INTRODUCTION
Trypanosoma cruzi is a protozoan parasite that causes Chagas disease, a parasitosis that affects around 18 million people in Latin America (WHO, 2005). T. cruzi presents invertebrate and vertebrate hosts during its life cycle: Triatominae insect vectors and a wide variety of mammals. In the vector, the parasites are present as epimastigotes that differentiate into metacyclic trypomastigotes, forms infective to mammal hosts, which circulate in wild and domestic environments (Morel et al. Reference Morel, Deane and Gonçalves1986). Based on molecular markers derived from constitutive genes, such as the 24Sα rRNA and the mini-exon genes, two major phylogenetic groups T. cruzi I (TCI) and T. cruzi II (TCII) were defined (Fernandes et al. Reference Fernandes, Souto, Castro, Pereira, Fernandes, Junqueira, Naiff, Barret, Degrave, Campbell and Coura1998), which coincide with the isoenzymatic dichotomy early proposed (Tibayrenc and Ayala, Reference Tibayrenc and Ayala1988; Souto et al. Reference Souto, Fernandes, Macedo, Campbell and Zingales1996). A putative association of these main genotypes with a given host, human disease or biome still needs to be confirmed. TCI has been mainly associated with the sylvatic transmission cycle, infecting Didelphis as well as placental mammals, and presents a broader distribution in the wild, but has also been observed in domestic cycles (Brisse et al. Reference Brisse, Barnabé and Tibayrenc2000). TCII parasites are proposed to be associated with the domestic transmission cycle, infecting mainly placental mammals (Tibayrenc and Ayala, Reference Tibayrenc and Ayala1988; Andrade, Reference Andrade1999; Buscaglia and Di Noia, Reference Buscaglia and Di Noia2003). However, the genotype TCII has already been described infecting several sylvatic mammalian species (Lisboa et al. Reference Lisboa, Pinho, Monteiro and Jansen2007).
Characterizing parasite populations found in nature is crucial to the elucidation of the Chagas disease complex transmission scenario. Biological, biochemical and molecular tools have demonstrated that different strains from endemic areas might be responsible for distinct clinical manifestations and chemotherapy response (Andrade, Reference Andrade1999). Outbreaks of acute Chagas disease due to oral transmission culminating with human infections by T. cruzi wild strains (Secretaria de Vigilância em Saúde, 2005), reinforce the importance of a better understanding of the parasite transmission cycles in nature. The in vitro engagement of the trypomastigote metacyclic gp82 molecule onto the gastric mucosal epithelium during invasion, associated to a gp90 glycoprotein down-modulating effect which has already been demonstrated. However, a T. cruzi isolate from an orally infected patient expressed high levels of gp90, and produced high parasitaemia and mortality when orally inoculated into mice (Covarrubias et al. Reference Covarrubias, Cortez, Ferreira and Yoshida2007).
