Published online by Cambridge University Press: 06 May 2004
Phylogenetic relationships within the Capsalidae (Monogenea) were examined using large subunit ribosomal DNA sequences from 17 capsalid species (representing 7 genera, 5 subfamilies), 2 outgroup taxa (Monocotylidae) plus Udonella caligorum (Udonellidae). Trees were constructed using maximum likelihood, minimum evolution and maximum parsimony algorithms. An initial tree, generated from sequences 315 bases long, suggests that Capsalinae, Encotyllabinae, Entobdellinae and Trochopodinae are monophyletic, but that Benedeniinae is paraphyletic. Analyses indicate that Neobenedenia, currently in the Benedeniinae, should perhaps be placed in a separate subfamily. An additional analysis was made which omitted 3 capsalid taxa (for which only short sequences were available) and all outgroup taxa because of alignment difficulties. Sequence length increased to 693 bases and good branch support was achieved. The Benedeniinae was again paraphyletic. Higher-level classification of the Capsalidae, evolution of the Entobdellinae and issues of species identity in Neobenedenia are discussed.
The Capsalidae comprises approximately 200 monogenean species most of which are ectoparasitic on marine fishes. Some capsalids are important pathogens of cultivated fishes (e.g. Ogawa et al. 1995a,b; Whittington, 1996; Deveney, Chisholm & Whittington, 2001; Whittington et al. 2001a) and a few have been responsible for epizootics of wild fishes (Bauer & Hoffman, 1976; Paperna & Overstreet, 1981). The capsalid Entobdella soleae is arguably the most studied and best known of all monogeneans due to detailed research by Kearn (e.g. see references in Whittington, 1994; Kearn, 1998). Despite the familiarity of some genera (e.g. Benedenia, Entobdella, Neobenedenia), higher-level capsalid classification remains unresolved (Klassen, Beverley-Burton & Locke, 1989; Kritsky & Fennessy, 1999; Whittington, Deveney & Wyborn, 2001b). Yamaguti (1963) divided the Capsalidae into 5 subfamilies (Table 1). This classification was widely accepted, but did not recognize the Dioncinae or the Entobdellinae (Table 1). Instead, Yamaguti (1963) followed Bychowsky (1957) who gave Dioncinae familial status (as Dioncidae) and he moved members of the Entobdellinae to the Benedeniinae. With the proposal of Pseudonitzschiinae and Interniloculinae and the subsequent reinstatement of Dioncinae (see Timofeeva, 1990; Egorova, 2000a) and Entobdellinae (see Egorova, 1999), the Capsalidae currently comprises 9 subfamilies (Table 1).

Kritsky & Fennessy (1999) commented that the 5 subfamilies of Yamaguti (1963) had not been shown to be monophyletic. However, the analysis of Mollaret, Jamieson & Justine (2000) which used large subunit ribosomal DNA (lsrDNA) data in an attempt to resolve higher level phylogenetic relationships within the Monogenea gave preliminary insight into relationships within the Capsalidae. Using sequences of 6 capsalid species spanning 6 genera and 4 subfamilies, they demonstrated that the Capsalidae was monophyletic and concluded that the Capsalinae, Encotyllabinae and Trochopodinae were valid. Their results, however, indicated that the Benedeniinae was paraphyletic and that Entobdella species should be placed in a separate subfamily, the Entobdellinae after Bychowsky (1957). Mollaret et al. (2000) were unaware of work by Egorova (1999) who reached the same conclusion based on morphological features. It was noted that relationships within the Capsalidae should be re-analysed using molecular data from additional taxa (Mollaret et al. 2000).
Many of the approximately 40 genera in the Capsalidae are poorly defined, especially those in the Trochopodinae and, to a lesser extent, the Capsalinae and some have been moved between subfamilies. Phylogenetic treatment of the family based on morphological methods, therefore, is pointless until a critical reexamination of most genera is achieved, including a study of available type material and preferably examination of living specimens. A phylogeny, however, is needed to provide an improved understanding of the origins, radiation and evolution of this monogenean family, members of which parasitize elasmobranch and teleost fishes. This study uses existing and new lsrDNA sequence data from 17 capsalid species spanning 7 genera in 5 of the currently recognized subfamilies to construct preliminary phylogenetic hypotheses for the Capsalidae.
