kocken et al. 1998).
Whilst the release of the complete annotated sequence of the P. falciparum genome will undoubtedly provide many clues as to putative factors constituting the secretory and trafficking machinery, caution should be taken to ensure that experimental evidence is provided to verify hypothetical pathways. The development of various tools for transfection of P. falciparum has provided researchers with the tools necessary to begin an earnest investigation into the nature of trafficking pathways and the signals destining proteins to certain locations within the parasite and its host erythrocyte. Recent advances in transfection technology, such as the transfection vector pARL1a+ (Crabb et al. 2004; Wrenger & Müller, 2004), the inclusion of rep20 repeats in transfection vectors (O'Donnell et al. 2002), and the use of alternative methods of transfection (Deitsch, Driskill & Wellems, 2001) has reduced the time required to establish stable transfectant lines from up to 60 days (for GFP expressing lines) to just over two weeks, and will continue to speed the pace of progress. Reporter proteins such as GFP, which have been used to great effect to delineate trafficking pathways and signals in other systems will play an ever increasing role in future studies. To sum up, although much progress has been made in understanding the complex trafficking system in P. falciparum, we are still faced with more questions than answers. Addressing these questions will require years of careful research, but can be expected to yield more than the odd surprise.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft and the European Commission (BioMalPar). We apologize to all of our colleagues whose work we have neglected to cite, due to space constraints.

Table 1. Localization and possible targeting signals of Plasmodium falciparum proteins

Fig. 1. Putative transport mechanisms in Plasmodium falciparum-infected erythrocytes. (1) Entry to the secretory pathway via the ER, mediated by an N-terminal signal peptide. The default pathway carries proteins through the secretory system, which may include a functional Golgi, via vesicles to the parasite plasma membrane. (2) At this point, proteins may travel through the parasitophorous vacuole in vesicles or as soluble, possibly chaperone bound, complexes. (3) Exit from the parasitophorous vacuole seems to be mediated by a ‘translocation’ domain, possibly involving putative protein transporters or coated vesicles. (4) Transport of coated vesicles to and from Maurer's clefts, which serve as an intermediate compartment where the cytoadhesion complex consisting of PfEMP1 and KAHRP appears to form. Maurer's clefts are membrane assemblies of parasite origin. (5) Chaperone-bound protein complexes may travel through the host cell cytosol before spontaneously inserting into or attaching to target membranes. (6) Integral membrane proteins may move by lateral diffusion from the PVM along a continuous intra-erythrocytic membrane network to the Maurer's clefts. This membrane network may involve the TVM or extensions of Maurer's clefts that are in contact with the PVM. Having reached the clefts, proteins such as PfEMP1 may travel to the surface of the infected red blood cell (7) by vesicular transport, or (8) at points of temporary and local fusion between Maurer's clefts and the erythrocyte plasma membrane. (9) Proteins may be transported by a translocon that bridges both the parasite plasma membrane and the PVM, similar to the major protein transport pathways across the outer membrane in certain bacteria (Nikaido, 2003). (10) Proteins trafficked to the food vacuole may be sorted into cytostomal vesicles which fuse with the membrane of the food vacuole. Import to the apicoplast (AP) and mitochondria (Mt) may involve translocases of TIC/TOC or TIM/TOM type (see other systems). ER, endoplasmic reticulum; N, nucleus; sERA, secondary ER of Apicomplexa; FV, food vacuole; PPM, parasite plasma membrane; PVM, parasitophorous vacuolar membrane; EM, erythrocyte plasma membrane; TVM, tubovesicular membrane network; Exp1, exported protein 1; MC, Maurer's cleft; PfEMP1, P. falciparum erythrocyte membrane protein 1; KAHRP, knob-associated histidine-rich protein; MESA, major erythrocyte surface antigen; RESA, ring erythrocyte surface antigen; STEVOR, subtelomeric variable open reading frame; Pfsec23, P. falciparum homologue of yeast sec23; PfSAR1p, P. falciparum homologue of yeast sar1p.
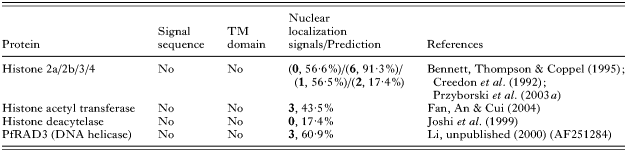
Table 2. Nuclear localization signals (NLS) of nuclear targeted proteins of Plasmodium falciparum