INTRODUCTION
Malaria is an infectious vector-borne disease responsible for significant global morbidity and mortality (Snow et al. Reference Snow, Guerra, Noor, Myint and Hay2005). The vast majority of the fatal cases of malaria are caused by Plasmodium falciparum, although Plasmodium vivax, which is itself responsible for huge morbidity (Mendis et al. Reference Mendis, Sina, Marchesini and Carter2001), may also, although rarely, be severe and fatal (Kochar et al. Reference Kochar, Saxena, Singh, Kochar, Kumar and Das2005; Rogerson and Carter, Reference Rogerson and Carter2008; Suwanarusk et al. Reference Suwanarusk, Chavchich, Russell, Jaidee, Chalfein, Barends, Prasetyorini, Kenangalem, Piera, Lek-Uthai, Anstey, Tjitra, Nosten, Cheng and Price2008; ter Kuile and Rogerson, Reference ter Kuile and Rogerson2008; Tjitra et al. Reference Tjitra, Anstey, Sugiarto, Warikar, Kenangalem, Karyana, Lampah and Price2008; Andrade et al. Reference Andrade, Reis-Filho, Souza-Neto, Clarencio, Camargo, Barral and Barral-Netto2010). The sequestration of parasitized erythrocytes in the deep vasculature is the main cause of the pathology of severe falciparum malaria. Mature trophozoites and schizonts sequester in the peripheral circulation due to the adhesion of infected erythrocytes to endothelial cells (cytoadherence) and with uninfected erythrocytes (rosetting) leading to significantly impaired blood circulation (Miller et al. Reference Miller, Good and Milon1994). Infected erythrocytes also become more rigid and adhere to different cell types (Raventos-Suarez et al. Reference Raventos-Suarez, Kaul, Macaluso and Nagel1985).
The malaria parasite modifies its host-cell environment, presumably to enhance its own survival, and this leads to pathological consequences for the host. While all stages of the parasite modify their host cell to a certain extent, infected erythrocytes are subject to extensive modifications that are vital for parasite survival (Miller et al. Reference Miller, Good and Milon1994). Human erythrocytes lack protein trafficking machinery, so, following invasion, P. falciparum first has to establish a trafficking pathway to export various proteins to the surface of the infected cell.
HOST-CELL MODIFICATION AND PROTEIN TRAFFICKING
The modification of the erythrocyte from a free-flowing and essentially non-adhesive cell to one that is capable of adhering to endothelial cells and non-infected erythrocytes (Miller et al. Reference Miller, Good and Milon1994) highlights the dramatic modification that occurs following the invasion of the malaria parasite. Major changes that occur in the infected erythrocyte include the formation of small protrusions on the surface of the cell (knobs), alterations in ion channel behaviour (Decherf et al. Reference Decherf, Egee, Staines, Ellory and Thomas2004; Staines et al. Reference Staines, Alkhalil, Allen, De Jonge, Derbyshire, Egee, Ginsburg, Hill, Huber, Kirk, Lang, Lisk, Oteng, Pillai, Rayavara, Rouhani, Saliba, Shen, Solomon, Thomas, Verloo and Desai2007), the formation of novel channels for nutrient import (Saliba et al. Reference Saliba, Horner and Kirk1998; Desai et al. Reference Desai, Bezrukov and Zimmerberg2000; Staines et al. Reference Staines, Powell, Thomas and Ellory2004), membrane rigidity and cell deformability (Glenister et al. Reference Glenister, Coppel, Cowman, Mohandas and Cooke2002) and altered behaviour of infected erythrocytes in the microcirculation (Diez-Silva et al. Reference Diez-Silva, Park, Huang, Bow, Mercereau-Puijalon, Deplaine, Lavazec, Perrot, Bonnefoy, Feld, Han, Dao and Suresh2012). These modifications occur as a result of the export of various effector proteins in the infected erythrocyte. Mature erythrocytes are devoid of any endogenous vesicle trafficking machinery; therefore, for the parasite to export proteins, it needs to establish its own trafficking pathway. The major obstruction for protein export in the infected erythrocyte is the interface of the parasite and the host-cell cytoplasm, i.e. the parasitophorous vacuole (PV) and its associated membrane (parasitophorous vacuole membrane (PVM)). In order to alter the host cell from a hostile environment to one that is conducive for parasite survival, parasite-encoded proteins have to traverse the PV and the PVM and enter the erythrocyte.