Table 2 lists the capsalid species used in this study (10 previously sequenced species; new sequences for 7 species) with their host species, site, locality, collector(s) and GenBank Accession details. Specimens sequenced in the present study were fixed in 95% analytical grade ethanol for DNA analysis. Additional specimens from each collection were preserved in 10% formalin and mounted in Canada balsam on slides beneath a cover-slip for species identification. Two mounted voucher specimens of each newly sequenced capsalid species (Table 2) are deposited in the Australian Helminthological Collection (AHC) of the South Australian Museum (SAMA), Parasitology Section, North Terrace, Adelaide, South Australia 5000, Australia. SAMA registration details follow each species: Entobdella australis (AHC 28422-3); E. hippoglossi (AHC 28424-5); E. soleae (AHC 28426-7); Entobdella sp. 1 ex Himanturafai (AHC 28428-9); Entobdella sp. 2 ex Pastinachussephen (AHC 28430-1); Neobenedenia sp. 1 ex Oreochromis sp. (AHC 28432-3); Neobenedenia sp. 2 ex Sphoeroides annulatus (AHC 28434-5).
Table 2. The 17 capsalid species (listed alphabetically) used in this study, including host species, site, locality details and GenBank number for the lsrDNA sequences examined (Chisholm and Whittington collected all new material unless indicated otherwise.)

DNA was extracted from single individuals following protocols in Chisholm et al. (2001a). lsrDNA was amplified by PCR using the methods and primers presented in Chisholm et al. (2001a,b). Multiple lsrDNA sequences up to 850 base pairs long were aligned using ClustalX, Vers 1.8 (Thompson et al. 1997), then edited by eye in GeneDoc Vers 2.6 (Nicholas, Nicholas & Deerfield, 1997). Two data sets were analysed.
Data Set A consisted of a reduced set of 315 characters. This reduction was necessary to accommodate some shorter capsalid sequences (for Capsala onchidiocotyle, Tristoma integrum and Trochopus pini) available on GenBank and because the more distant taxa (Dendromonocotyle ardea and Calicotyle kroyeri [Monogenea: Monopisthocotylea: Monocotylidae]; Udonella caligorum [Monogenea: Monopisthocotylea: Monocotylidae]) (GenBank details AF348351, AF279747 and AJ228803, respectively) could not be aligned reliably with the capsalid taxa through the 3′ half of the sequence. The total number of species included in Data Set A was the 2 outgroup species (D. ardea and C. kroyeri) plus U. caligorum (shown by Olson & Littlewood (2002) to be a close sister taxon to the capsalids) and 17 ingroup species including all previously sequenced capsalid taxa and the capsalid species newly-sequenced in the present study (see Table 2 for GenBank details).
Greater power for resolving relationships among the capsalid taxa was achieved by including more characters in the data set. To do this, species for which only short sequences were available were removed and taxa which were difficult to align with confidence were excluded. While 847 bases were sequenced, alignment of Data Set B remained poor through highly variable regions. Therefore, 154 of the 847 characters were removed leaving 693 bases for phylogenetic analyses. Excluded characters were positions 441–555, 637–649 and 802–827. Since outgroup taxa were removed, tree rooting was based on the results from Data Set A.
All phylogenetic analyses were carried out using PAUP* Vers 4.0b10 (Swofford, 2001). Trees were generated using maximum parsimony (P), maximum likelihood (L) and distance matrix (D, minimum evolution) analyses. Unweighted trees were found using heuristic searches with random sequence addition. For the parsimony analysis, gaps were treated as missing data. Other settings used were Mulpars in effect, Maxtrees set to 100 (limited by computational time), 10 heuristic search repetitions for P (one for D and L) and tree-bisection-reconnection (TBR) branch swapping.
For Data Sets A and B, a series of likelihood-ratio tests were completed using Modeltest Vers 3.04 (Posada & Crandall, 1998) to determine the best nucleotide substitution model to use for L and D analyses. The likelihood-ratio test statistic is calculated as twice the difference between the log likelihood scores of the 2 models being contrasted. When the models compared are nested, the test statistic fits a Chi-squared distribution with degrees of freedom equal to the number of taxa minus 2 (Huelsenbeck & Crandall, 1997; Posada & Crandall, 1998). Data Set A was best described with a transversional model (TVM) with among site heterogeneity (G), summarized as TVM+G. Data Set B was described with a Hasegawa–Kishino–Yano-1985 model (HKY) with among site heterogeneity (G), plus estimates of invariant sites (I), as HKY+I+G. For all inferred trees, branch support was tested using bootstrap analysis with 1000 replicates for P and D and 100 replicates for L.