A few studies have focused on the differential gene expression of factors involved in key events of the parasite biology between TCI and TCII groups (Ruiz et al. Reference Ruiz, Favoreto, Dorta, Oshiro, Ferreira, Manque and Yoshida1998; Risso et al. Reference Risso, Garbarino, Mocetti, Campetella, Cappa, Buscaglia and Leguizamón2004; Di Noia et al. Reference Di Noia, Buscaglia, de Marchi, Almeida and Frasch2002; Dutra et al. Reference Dutra, Couto, Lopes and Meyer-Fernandes2006; Mathieu-Daudé et al. Reference Mathieu-Daudé, Bosseno, Garzon, Lelièvre, Sereno, Ouaissi and Brenière2007). A correlation between the expression of a sialidase-homologue gene and parasite virulence in mice (Risso et al. Reference Risso, Garbarino, Mocetti, Campetella, Cappa, Buscaglia and Leguizamón2004) or between the expression of surface glycoproteins involved in Ca2+ mobilization and the ability of the parasite to enter mammalian cells (Ruiz et al. Reference Ruiz, Favoreto, Dorta, Oshiro, Ferreira, Manque and Yoshida1998) was observed. The trypomastigote small surface antigen (TSSA), a surface glycosylphosphatidyl inositol (GPI) – anchored mucin-like protein, revealed a dimorphism that matches the two T. cruzi lineages. The seroprevalence for TSSA in Chagas patients is restricted to T. cruzi II isoform (Di Noia et al. Reference Di Noia, Buscaglia, de Marchi, Almeida and Frasch2002). Differences in ecto-phosphatase activities (Dutra et al. Reference Dutra, Couto, Lopes and Meyer-Fernandes2006), as well as sequence differences and expression levels of Tc52, an immuno-regulatory parasitic protein (Mathieu-Daudé et al. Reference Mathieu-Daudé, Bosseno, Garzon, Lelièvre, Sereno, Ouaissi and Brenière2007), were also demonstrated. Finally, we have been showing that TCI and II groups activate the complement system differentially, the latter being more resistant to complement system mediated-lysis (Cestari et al. Reference Cestari, Evans-Osses, Freitas, Inal and Ramirez1998). Proteases have been implicated in host-parasite interactions, infectivity, pathogenicity, virulence, intracellular survival, replication, differentiation, immune evasion and nutrition (Sajid and McKerrow, Reference Sajid and McKerrow2002; Santos et al. Reference Santos, Branquinha and D'Avila-Levy2006). T. cruzi contains several proteolytic activities, among them, cysteine, serine, threonine and metalloproteases (Cazzulo, Reference Cazzulo2002; Cuevas et al. Reference Cuevas, Cazzulo and Sanchez2003). However, the most abundant is cruzipain, a cysteine-protease expressed as a complex mixture of isoforms by the major developmental stages of the parasite. Inhibitors of cruzipain kill the parasite and cure infected mice, thus making the enzyme a very promising target for the development of drugs against Chagas disease (Meirelles et al. Reference Meirelles, Juliano, Carmona, Silva, Costa, Murta and Scharfstein1992). Differences in protease expression between TCI and TCII phylogenetic groups have not been investigated so far.
We have interest in understanding the biological differences between TCI and TCII phylogenetic groups. In this study we have worked with 16 T. cruzi low-passage field-available strains, isolated from different Brazilian states. The protease expression profile is analysed to identify molecules that can be correlated to TCI or TCII lineages and their contribution to wild isolates biological differences in competence.
MATERIALS AND METHODS
T. cruzi strains, hosts and geographical location
We utilized TCII CL Brener and TCI Dm28c strains (Coleção de Tripanosomatídeos, Instituto Oswaldo Cruz, Rio de Janeiro). The other 16 T. cruzi field-isolated strains differ according to the phylogenetic group (TCI or TCII), hosts and origin biome, as shown in Table 1.
Table 1. Trypanosoma cruzi I and II field isolates
(Utilized isolates according to T. cruzi phylogenetic groups (TCI or TCII), their origin hosts and biomes.)

Parasite growth conditions
T. cruzi epimastigotes were cultivated in LIT culture medium (bovine liver infusion at 5 g/l, tryptose at 5 g/l, NaCl at 4 g/l, KCl at 0·4 g/l; Na2HPO4 at 8 g/l; glucose at 2 g/l; pH 7·2) supplemented with hemin at 10 mg/l and bovine fetal serum 10% at 27°C.
Parasite extracts
Briefly, 5-day parasite cultures were harvested by centrifugation at 4000 g for 5 min, and washed 3 times with cold PBS (150 mm NaCl, 20 mm sodium phosphate buffer; pH 7,2). Cells were resuspended in 200 μl of distilled water, submitted to vortex for 10 sec and transferred to ice. Then 20 μl of SDS 10% (sodium dodecyl sulfate) were added, and cells submitted to 3 cycles, alternating vortex for 30 sec and incubation on ice for 1 min. Extracts were then centrifuged at 15 300 g for 10 min. Supernatants were transferred to clean microcentrifuge tubes and kept at −20°C. The proteins were measured according the Lowry et al. (Reference Lowry, Rosebrough, Farr and Randall1951) method.