How, then, does the parasite achieve this? To understand protein trafficking beyond the PVM, two pathways of protein export have been proposed: one is vesicle mediated (Gormley et al. Reference Gormley, Howard and Taraschi1992; Foley and Tilley, Reference Foley and Tilley1998; Taraschi et al. Reference Taraschi, Trelka, Martinez, Schneider and O'Donnell2001) and the other channel mediated (Gormley et al. Reference Gormley, Howard and Taraschi1992; Schatz and Dobberstein, Reference Schatz and Dobberstein1996; Foley and Tilley, Reference Foley and Tilley1998; Schnell and Hebert, Reference Schnell and Hebert2003). Plasmodium falciparum proteins thought to be exported via vesicles include PfEMP-1, PfSar1p, Pfsec31p, etc. (Gormley et al. Reference Gormley, Howard and Taraschi1992; Ansorge et al. Reference Ansorge, Benting, Bhakdi and Lingelbach1996; Trelka et al. Reference Trelka, Schneider, Reeder and Taraschi2000; Taraschi et al. Reference Taraschi, Trelka, Martinez, Schneider and O'Donnell2001, Reference Taraschi, O'Donnell, Martinez, Schneider, Trelka, Fowler, Tilley and Moriyama2003), while soluble proteins such as KAHRP, PHIST, MESA, PfEMP-3, FIKK kinase, etc. are thought to be exported via the channel pathway (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004; Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006). The channel-mediated protein export pathway in P. falciparum is discussed here in detail. First, a signal sequence directs the protein for export to the lumen of the endoplasmic reticulum (ER) where the export signal is recognized and processed for entry into the secretory pathway. Through this secretory pathway, the protein crosses the PV and then the PVM through translocons present in the membrane (de Koning-Ward et al. Reference de Koning-Ward, Gilson, Boddey, Rug, Smith, Papenfuss, Sanders, Lundie, Maier, Cowman and Crabb2009). The protein then enters the erythrocyte cytosol and is finally directed to its destination, which may be the host-cell cytosol, Maurer's clefts (MCs), the erythrocyte membrane or the surface of the erythrocyte (Fig. 1) (Cooke et al. Reference Cooke, Lingelbach, Bannister and Tilley2004).

Fig. 1. Diagrammatic representation of channel-mediated soluble protein trafficking in the P. falciparum-infected erythrocyte. The figure shows exported proteins most likely exported via the secretory vesicle pathway. These proteins are recognized and processed (signal sequence and PEXEL) in Golgi-like bodies and then accumulate in small secretory vesicles that extend towards the parasite plasma membrane (PPM). After reaching PPM, a secretory vesicle becomes fused with PPM and discharges its soluble proteins in PV. The exported proteins then translocate the PVM with the help of a translocon present in PVM and enter its destinations in erythrocytes such as red cell cytoplasm, MCs, red cell membrane and the surface of red cells. TVN, tubulo-vesicular network; PV, parasitophorous vacuole; PVM, parasitophorous vacuole membrane; PM, parasite membrane; ER, endoplasmic reticulum; MCs, Maurer's clefts. The modelling channel-mediated trafficking of exported protein in infected erythrocyte is a modified version of Cooke et al. (2004).
Recently, several key issues in protein export have been elucidated. These include the identification of an export signal (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004), the mechanism by which the export signal is processed (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008; Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009, Reference Boddey, Hodder, Gunther, Gilson, Patsiouras, Kapp, Pearce, de Koning-Ward, Simpson, Crabb and Cowman2010; Russo et al. Reference Russo, Babbitt, Muralidharan, Butler, Oksman and Goldberg2010; Bhattacharjee et al. Reference Bhattacharjee, Stahelin, Speicher, Speicher and Haldar2012), the physical nature of exported proteins in the PV (Gehde et al. Reference Gehde, Hinrichs, Montilla, Charpian, Lingelbach and Przyborski2009), the translocon machinery (de Koning-Ward et al. Reference de Koning-Ward, Gilson, Boddey, Rug, Smith, Papenfuss, Sanders, Lundie, Maier, Cowman and Crabb2009) and the role of MCs in directing proteins to the surface of the host cell (Bhattacharjee et al. Reference Bhattacharjee, van Ooij, Balu, Adams and Haldar2008). Based on these breakthroughs, the channel-mediated protein export pathway has been modelled (Fig. 1) and advances in our current understanding of the various steps involved in protein trafficking are discussed in detail below.