Nucleotide data from the lsrDNA region of the 7 newly sequenced capsalid species produced an alignment of approximately 850 characters including domains C2 and D1 and partial domains C1 and D2 as defined by Hassouna, Michot & Bachellerie (1984).
The 3 tree building methods were congruent in topology (e.g. Fig. 1), but were unable to resolve the relationship among Entobdella sp. 2, E. australis and Entobdella sp. 1. Single trees were obtained for maximum likelihood analysis (−Ln likelihood=2147·44579) and distance analysis (D score=1·75192). Parsimony analysis found 2 trees, each of 377 steps (CI=0·6109) that differed in their grouping of the 3 Entobdella species mentioned above. Results from this analysis support the monophyly of the Capsalinae, Encotyllabinae, Entobdellinae and Trochopodinae (based on a single species), but suggest that the Benedeniinae is paraphyletic (Fig. 1). Results also indicate that the validity of Tristoma integrum needs to be investigated further since it nested between the 2 Capsala species.

Fig. 1. Maximum likelihood analysis tree of the Capsalidae inferred from lsrDNA sequences in Data Set A (315 characters). * indicates [ges ]70% support for all tree generation methods: maximum likelihood (L), distance (D) and parsimony analysis (P). Letter indicates [ges ]70% support for the type of analysis indicated. Shaded regions indicate current capsalid subfamilial designations.
Results of the maximum likelihood analysis (−Ln likelihood=4358·33311) of the longer data set are shown in Fig. 2. Distance analysis returned a D score of 1·66426. Parsimony analysis found 1 tree of 805 steps (CI=0·6845). Even with the additional characters, resolution among 3 Entobdella species (Entobdella sp. 2, E. australis and Entobdella sp. 1) remained poor. Bootstrap support for all genus-level clades is excellent (Fig. 2). However, the current concept of the Benedeniinae (represented here by Neobenedenia and Benedenia) is paraphyletic (Fig. 2). The Entobdellinae is monophyletic and is the sister group to Capsala martinieri (Capsalinae).

Fig. 2. Maximum likelihood tree of the Capsalidae inferred from lsrDNA sequences from Data Set B (693 characters). Tree root based on topology in Fig. 1. * indicates [ges ]70% support for all tree generation methods: maximum likelihood (L), distance (D) and parsimony analysis (P). Letter indicates [ges ]70% support for the type of analysis indicated. Shaded regions indicate current capsalid subfamilial designations.
Two Neobenedenia ‘isolates’ (ex Oreochromis sp., Red Sea, Israel and ex Sphoeroidesannulatus, Sea of Cortez, Mexico) identified morphologically (from mounted material) as N. ‘melleni’ were sequenced in this study. These ‘isolates’ differed by 9·52% (66 base pairs over 693 sites).
The Capsalidae are mostly ectoparasitic on marine fishes. This is an exceptional family because species span major fish groups including the sharks, batoids, acipenserids (Nitzschia) and numerous teleost species. The dynamics of capsalid systematics over the last 40 years has caused confusion because several new genera and many species have been added while opinions on higher capsalid classification have differed. This has led to a lack of awareness of constituent taxa (Klassen et al. 1989; Kritsky & Fennessy, 1999) and considerable disorder because of blurred distinctions between some taxa. However, recently steps have been made to address these issues.
Egorova (1989, 1994a,b, 1997, 1999, 2000a,b,c) has methodically revised subfamilial and generic diagnoses within the Benedeniinae, Capsalinae, Dioncinae, Encotyllabinae, Entobdellinae and Trochopodinae and has provided lists of valid species based on morphological assessments derived mostly from published descriptions. Concurrent studies by Timofeeva (1990) and Whittington (e.g. Horton & Whittington, 1994; Whittington & Horton, 1996; Whittington et al. 2001b) have made comprehensive morphological evaluations of particular genera (Dioncus; Metabenedeniella; Neobenedenia; Benedenia and Menziesia) and as a result, several genera and subfamilies are now better defined. In particular, Timofeeva (1990) included dioncids in the Capsalidae as the Dioncinae, based on haptoral characteristics and morphology of reproductive structures. Inclusion of the Dioncinae in the Capsalidae provides the family with a unique morphological synapomorphy, the presence of accessory sclerites on the haptor (modified hooklets I; see Kearn, 1963). This character is absent (presumably a secondary loss?) in only Pseudonitzschia uku and Calicobenedenia polyprioni. The presence of a clearly homologous structure across the majority of species in the family is significant because so few morphological features are shared broadly among species within the family.