Protease activity assay
Proteases were assayed and characterized by electrophoresis on 10% SDS-PAGE with 0·1% co-polymerized gelatin as substrate (Heussen and Dowdle, Reference Heussen and Dowdle1980). The gels were loaded with 25 or 100 μg of protein per slot, for pH 5·5 and 10, respectively. Electrophoresis was performed at a constant current of 120 V at 4°C and gels were then incubated at room temperature for 1 h in 10 vols of 2·5% Triton X-100. The gels were then incubated for 24 h at 37°C in 50 mm sodium phosphate buffer, pH 5·5, supplemented with 2 mm DTT, or for 48 h in 50 mm glycine-NaOH buffer, pH 10, in the presence or absence of proteolytic inhibitors (10 mm 1,10-phenanthroline or 10 μm E-64. Sigma Chemical Co., St Louis, MO, USA). The gels were stained for 2 h in 0·2% Comassie Brilliant Blue R-250 in methanol-acetic acid-water (50:10:40), and destained in the same solvent. Molecular mass was calculated from the mobility of molecular weight standards (Hanover, MD, USA).
Immunoblotting
Expression of cruzipain among T. cruzi field-isolated strains were compared by Western-blotting. Protein extracts equivalent to 20 μg per slot were separated in 12% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was blocked in 5% milk/PBS containing 0·5% Tween (PBS-Tween) at 4°C overnight. Then, membranes were washed 3 times (10 min each) with the blocking solution and incubated with anti-cruzipain at 1:5000 dilution (kindly provided by Dr J. J. Cazzullo) for 1 h. The secondary antibody used was peroxidase-conjugated goat anti-rabbit IgG 1:2500 for 1 h. Immunoblots were exposed to X-ray films after reaction with ECL reagents (kit from Pierce, USA) for chemiluminescence.
Phenetic analysis
Proteases activity and cruzipain expression band patterns were collected into a matrix indicating the presence or absence of specific polypeptide bands (scored as 1 or 0, respectively). Simple matrices were obtained using a similarity coefficient, and TCI versus TCII profile dendograms were constructed using an unweighted pair group method analysis (UPGMA). For these analyses, the NTSYS software package (Version 2.02, Exeter Software, Setauket, NY, USA) was used.
RESULTS
Protease expression profile in T. cruzi field-isolated strains
In order to compare the protease general expression patterns among the 16 field-isolated TCI and TCII strains, epimastigote total cellular extracts were submitted to gelatin-SDS-PAGE.
At pH 10·0, almost all TCI isolates presented active proteases around 66 kDa (Fig. 1A). Gambá 05 isolate presented a second protease of 45 kDa (Fig. 1A, lane 7). On the other hand, all TCII isolates presented 2 proteases around 66 and 45 kDa (Fig. 1B). Active proteases at pH 5·5 were also investigated (Fig. 1C, D). All TCI isolates presented 2 protease bands around 66 and 45 kDa (Fig. 1C), while TCII isolates exhibited different patterns with 2 (860 and MLCD88 isolates), 3 (750, 840, R4 and M3 isolates) or 4 (812, 832 and JCT1 isolates) protease activities/bands (Fig. 1D).

Fig. 1. Zymogram of Trypanosoma cruzi isolates. Proteolytic enzymes in T. cruzi field-isolated TCI (A, C: lanes:1- 4264; 2- 8612; 3- 8622; 4- 8628; 5- 8636; 6- 8659; 7- Gambá 05) and TCII (B, D: lanes: 1- 750; 2- 812; 3- 832; 4- 840; 5- 860; 6- R4; 7- JCT1; 8- MLCD88; 9- M3) isolates analysed on gelatin-SDS-PAGE. Gels were incubated for 24 h at 37°C in 50 mm sodium phosphate, pH 5·5 supplemented with 2 mm DTT (C, D) or for 48 h in glycine-NaOH (50 mm), pH 10·0 (A, B).