Identification of the export signal and prediction of exported proteins
A major advance in our understanding of the protein trafficking mechanisms of malaria parasites was the identification of an export signal sequence, and the use of this to predict which proteins the parasite exports (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004). These studies revealed a consensus conserved amino acid motif downstream of the N-terminal sequences of many exported proteins, which was termed the Plasmodium export element (PEXEL) or the vacuole transport signal (VTS) (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004), which is conserved structurally and functionally across the Plasmodium species infecting humans and birds (Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004). The PEXEL/VTS motif generally lies within a 20–60 amino-acid stretch downstream of the N-terminal sequence of many exported proteins (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004; Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006) (Fig. 2). It consists of five amino acids RxLxE/Q/G, of which arginine (R) and lysine (L) are essential for recognition whereas the fifth amino acid glutamate (E) is not essential and can be replaced with glutamine (Q) (frequently) or glycine (G) (rarely) (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004) (x could be any amino acid). Further, the PEXEL processed proteins get acetylated at the new N-terminus, which appears to be crucial for recognition and transport of exported proteins to red cells (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008; Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009). A recent study has identified that many exported proteins can have a relaxed PEXEL motif (RxLxxE), which is functional and processed by the same mechanism used for canonical PEXEL (Boddey et al. Reference Boddey, Carvalho, Hodder, Sargeant, Sleebs, Marapana, Lopaticki, Nebl and Cowman2013). Based on the updated information of the relaxed PEXEL motif, ExportPred version 1.0 (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006) has been re-designed (version 2.0), which identified 73 additional exported proteins from the falciparum genome (Boddey et al. Reference Boddey, Carvalho, Hodder, Sargeant, Sleebs, Marapana, Lopaticki, Nebl and Cowman2013).

Fig. 2. Diagrammatic representation of P. falciparum PEXEL and PNEP protein structure. The figure represents a typical structure of an exported protein. The arrow indicates ER resident enzyme Plasmepsin-V that cleaves the PEXEL motif. Scenarios of export of PEXEL-negative exported proteins (PNEPs) are also presented. The bar above the colour indicates hydrophobic regions potentially involved in export and the figure is a modified version of Spielmann and Gilberger (Reference Spielmann and Gilberger2010).
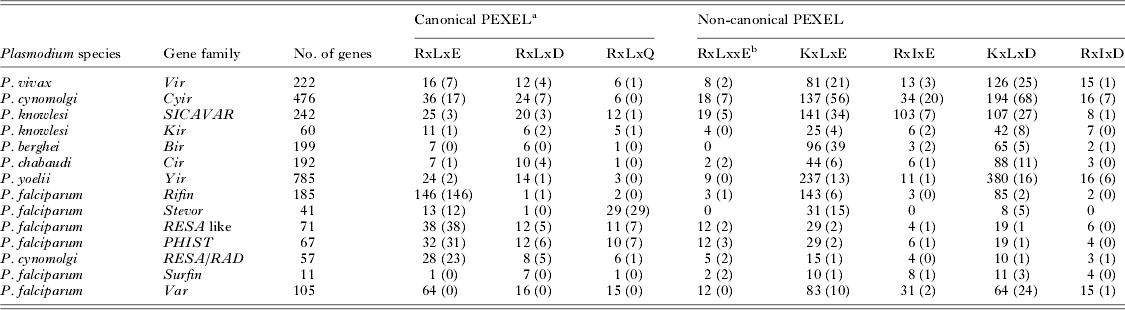
The PEXEL/VTS motif does not appear to be essential for a protein to enter the export pathway, however, as several exported proteins do not possess such a motif. The exported proteins targeted to the host-cell cytoplasm that lack a well-defined PEXEL/VTS motif are denoted as PEXEL negative export proteins (PNEPs). These PNEPs include skeleton binding protein-1 (SBP-1), membrane-associated histidine-rich protein-1 (MAHRP-1), and ring export protein-1 and -2 (REX-1 and REX-2) (Blisnick et al. Reference Blisnick, Morales Betoulle, Barale, Uzureau, Berry, Desroses, Fujioka, Mattei and Braun Breton2000; Spycher et al. Reference Spycher, Klonis, Spielmann, Kump, Steiger, Tilley and Beck2003; Hawthorne et al. Reference Hawthorne, Trenholme, Skinner-Adams, Spielmann, Fischer, Dixon, Ortega, Anderson, Kemp and Gardiner2004; Spielmann et al. Reference Spielmann, Hawthorne, Dixon, Hannemann, Klotz, Kemp, Klonis, Tilley, Trenholme and Gardiner2006; Haase et al. Reference Haase, Herrmann, Gruring, Heiber, Jansen, Langer, Treeck, Cabrera, Bruns, Struck, Kono, Engelberg, Ruch, Stunnenberg, Gilberger and Spielmann2009; Saridaki et al. Reference Saridaki, Frohlich, Braun-Breton and Lanzer2009), which are all resident proteins of MCs. The PNEPs (REX-2, SBP-1 and MAHRP-1) contain a conserved transmembrane domain but lack a signal sequence, whereas REX-1 has a recessed signal sequence (Dixon et al. Reference Dixon, Hawthorne, Spielmann, Anderson, Trenholme and Gardiner2008). These PNEPs have ER intermediates, suggesting