Despite these advances, relationships between some other capsalid subfamilies (Capsalinae, Nitzschiinae, Trochopodinae) continue to be difficult to assess because many defining features are shared between groups (e.g. Klassen et al. 1989; Kritsky & Fennessy, 1999). Many characters have little obvious evolutionary connection between species; in fact, it is unclear whether some structures that have been regarded traditionally as taxonomically important are truly homologous. No comprehensive phylogeny has been proposed for the Capsalidae and indeed some have questioned the monophyly of the family (Kritsky & Fennessy, 1999). The present study has generated initial phylogenetic hypotheses for the Capsalidae using lsrDNA data and extends the 6 species studied by Mollaret et al. (2000) to 17 capsalid species representing 7 genera and 5 subfamilies.
One tree was generated from short (315 bases; Data Set A) and 1 tree from long (693 bases; Data Set B) data sets. Analysis of Data Set A, including distantly related taxa, supports the monophyly of the Capsalidae and the subfamilies Capsalinae, Encotyllabinae and Entobdellinae (Fig. 1). The Trochopodinae also appear monophyletic, but this is based on the short sequence available for a single exemplar species, Trochopus pini. As stated above, the taxonomy of the Trochopodinae is in a state of great confusion and many of the 17 genera and approximately 50 species in the subfamily are in need of careful revision. The Capsalinae currently comprises approximately 55 species in 7 genera and also clearly requires re-evaluation because Tristoma integrum falls between Capsala martineri and C. onchidiocotyle in the tree generated from Data Set A.
The Benedeniinae (represented by 4 previously sequenced Benedenia species and 2 newly sequenced Neobenedenia ‘isolates’) is paraphyletic in the trees generated from Data Sets A and B. The molecular phylogeny of Mollaret et al. (2000) also indicated that the Benedeniinae was paraphyletic, but this was because Benedenia lutjani and Entobdella australis were in different clades; no species of Neobenedenia were included in their analysis. They used the classification of Yamaguti (1963) who considered Entobdella to be part of the Benedeniinae and Mollaret et al. (2000) were unaware that the Entobdellinae (including species of Entobdella and Pseudoentobdella) had already been resurrected based on morphological analyses (see Egorova, 1997, 1999). The issue of paraphyly in the Benedeniinae is not simple to resolve. We have demonstrated excellent bootstrap support for the possible proposal of a new subfamily to include Neobenedenia species and the absence of a vagina in this genus provides additional morphological support for this option. Alternatively, synonymy of the Encotyllabinae with the Benedeniinae as conceived currently would also address the issue of paraphyly in the Benedeniinae, but there are robust morphological characters that support the Encotyllabinae (e.g. shape of body and haptor). We believe it is premature to propose the erection of a new subfamily or synonymy of existing subfamilies to address the problem of paraphyly in the Benedeniinae because sequence data from exemplars of only 2 of the 11 genera assigned currently to the subfamily are available. Further resolution in the apparently closely related Trochopodinae is also necessary.
The Entobdellinae is an especially interesting subfamily because species have been recorded from the skin of teleosts and elasmobranchs. Monophyly of the newly resurrected Entobdellinae (see Egorova, 1999) is supported by our molecular analyses and there is also good support morphologically for the subfamily (Whittington, unpublished data). In analyses of both data sets, the Entobdella species from teleosts cluster separately from Entobdella species from elasmobranchs although resolution of the relationship among species from elasmobranchs is equivocal. In the analysis of Data Set B, the Entobdellinae is placed as the sister group to Capsala martinieri (Capsalinae) from the teleost, Mola mola. Therefore, our hypothesis implies that capsalid species from elasmobranchs evolved relatively recently from capsaline relatives infecting teleosts. This supports the views on coevolution by Boeger & Kritsky (1997) who proposed that capsalids evolved and radiated on neopterygiians and secondarily dispersed to sturgeons, sharks and rays.