TCI 8628 and TCII M3 isolates were analysed by gelatin-SDS-PAGE, at different points of culture growth: pre-log, log and stationary phase epimastigotes as well as in vitro differentiated metacyclic trypomastigotes. Their respective patterns of 2 (8628) and 3 (M3) bands at pH 5·5 were maintained at all culture time-points, indicating that differential TCI and TCII protease expression is independent of culture growth phase (not shown). Blood-stage trypomastigotes and intracellular amastigotes were not investigated in this study.
Previously, the M3 isolate had been typed as TCI (Herrera et al. Reference Herrera, D'Andrea, Xavier, Mangia, Fernándes and Jansen2005). However, when compared to TCI isolates, it showed the TCII protease expression pattern at both pH 5·5 and 10 (Fig. 1). Gambá 05 TCI isolate also showed the TCII protease expression pattern, but only at pH 10 (Fig. 1). To test the hypothesis that M3 and Gambá 05 presented mixed TCI/TCII populations, after limiting dilution cloning, both isolates and their individual clones were molecularly re-typed (utilizing the mini-exon gene marker). T. cruzi isolate Gambá 05 was confirmed as corresponding to the TCI genotype, while M3 was re-classified and corresponded to TCII genotype (data not shown).
Identification of protease classes differentially expressed between T. cruzi I and II field-isolated strains
We observed that TCII isolates present a more complex protease expression pattern at pH 5·5 and 10 than TCI isolates. With the goal of identifying which protease classes the TCI and TCII groups express, activity gels were incubated with cysteine and metalloprotease inhibitors (E-64 or 1,10-phenanthroline, respectively). In these assays, we utilized the following representative strains: CL Brener (TCII) and Dm28c (TCI) strains as controls; TCII 860 (2 bands at pH 5·5), TCII 750 (3 bands at pH 5·5), TCII 832 (4 bands at pH 5·5); TCI 8628 (representing all TCI isolates, once they have the same active protease patterns at pH 5·5 and pH 10). We also utilized TCI Gambá 05, for being an exception, exhibiting 2 bands at pH 10, instead of 1 (Fig. 1A), and TCII M3, once it had been previously typed as TCI.
As observed in Fig. 2, after incubation of protease activity gels at pH 5·5 buffer supplemented with E-64, all TCI and TCII proteolytic activities were inhibited (Fig. 2A and C). The same was observed when gels were incubated with pH 10 buffer supplemented with 1,10-phenantroline (Fig. 2B and D). However, E-64 had no effect at pH 10, and 1,10-phenantroline had no inhibitory effect at pH 5,5 at the tested concentrations (data not shown). These results suggest that in all the T. cruzi strains utilized, the active proteases at acidic pH are cysteine-proteases, and all active proteases at alkaline pH are metalloproteases.

Fig. 2. Zymogram of Trypanosoma cruzi isolates with proteolytic inhibitors. Gelatin-SDS-PAGE showing the modulation of proteolytic activity from T. cruzi I (A, B) and II (C, D) strains, when gels were incubated in the absence (control) or the presence (*) of proteolytic inhibitors: 10 μm E-64 at pH 5·5 (A, C) or 10 mm 1,10-phenanthroline at pH 10 (B, D).
The quantity of parasites needed to detect metalloprotease activities in T. cruzi epimastigotes isolates was 4 times higher than that for cysteine-protease activities (25 μg×100 μg proteins/slot, respectively), as shown in Figs 1 and 2.