export via a classical secretory pathway (Spycher et al. Reference Spycher, Rug, Klonis, Ferguson, Cowman, Beck and Tilley2006; Dixon et al. Reference Dixon, Hawthorne, Spielmann, Anderson, Trenholme and Gardiner2008; Saridaki et al. Reference Saridaki, Sanchez, Pfahler and Lanzer2008; Haase et al. Reference Haase, Herrmann, Gruring, Heiber, Jansen, Langer, Treeck, Cabrera, Bruns, Struck, Kono, Engelberg, Ruch, Stunnenberg, Gilberger and Spielmann2009). It is very likely that processing of a signal peptide could also generate a similar N-terminus of PNEPs as generated by Plasmepsin-V; therefore, it has been proposed (Spielmann and Gilberger, Reference Spielmann and Gilberger2010) that following processing by a signal peptidase, the trafficking of PNEPs may converge with PEXEL proteins at the translocon. The functional evaluation of Plasmodium export signals in Plasmodium berghei suggests that there may be multiple pathways of protein export for PEXEL and PNEP proteins in non-falciparum malaria parasites (Sijwali and Rosenthal, Reference Sijwali and Rosenthal2010). Based on the quantity of PEXEL/VTS positive proteins, it is apparent that the majority of proteins exported by P. falciparum are PEXEL/VTS positive, whereas in non-falciparum Plasmodium species, it is the PEXEL/VTS-independent proteins that make up the majority of the exportome (Table 1). The most well-characterized example of PEXEL/VTS-independent export in a non-P. falciparum malaria parasite is the export of the variant surface proteins of P. vivax, Plasmodium cynomolgi, Plasmodium knowlesi and Plasmodium yoelii/P. berghei/Plasmodium chabaudi (Table 1). Only a few members of the above variant gene families (Table 1) possess a canonical PEXEL/VTS motif near their signal sequences. Plasmodium vivax VIR proteins provide direct evidence for their export at the surface of infected red blood cells (iRBC; Bernabeu et al. Reference Bernabeu, Lopez, Ferrer, Martin-Jaular, Razaname, Corradin, Maier, Del Portillo and Fernandez-Becerra2012; Lopez et al. Reference Lopez, Bernabeu, Fernandez-Becerra and del Portillo2013). Indirect evidence for export of VIR proteins can be inferred from the structural similarity of VIR subfamilies A and D (Merino et al. Reference Merino, Fernandez-Becerra, Durham, Ferreira, Tumilasci, d'Arc-Neves, da Silva-Nunes, Ferreira, Wickramarachchi, Udagama-Randeniya, Handunnetti and Del Portillo2006) with the exported proteins of P. falciparum SURFIN and two transmembrane (Pf2TM) proteins respectively that lack a canonical PEXEL (Sam-Yellowe et al. Reference Sam-Yellowe, Florens, Johnson, Wang, Drazba, Le Roch, Zhou, Batalov, Carucci, Winzeler and Yates2004; Winter et al. Reference Winter, Kawai, Haeggstrom, Kaneko, von Euler, Kawazu, Palm, Fernandez and Wahlgren2005; Alexandre et al. Reference Alexandre, Yahata, Kawai, Torii and Kaneko2011). This suggests that the non-falciparum Plasmodium species may have different mechanisms for the export of proteins necessary for host-cell remodelling and virulence, and corroborates a hypothesis that canonical PEXEL/VTS exported proteins have undergone lineage-specific expansion in the falciparum parasite (Pick et al. Reference Pick, Ebersberger, Spielmann, Bruchhaus and Burmester2011). Further, it is unclear whether the greater abundance of canonical PEXEL/VTS proteins in P. falciparum is due to the fact that P. falciparum requires a high degree of host-cell remodelling compared with non-falciparum malaria parasite species, and so exports more proteins, or to the fact that protein export in the non-falciparum species is mainly mediated through PEXEL/VTS-independent pathways (Pick et al. Reference Pick, Ebersberger, Spielmann, Bruchhaus and Burmester2011).
Table 1. Canonical and non-canonical PEXEL motifs in exported proteins from Plasmodium species

Data obtained from the Plasmodium genome database (www.plasmodb.org). Canonical and non-canonical PEXEL motifs in the most likely exported proteins were estimated using the sequence pattern search tool (http://www-archbac.u-psud.fr/genomics/patternSearch.html). Number indicates number of paralogs in a gene family. Values in parentheses indicate the actual number of gene family members having a defined canonical or non-canonical PEXEL motif.
a Canonical PEXEL cleaved by Plasmepsin-V.
b Non-canonical PEXEL, which is also processed by Plasmepsin-V.