The concept of species identity in the Capsalidae, especially with respect to pathogenic species, is of considerable interest. Neobenedenia melleni is a particularly notorious, widespread pathogen of teleosts in aquaria and aquaculture and is aberrant among Monogenea because of its broad host-specificity (>100 host teleost species; Whittington & Horton, 1996; Deveney et al. 2001). Extensive morphological variation for N. melleni from different host species is reported and attributed to host induced morphological variation (Whittington & Horton, 1996). Our study incorporated 2 ‘N. melleni’ ‘isolates’ (identified morphologically) from different host species and localities (ex Oreochromis sp. [Cichlidae], Red Sea, Israel and ex Sphoeroidesannulatus [Tetraodontidae], Sea of Cortez, Mexico). The lsrDNA sequences for these 2 capsalids differ by 9·52% (66 base pairs over 693 sites). Benedenia lutjani and B. rohdei, congeners on the same host species, Lutjanus carponotatus, are morphologically distinct and differ by only 3·17% (22 base pairs over 693 sites). Therefore, the 2 ‘isolates’ of ‘N. melleni’ sequenced here appear to be different species and a reexamination is necessary to determine whether morphological differences have been overlooked. These findings have considerable implications for quarantine and disease management because species within a complex may vary widely in life-cycle features, fecundity and pathogenicity. The taxonomy of some Neobenedenia species has been contentious (e.g. see Ogawa et al. 1995a; Whittington & Horton, 1996) and it is unlikely that substantial resolution will be achieved without further in-depth studies. The identity of Neobenedenia ‘melleni’, in particular, must be reevaluated critically using morphological and molecular techniques.
In summary, a comprehensive study of the Capsalidae, combining both morphological and molecular methods (as done for the Monocotylidae, see Chisholm et al. 2001b), should help resolve the issues of paraphyly in the Benedeniinae and address the validity of other subfamilies, genera and species not represented in our analysis. Specifically, some of the subfamilies containing only 1 or 2 genera require careful review. We hope to pursue these avenues of research in the future. More lsrDNA sequences from more species will help, but it is likely that other genetic markers will be needed to pin down the ancestral taxa.
We thank the Director and staff at Heron Island Research Station, The University of Queensland for access to facilities and assistance during field studies. We are grateful to the following colleagues for providing specimens (Table 2): Dr Angelo Colorni (National Centre for Mariculture, Israel Oceanographic and Limnological Research Ltd); Dr Emma Fajer-Avila (Centro de Investigación en Alimentación y Desarollo (CIAD), A. C. Unidad de Investigación en Acuicultura y Manejo Ambiental del CIAD, Mazatlán, Sinaloa, Mexico); Dr Graham Kearn (University of East Anglia, Norwich, UK); Dr Andy Shinn (Institute of Aquaculture, University of Stirling, Scotland). Two anonymous referees made valuable suggestions on an earlier draft of this paper. Funding was provided in part by Australian Research Council Small Grant nos. 97/ARCS146G awarded to I.D.W. and R.D.A. and 99/ARCS011G awarded to L.A.C., I.D.W. and R.D.A.

Table 1. Subfamilial composition of the Capsalidae proposed by Yamaguti (1963) and the current composition based on actions and opinions of various authors (final column)
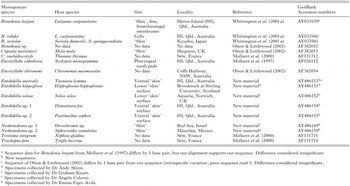
Table 2. The 17 capsalid species (listed alphabetically) used in this study, including host species, site, locality details and GenBank number for the lsrDNA sequences examined

Fig. 1. Maximum likelihood analysis tree of the Capsalidae inferred from lsrDNA sequences in Data Set A (315 characters). * indicates [ges ]70% support for all tree generation methods: maximum likelihood (L), distance (D) and parsimony analysis (P). Letter indicates [ges ]70% support for the type of analysis indicated. Shaded regions indicate current capsalid subfamilial designations.

Fig. 2. Maximum likelihood tree of the Capsalidae inferred from lsrDNA sequences from Data Set B (693 characters). Tree root based on topology in Fig. 1. * indicates [ges ]70% support for all tree generation methods: maximum likelihood (L), distance (D) and parsimony analysis (P). Letter indicates [ges ]70% support for the type of analysis indicated. Shaded regions indicate current capsalid subfamilial designations.