Comparison of cruzipain expression among T. cruzi I and II filed-isolated strains
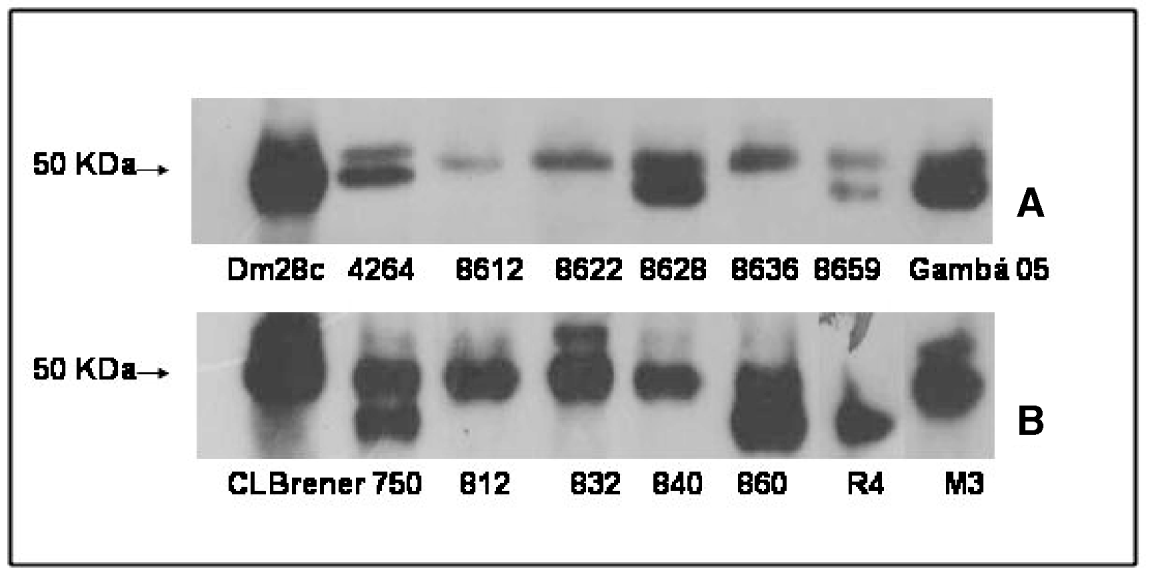
The expression of cruzipain among TCI and TCII field strains was evaluated by Western blotting, as shown in Fig. 3 (A, B). Single or double bands around 50 kDa were detected, as previously described (Campetella et al. Reference Campetella, Martínez and Cazzulo1990), independent of the phylogenetic TCI or TCII background (Fig. 3A, B).

Fig. 3. Immunoblotting of Trypanosoma cruzi isolates. Cruzipain expression at the expected size of 50 kDa (arrow) by Western-blotting in TCI (A) and TCII (B) field isolates. Twenty μg of protein per slot were separated in SDS-PAGE, transferred to nitrocellulose membranes, and then incubated with anti-cruzipain at 1:5000 followed by labelling with goat anti-rabbit-peroxidase at 1:2500. Immunoblots were exposed to X-ray after reaction with ECL reagents.
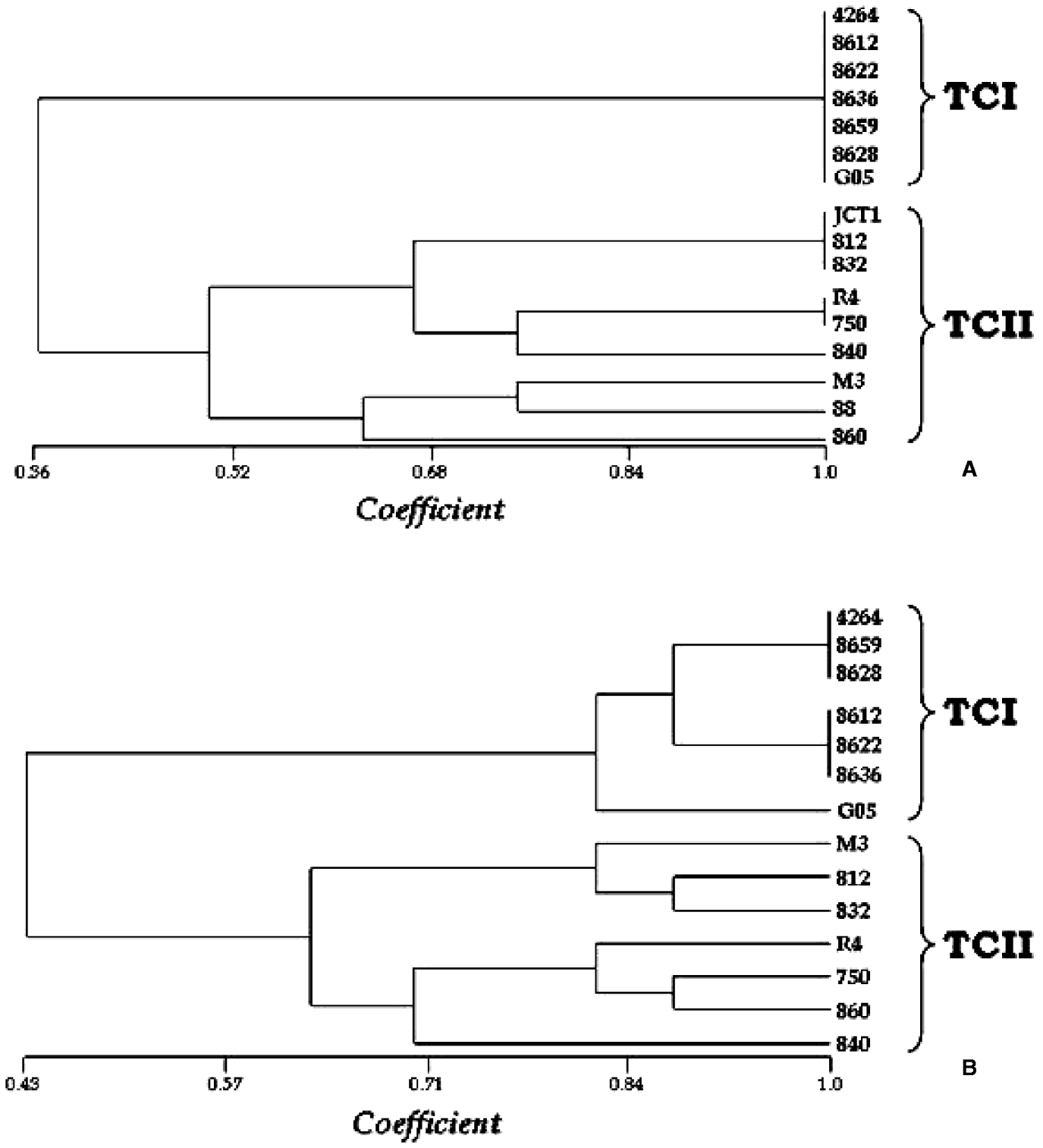
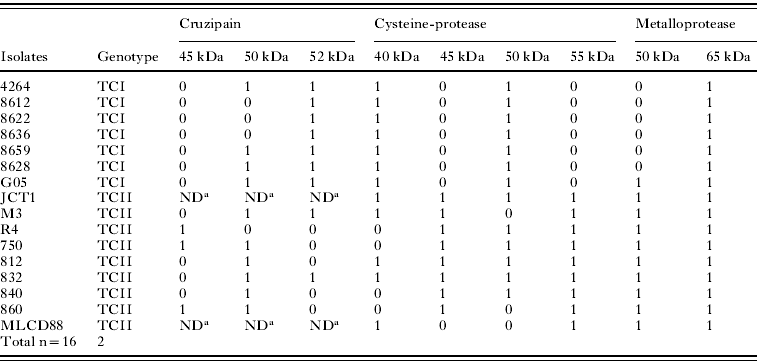
Considering the differential heterogeneity amongst the TCI and TCII isolate protease patterns, a similarity analysis was applied, with the construction of UPGMA-based dendograms. Markers were defined as the bands present or absent after protease activity gels, and cruzipain Western blotting analyses, detected in the present investigation, according to Table 2. The results were expressed as dendograms that separated TCI from TCII genotypes (Fig. 4A, B). When only the cysteine-protease activities were considered, the 2 major groups TCI and TCII were separated at about 50% similarity (Fig. 4A). In this tree, we observed, as expected, that the TCI group is homogenous, since all these isolates exhibited 2 cysteine-protease activity bands. The TCII group, on the other hand, consisted of subdivided groups, as a consequence