Recognition and processing of the export signal
Canonical PEXEL/VTS-mediated protein export requires the recognition and processing of the export signal in the ER, the first step of entry of exported proteins into the trafficking/secretory pathway. The canonical PEXEL/VTS motif was initially thought to mediate the trafficking of exported protein across the PV; however, it was later identified as a cleavage site for a parasite protease within the ER (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008; Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009). The cleavage of the PEXEL/VTS motif in exported proteins directs access to the host-cell cytosol (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008; Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009). The PEXEL/VTS cleaving protease has been identified as Plasmepsin-V, an aspartic protease localized in the lumen of ER (Klemba and Goldberg, Reference Klemba and Goldberg2005), by two independent studies conducted with P. falciparum (Boddey et al. Reference Boddey, Hodder, Gunther, Gilson, Patsiouras, Kapp, Pearce, de Koning-Ward, Simpson, Crabb and Cowman2010; Russo et al. Reference Russo, Babbitt, Muralidharan, Butler, Oksman and Goldberg2010). Plasmepsin-V recognizes the conserved PEXEL/VTS sequence (RxLxE/Q/D) and specifically cleaves between the lysine (L) and glutamate (E) residues (Fig. 2). The cleaved fragment carries an xE/Q/D at the N-terminal of the processed protein that is further N-acetylated (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008; Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009) and then recruited into the secretory pathway (Chang et al. Reference Chang, Falick, Carlton, Sedat, DeRisi and Marletta2008). The conservation of the xE/D/Q residue in a processed protein seems essential for export but not for processing (Boddey et al. Reference Boddey, Moritz, Simpson and Cowman2009). Plasmepsin-V can process canonical PEXEL and relaxed PEXEL as well, such as RxLxxE (Boddey et al. Reference Boddey, Carvalho, Hodder, Sargeant, Sleebs, Marapana, Lopaticki, Nebl and Cowman2013), indicating that this export signal cleaver is capable of processing a larger number of exported proteins (>400). However, genome analysis of Plasmodium species reveals several fixed patterns (relaxed PEXEL) in the N-terminus of various exported proteins (Table 1). Assuming that these non-canonical PEXEL could be processed by a parasite, as these are exported proteins, we can expect that the parasite might have additional ER resident proteases other than the well-known canonical PEXEL cleaver Plasmepsin-V.
Physical nature of exported proteins in the PV
Shared physical characteristics of exported proteins that traverse the parasite membrane (PM), PV and PVM may offer clues to aid the elucidation of the export/secretory pathway. It has been shown that exported proteins traverse the PV in an unfolded form (Gehde et al. Reference Gehde, Hinrichs, Montilla, Charpian, Lingelbach and Przyborski2009) (Fig. 3). The study was performed with a transgenic parasite expressing a chimeric protein in which a functional export signal is fused to green fluorescent protein (GFP) and to murine dihydrofolate reductase (mDHFR). In the presence of WR99210, all chimeric GFP–mDHFR fusion proteins were found to be accumulated in the PV; on the removal of WR99210, successful entry of the chimeric protein was observed in the host-cell cytoplasm. These unfolded proteins must then be refolded in the iRBC and then trafficked to their final destination (Gehde et al. Reference Gehde, Hinrichs, Montilla, Charpian, Lingelbach and Przyborski2009). This study confirms that exported proteins traverse the PV and PVM in an unfolded form and further underlines the putative role of heat shock proteins and molecular chaperones in unfolding exported proteins for successful translocation and refolding in the iRBC. Proteome analysis of P. falciparum revealed that the PV and iRBC are enriched with a number of heat shock proteins (Florens et al. Reference Florens, Washburn, Raine, Anthony, Grainger, Haynes, Moch, Muster, Sacci, Tabb, Witney, Wolters, Wu, Gardner, Holder, Sinden, Yates and Carucci2002, Reference Florens, Liu, Wang, Yang, Schwartz, Peglar, Carucci, Yates and Wub2004; Nyalwidhe and Lingelbach, Reference Nyalwidhe and Lingelbach2006; Acharya et al. Reference Acharya, Kumar and Tatu2007). Genome sequence analysis has revealed a large repertoire of exported proteins in P. falciparum that are either heat shock proteins (Hiller et al. Reference Hiller, Bhattacharjee, van Ooij, Liolios, Harrison, Lopez-Estrano and Haldar2004; Marti et al. Reference Marti, Good, Rug, Knuepfer and Cowman2004; Nyalwidhe and Lingelbach, Reference Nyalwidhe and Lingelbach2006; Shonhai et al. Reference Shonhai, Boshoff and Blatch2007) or contain the DNA-J domain (which encodes for a molecular chaperone), for example, the seven members of the PHIST-b protein and Hsp40 families (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006; Acharya et al. Reference Acharya, Kumar and Tatu2007, Reference Acharya, Chaubey, Grover and Tatu2012), and ring-infected erythrocyte surface antigen (RESA) protein (Favaloro et al. Reference Favaloro, Coppel, Corcoran, Foote, Brown, Anders and Kemp1986). These proteins emphasize the importance of chaperone molecules in malaria parasite protein export pathways, and offer a relatively unexplored avenue for exportome research.

Fig. 3. Schematic representation of an exported protein in the PV in a P. falciparum-infected erythrocyte. Exported proteins enter PV, get unfolded and cross PVM and enter erythrocyte cytoplasm with the help of translocons. In erythrocyte cytoplasm, exported proteins first get refolded with the help of heat shock protein and then reach subcellular locations in erythrocytes. PVM, parasitophorous vacuole membrane; PM, parasite membrane. This image showing translocation of exported protein from PVM is drawn on the basis of work by Gehde et al. (Reference Gehde, Hinrichs, Montilla, Charpian, Lingelbach and Przyborski2009).
Identification of translocon machinery in the PVM
Channel-mediated delivery is a translocon. As channel-mediated protein trafficking necessitates machinery within the PVM to facilitate protein movement through the membrane, so there must exist translocon(s) embedded within the PVM (Schatz and Dobberstein, Reference Schatz and Dobberstein1996; Schnell and Hebert, Reference Schnell and Hebert2003). Such a translocon has recently been described in P. falciparum (de Koning-Ward et al. Reference de Koning-Ward, Gilson, Boddey, Rug, Smith, Papenfuss, Sanders, Lundie, Maier, Cowman and Crabb2009). It appears that the P. falciparum translocon machinery consists of a complex of parasite-encoded proteins embedded within the PVM, termed the Plasmodium translocon of export protein (PTEX). The PTEX complex is an ATP-powered machine and consists of two PTEX proteins (PTEX150 and PTEX88), a heat shock protein (Hsp101), thioredoxin protein-2 (TRX2) (Matthews et al. Reference Matthews, Kalanon, Chisholm, Sturm, Goodman, Dixon, Sanders, Nebl, Fraser, Haase, McFadden, Gilson, Crabb and de Koning-Ward2013) and parasite export protein-2 (EXP2) (de Koning-Ward et al. Reference de Koning-Ward, Gilson, Boddey, Rug, Smith, Papenfuss, Sanders, Lundie, Maier, Cowman and Crabb2009; Bullen et al. Reference Bullen, Charnaud, Kalanon, Riglar, Dekiwadia, Kangwanrangsan, Torii, Tsuboi, Baum, Ralph, Cowman, de Koning-Ward, Crabb and Gilson2012; Riglar et al. Reference Riglar, Rogers, Hanssen, Turnbull, Bullen, Charnaud, Przyborski, Gilson, Whitchurch, Crabb, Baum and Cowman2013). A recent study has shown that a homodimer of EXP2 oligomerizes, forms a pore-like shape and attaches to the remainder of the PTEX complex in PVM (Bullen et al. Reference Bullen, Charnaud, Kalanon, Riglar, Dekiwadia, Kangwanrangsan, Torii, Tsuboi, Baum, Ralph, Cowman, de Koning-Ward, Crabb and Gilson2012) (Fig. 4). Since P. falciparum exports hundreds of highly diverse remodelling and virulence-associated proteins, it is proposed that the parasite may utilize structurally related PVM translocons that may have different accessory proteins or exported proteins may use different cargo. Thus, functional dissection of translocon complexes and modelling of translocon machineries would provide further insights into protein trafficking in P. falciparum.

Fig. 4. Molecular composition and structure of the translocon in the PVM of the P. falciparum-infected erythrocyte. PTEX150, Plasmodium translocon of exported protein-150; PTEX88, Plasmodium translocon of exported protein-88; TRX-2, thioredoxin-2; HS101, heat shock protein-101; EXP2, exported protein-2. This figure is a modified version of work by de Koning-Ward et al. (Reference de Koning-Ward, Gilson, Boddey, Rug, Smith, Papenfuss, Sanders, Lundie, Maier, Cowman and Crabb2009) and Bullen et al. (Reference Bullen, Charnaud, Kalanon, Riglar, Dekiwadia, Kangwanrangsan, Torii, Tsuboi, Baum, Ralph, Cowman, de Koning-Ward, Crabb and Gilson2012).