of heterogeneous cysteine-proteases activity. There was a variation amongst TCII isolates with 2–4 bands, and not necessarily the same bands were present in each group (2, 3 or 4 bands) (Fig. 4A). When all protease (cysteine and metallo) activity and cruzipain bands were used to generate a matrix of absence or presence, the resulting tree also showed a separation between the TCI and TCII groups at about 60% similarity (Fig. 4B). Nevertheless, in this case, the TCI isolates presented a low-level heterogeneity. This can be explained because, in spite of their homogenous 2 cysteine-protease activity bands, they showed 1–2 cruzipain expression band variation, as well as TCII isolates (Fig. 4B). In the case of metalloprotease activities, the separation of T. cruzi major phylogenetic groups would be expected, since all TCII have 2 bands, and all TCI have 1 band, expect for Gambá 05, with 2 activities. Interestingly, Gambá 05 is in a separated branch from other TCI isolates at about 82% similarity (Fig. 4B).

Fig. 4. Dendogram (UPGMA) of TCI and TCII-proteases. The phylogenetic tree shows a separation between TCI and TCII isolates based on cysteine-protease activity bands (A), or cruzipain expression and cysteine and metalloproteases activity bands (B). The x-axis represents the coefficient of similarity.
Table 2. Cysteine-proteases and cruzipain bands amongst TCI and TCII isolates
(Trypanosoma cruzi field-isolated strains captured in different biomes in Brazil: analysis of protease expression (1- presence or 0- absence of bands in their approximate kDa).)

a ND=not done.
We intend to purify additional TCII active cysteine-proteases and evaluate their role in interaction with vertebrate and invertebrate hosts.
DISCUSSION
As mentioned above, T. cruzi isolates typed as TCII present more complex protease activity patterns in comparison with TCI. Based on isoenzymes and RAPD analysis tools, other authors (Brisse et al. Reference Brisse, Barnabé and Tibayrenc2000) have reported the genotype TCII as a more diverse group. It can be partitioned into 5 sublineages (IIa–e) or discrete typing units (DTUs). The different DTUs display distinct geographical and ecological variations. In contrast, TCI can be no longer subdivided according to these criteria (Brisse et al. Reference Brisse, Barnabé and Tibayrenc2000), despite being subdivided into 3 groups by clustering the intergenic mini-exon gene sequences (O' Connor et al. Reference O' Connor, Bosseno, Barnabé, Douzery and Brenière2007).
Lowdes et al. (Reference Lowdes, Bonaldo, Thomaz and Goldenberg1996) comparing different strains of T. cruzi epimastigote and metacyclic trypomastigote forms, showed metalloprotease heterogeneity, but highly conserved cysteine-protease expression patterns (Lowdes et al. Reference Lowdes, Bonaldo, Thomaz and Goldenberg1996), corroborated by other data (Campetella et al. Reference Campetella, Martínez and Cazzulo1990). We showed a homogeneous expression pattern of both metallo and cysteine-proteases in TCI isolates, and a heterogeneous expression of cysteine-proteases in TCII field-isolated epimastigotes. Metalloprotease expression in TCII isolates is characterized by an additional activity in relation to TCI isolates, but is a homogeneous intra-TCII group. Cysteine-protease activities are stronger in T. cruzi isolates than metalloprotease activities, since higher amounts of parasites are needed in the latter case for activity detection. Cuevas et al. (Reference Cuevas, Cazzulo and Sanchez2003) showed that T. cruzi metalloprotease activities are also weaker when compared to Leishmania mexicana mexicana promastigotes.