Trafficking of exported proteins within the erythrocyte cytoplasm
The fate of exported proteins and the mechanisms behind their journey following their exit from the PVM remains relatively poorly understood. A number of studies have suggested an important role of MCs in the sorting and further trafficking of exported proteins to the surface of infected erythrocytes (Lanzer et al. Reference Lanzer, Wickert, Krohne, Vincensini and Braun Breton2006; Wickert and Krohne, Reference Wickert and Krohne2007; Bhattacharjee et al. Reference Bhattacharjee, van Ooij, Balu, Adams and Haldar2008; Tilley et al. Reference Tilley, Sougrat, Lithgow and Hanssen2008). MCs are parasite-induced, flattened membranous structures scattered throughout the cytoplasm of infected erythrocytes (Spycher et al. Reference Spycher, Rug, Klonis, Ferguson, Cowman, Beck and Tilley2006). However, it remains to be elucidated how the sorting of proteins to the RBC membrane and the surface of the iRBC occurs following protein traversal of the PVM. The resident proteins of MCs have been shown to play an important role in the sorting and trafficking of PfEMP-1 from MCs to the erythrocyte surface. These resident proteins include Pf332, PfEMP-3, PfSBP-1 and MAHRP-I (Waterkeyn et al. Reference Waterkeyn, Wickham, Davern, Cooke, Coppel, Reeder, Culvenor, Waller and Cowman2000; Spycher et al. Reference Spycher, Klonis, Spielmann, Kump, Steiger, Tilley and Beck2003; Maier et al. Reference Maier, Rug, O'Neill, Beeson, Marti, Reeder and Cowman2007; Hodder et al. Reference Hodder, Maier, Rug, Brown, Hommel, Pantic, Puig-de-Morales-Marinkovic, Smith, Triglia, Beeson and Cowman2009). Previously, it was believed that MCs are responsible for the display of exported proteins on the surface of infected erythrocytes by a mechanism in which MCs ultimately merge with the host-cell membrane (Trelka et al. Reference Trelka, Schneider, Reeder and Taraschi2000). Later studies described the role of MCs as a secretory organelle that accumulates parasite proteins and delivers them to the surface of the erythrocyte (Bhattacharjee et al. Reference Bhattacharjee, van Ooij, Balu, Adams and Haldar2008). Further, a large-scale gene knockout study confirmed their role in the trafficking of PfEMP-1 to the surface of the infected erythrocyte (Maier et al. Reference Maier, Rug, O'Neill, Brown, Chakravorty, Szestak, Chesson, Wu, Hughes, Coppel, Newbold, Beeson, Craig, Crabb and Cowman2008). The export of PfEMP-1, one of the most well-characterized virulence-associated proteins, to the surface of the infected erythrocyte has been proposed to be completed in three phases, early, middle and late, wherein each phase involves a number of other parasite-encoded proteins assisting the trafficking through protein–protein interactions (Maier et al. Reference Maier, Rug, O'Neill, Brown, Chakravorty, Szestak, Chesson, Wu, Hughes, Coppel, Newbold, Beeson, Craig, Crabb and Cowman2008). Other parasite-encoded proteins involved in PfEMP-1 trafficking to the surface of infected erythrocytes have been reviewed by Sam-Yellowe (Reference Sam-Yellowe2009). Further investigations are required in order to fully understand the mechanisms behind the transport of proteins beyond the PVM to their final destination in the host-cell cytoplasm and on to the RBC surface.
EXPANSION OF GENE FAMILIES INVOLVED IN PROTEIN TRAFFICKING
The number of predicted exported proteins is far fewer in non-falciparum malaria species than in P. falciparum when algorithm-based predictions are used (Pick et al. Reference Pick, Ebersberger, Spielmann, Bruchhaus and Burmester2011). The majority of predicted exported proteins belong to 27 well-characterized gene families, including stevor, pfemp-1, rifin, fikk kinases, surfin and pf2tm and 21 other novel gene families (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006; Boddey et al. Reference Boddey, Carvalho, Hodder, Sargeant, Sleebs, Marapana, Lopaticki, Nebl and Cowman2013). Some of the major novel exported protein-encoding gene families are PHIST, DNA-J and hydrolase proteins (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006). The majority of predicted exported proteins are hypothetical and require further functional characterization and annotation. Furthermore, phylogenetic studies suggest the occurrence of lineage-specific expansion of the phist, DNA-J and FIKK kinases gene families in the P. falciparum genome (Ward et al. Reference Ward, Equinet, Packer and Doerig2004; Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006). One member of the phist-b gene family has been shown to be involved in knob formation (Maier et al. Reference Maier, Rug, O'Neill, Brown, Chakravorty, Szestak, Chesson, Wu, Hughes, Coppel, Newbold, Beeson, Craig, Crabb and Cowman2008; Acharya et al. Reference Acharya, Chaubey, Grover and Tatu2012).