These wild recently-isolated TCI and TCII parasites investigated in this study had not previously been biologically characterized, except for 2 isolates (750 and R4, both typed as TCII) that caused parasitaemia with mortality in Swiss mice (Lisboa et al. Reference Lisboa, Pinho, Monteiro and Jansen2007). The diversification imparted by protease expression heterogeneity presented by TCII isolates in contrast to the homogenous TCI pattern may contribute to their adaptation to differential hosts. It is noteworthy that TCI and TCII infect the same number of host orders (Lisboa et al. Reference Lisboa, Mangia, de Lima, Martins, Dietz, Baker, Ramon-Miranda, Ferreira, Fernandes and Jansen2004).
In addition to cruzipain, a 30 kDa cathepsin B-like cysteine-protease expression increased after T. cruzi treatment with the Z-(SBz)Cys-Phe-CHN2 inhibitor, probably compensating the cruzipain inhibition and expression decrease (Yong et al. Reference Yong, Schimtz, Vannier-Santos, Lima, Lalmanach, Juliano, Gaultier and Scharfstein2000). The active cysteine-proteases detected in TCI and TCII isolates at the present study exhibit a molecular mass range of 40–55 kDa.
Cruzipain is a T. cruzi cysteine-protease with an important role in many parasitic processes, including host infection (Meirelles et al. Reference Meirelles, Juliano, Carmona, Silva, Costa, Murta and Scharfstein1992; Aparício et al. Reference Aparício, Scharfstein and Lima2004). The pattern observed in cysteine-protease activity gels was complex, where all TCI isolates exhibited 2 activities and TCII isolates exhibited from 2–4 proteolytic activities. Other groups described only 2 cysteine-proteases from different T. cruzi strains in activity assaying gels (Campetella et al. Reference Campetella, Martínez and Cazzulo1990; Lowdes et al. Reference Lowdes, Bonaldo, Thomaz and Goldenberg1996). It is possible that the additional activities observed in substrate-containing gels for wild isolates in this work, correspond to other cysteine-proteases rather than cruzipain. We intend to purify additional TCII active cysteine-proteases and evaluate their roles in interaction with vertebrate and invertebrate hosts.
These wild isolates had never been studied, and these extra cysteine-protease activities may have a role in the interaction with hosts in the environment where they circulate. Parasites may lose these protease activities as a consequence of selective pressures exerted by long-lasting maintenance in laboratory conditions. We showed that protease activities did not change between epimastigote and metacyclic trypomastigote forms among wild isolates; however, it would be interesting to investigate intracellular amastigotes and blood-stage trypomastigotes. By comparing different T. cruzi strains, a correlation between the level of cruzipain secreted by trypomastigotes and the capacity of invading cells was reported (Aparício et al. Reference Aparício, Scharfstein and Lima2004). T. cruzi G strain presented reduced levels of secreted cruzipain, being less infective, when compared to the Dm28c and X10/6 strains. Cruzipain-rich Dm28c trypomastigote supernatants enhanced G strain invasion capacity (Aparício et al. Reference Aparício, Scharfstein and Lima2004).
We also intend to investigate proteases in natural mixed infections by TCI and TCII genotypes, isolated from wild mammals. Little is known about these mixed infections, for example, whether there is competition or cooperation between these populations in host infections. Indeed, mammals (including humans) are more probably exposed to consecutive and variable inocula through distinct infection routes, rather than a single inoculum as is usually performed in the laboratory. Our goal is to associate protease expression and activity with the ecological relations of mixed parasite populations, and their outcome in successful infections in their hosts.
Proteases proved to be good markers for separating TCI isolates from TCII isolates, as shown by dendogram analyses.
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Universitária José Bonifácio (FUJB). Patrícia Fampa is a fellow from Fundação Oswaldo Cruz/Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Fiocruz/FAPERJ). The authors thank Dr Marcia Reed R. Coelho (Universidade Federal do Rio de Janeiro- UFRJ) for the valuable suggestions on the dendogram analyses.