The expansion of various gene families in P. falciparum but not in non-falciparum malaria species suggests that radiation of these gene families may have shaped the specific pathogenesis of this parasite (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006; Pick et al. Reference Pick, Ebersberger, Spielmann, Bruchhaus and Burmester2011). The high virulence of P. falciparum compared with non-falciparum malaria species is mainly mediated by the localization of PfEMP-1 on the surface of infected erythrocytes. Studies of the export of PfEMP-1 beyond the PVM have been carried out using the PfEMP-1 protein (Var2CSA) expressed by the CS-2 cloned line of P. falciparum that causes placental malaria (Salanti et al. Reference Salanti, Dahlback, Turner, Nielsen, Barfod, Magistrado, Jensen, Lavstsen, Ofori, Marsh, Hviid and Theander2004). The genome of this parasite has 59 different PfEMP-1 proteins expressed in a mutually exclusive way so that a single PfEMP-1 protein is expressed during each schizogonic cycle in the blood (Dzikowski et al. Reference Dzikowski, Frank and Deitsch2006a, Reference Dzikowski, Templeton and Deitschb). This suggests that the actual number of exported proteins required for the successful expression of PfEMP-1 at the surface of the erythrocyte may be larger than currently thought. A large-scale gene knockout study has described the involvement of various PHIST proteins in PfEMP-1 trafficking as well as in the modification of erythrocyte membrane rigidity (Maier et al. Reference Maier, Rug, O'Neill, Brown, Chakravorty, Szestak, Chesson, Wu, Hughes, Coppel, Newbold, Beeson, Craig, Crabb and Cowman2008). Recently, a single PHIST protein has been shown to have vital interaction with the ATS domain of PfEMP-1 (Mayer et al. Reference Mayer, Slater, Erat, Konrat and Vakonakis2012), suggesting a putative role for PHIST proteins in trafficking parasite-encoded proteins in the infected erythrocyte. Therefore, the lineage-specific expansion of PHIST and other export families (Sargeant et al. Reference Sargeant, Marti, Caler, Carlton, Simpson, Speed and Cowman2006) may be a specific requirement of P. falciparum relating to the trafficking of host-cell remodelling and virulence-associated proteins.
PERSPECTIVE
Key advances in analysing protein export beyond the PVM using parasite genetic modification and in vivo imaging technologies have resulted in a clearer understanding of protein trafficking pathways in P. falciparum. Despite these advances, critical parts of the parasite's protein transport mechanisms remain incompletely understood. Based on the current knowledge on protein trafficking in malaria-infected erythrocytes and exportome size, a schematic diagram of the protein trafficking pathway (Fig. 5) has been prepared that shows possible knowledge gaps. Many intriguing questions still remain to be answered. For example, does a parasite have multiple ER-resident proteases to process non-canonical PEXEL? Can translocons have different accessory proteins? How do unfolded exported proteins become refolded in the host-cell cytoplasm? How are PEXEL/VT-negative proteins exported beyond the PVM? Is expansion of gene families involved in protein trafficking related to exportome size of a malaria parasite?

Fig. 5. Diagrammatic representation of the protein export pathway in P. falciparum-infected red cells. The figure shows knowledge gaps in the protein trafficking pathway, which are (1) requirement of additional ER peptidases to process a non-canonical PEXEL signal, (2) requirement of additional translocons that may have structurally related composition as of a well-known translocon, (3) characterization of heat shock proteins that helps unfolding of diverse exported proteins in PV and refolding in infected erythrocyte cytoplasm and (4) characterization of signal in exported proteins for subcellular targeting in infected erythrocytes.
The trafficking of parasite-encoded proteins beyond the parasite and into the RBC cytosol and onwards onto the RBC surface is, presumably, unique to malaria parasites. Further characterization of these processes could provide useful knowledge for the design of therapeutics as well as for the elucidation of a fascinating aspect of the biology of the malaria parasite.
ACKNOWLEDGEMENTS
The authors would like to thank Ms Cherry L. Dykes and Ms Anushrita for editorial corrections. S.K.P. is an ICMR-Postdoctoral Fellow.
FINANCIAL SUPPORT
This work was supported by the Indian Council of Medical Research (ICMR), New Delhi, India.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.