Published online by Cambridge University Press: 05 December 2003
The population dynamics of Toxocara canis in pigs, and their immune response to a primary and a challenge infection, were studied by parasitological, haematological and serological parameters. Seventy pigs were divided into 4 groups; 35 pigs received a primary infection (group A), 15 pigs received both a primary and a challenge infection (group B), 15 pigs received the challenge infection only (group C), and 5 pigs served as helminth-free controls (group NC). A dose of 50000 eggs was administered for the primary infection (day 0) and a dose of 10000 eggs was given for the challenge infection (day 28). On days 7, 14, 21 and 28 p.i., 5 pigs of group A, and on days 35, 42 and 49 p.i., 5 pigs from each of groups A, B and C were necropsied. Numbers of recovered larvae varied widely among the 5 pigs of each group on all days of necropsy. Toxocara canis larvae were recovered predominantly from the lungs; migration of larvae to other organs or tissues from the lungs was restricted. In group A, the larval burden in the lungs peaked on day 14 p.i., and the larval densities decreased significantly over time. Thereafter, the majority of larvae were recovered from the lungs until the end of the experiment (day 49 p.i.). A few larvae were found in the muscles and brain until day 42 p.i., and 2 larvae were found in the eyes of 2 pigs on day 35 p.i. There was little evidence of protective immunity to a challenge infection in this experiment. The eosinophil levels tended not to increase in pigs receiving a challenge infection, in contrast to the challenge control pigs. The fact that T. canis larvae migrate and persist in the tissues of pigs for more than 1 month suggests a zoonotic risk in infected pigs. The relevance of these data to the population biology and immunology of porcine and human toxocarosis is discussed.
The round worm Toxocara canis (Werner, 1782) is a common intestinal nematode parasite of canids. This parasite also has a wide range of paratenic hosts, mammals, birds and even earthworms, in which larvae migrate in the visceral organs and the body tissues, and may survive for prolonged periods up to 10 years (in monkey, Beaver, 1966), without development. The major impact of this helminthosis is its zoonotic potential; toxocarosis is one of the most common helminth zoonoses in the US (Hotez, 2002). However, many features of the disease are still unclear, because human toxocarosis shows diverse clinical symptoms and signs.
Increasing companion animal ownership and growing fox populations in some urban areas (Willingham et al. 1996; Deplazes & Eckert, 2001) have resulted in the contamination of the environment with eggs, increasing the risk of infection in paratenic hosts, including humans. Apart from direct transmission to humans through ingestion of T. canis eggs on contaminated soil, indirect transmission may come from consumption of infected food animals, e.g. chickens (Nagakura, 1989), pigs or rabbits (Sturchler, Weiss & Gassner, 1990), and lambs (Salem & Schantz, 1992).
Pigs have many physiological similarities to humans, in addition to body size (Miller & Ullrey, 1987; Boes & Helwigh, 2000) and have, therefore, been used as an animal model for several human helminthoses (Willingham & Hurst, 1996; Pedersen & Saeed, 2001). Investigations of porcine toxocarosis could provide important information also for human toxocarosis. However, only a few studies have been carried out on T. canis in pigs. Done, Richardson & Gibson (1960) reported the migration behaviour after a single large infection with T. canis eggs in a limited number of pigs over the course of days 2–64 p.i. Recently, Helwigh, Lind & Nansen (1999) reported on the early (7–28 days p.i.) migration behaviour of T. canis larvae in male pigs after a single infection. These authors observed that the majority of larvae were recovered from the lymph nodes surrounding the intestine on day 7 p.i., and from the lungs on day 14 and 28 p.i. They also suggested that most larvae die relatively early after infection. No information on the persistence over time of T. canis larvae in pigs, and on the effect of host sex, or repeated infections, was produced from these previous studies. The lack of data on larval population dynamics in pigs receiving multiple exposures is especially important because such exposure patterns are most likely to occur in natural circumstances.
The objectives of the present study were to investigate the larval population dynamics, predilection sites, and the persistence of T. canis larvae in pigs of both sexes over a longer period after single or multiple infections, and to evaluate the immune response, including acquired protection, resulting from infection.
Seventy helminth-naive Danish Landrace/Yorkshire/Duroc cross-bred pigs of both sexes (31 males and 39 females), obtained from a specific pathogen-free (SPF) herd, were used for the experiment. At the beginning of the experiment, pigs were 8–10 weeks of age and their average weight was 22·5 kg (2·23 S.D.). All pigs were kept in the same parasite-free building and allocated into 14 separate pens according to sex (2[ratio ]3 or 3[ratio ]2 (male[ratio ]female) in a pen), weight, experimental group and necropsy date. Pigs had free access to water and were fed twice daily with a standard diet consisting of ground barley with protein supplements, minerals and vitamins. The pigs were allowed to adapt to pens and feed for 1 week prior to infection. The animals were treated in accordance with animal ethics laws of Denmark.
Toxocara canis eggs obtained from a naturally infected local dog were passed experimentally through foxes (Vulpes vulpes) for propagation of the eggs (Taira, Saeed & Kapel, 2002). Eggs were collected from the faeces of infected foxes by using sieves. After the egg suspension was centrifuged, the supernatant was poured off, 1·27 SG glucose NaCl solution was added and centrifuged for purification of the egg suspension. Floating eggs in the upper suspension were collected and washed with tap water. The concentrated suspension of eggs was immersed and stirred in 0·5% sodium hypochlorite for 10 min to achieve decoating, and then washed repeatedly with tap water until no chlorine odour was detected. Eggs were incubated in 0·1% formalin at 25 °C for 1–2 months to embryonate. After embryonation, eggs were held at room temperature for 1 month. Before infection the egg suspension was washed with tap water.
The infectivity of the eggs was pre-tested in a mouse bioassay. Five 6-week-old female NMRI mice were inoculated with 2000 embryonated eggs, and killed and skinned on day 3 p.i. The liver, lungs, brain and carcass were digested separately for larval recovery using the same digestion method described below. Larvae were found in all organs and tissues, and the total mean percentage of recovery was 7·9% (1·98 S.D.).
The design of the experiment is presented in Fig. 1.

Fig. 1. Experimental design. Group A received only a primary 50000 Toxocara canis eggs, group B received both the primary and a challenge of 10000 eggs given 28 days after primary infection, and group C received only the challenge infection (10000 eggs). Group NC served as a parasite-free control. Arrows indicate inoculations. Crosses indicate necropsy intervals.
To investigate the population dynamics and persistence of T. canis larvae after a single infection, pigs in group A (n=35) received 50000 T. canis eggs and 5 pigs were necropsied weekly until day 49 p.i. Five pigs were left uninfected throughout the experiment to serve as a negative control group (group NC) for pig weight gains, blood analysis and antibody response, and necropsied at the termination of the experiment.
The effect of the primary infection on a secondary infection was evaluated in an additional 30 pigs. Pigs in group B (n=15) received 50000 eggs at the same time as group A, and a challenge infection of 10000 eggs was given 28 days after the primary infection. Pigs in group C (n=15) received only the challenge infection and were therefore the challenge control group. Five pigs of each group were necropsied weekly after the challenge infection until day 49 post-primary infection. Group A constituted the primary infection control group.
Eggs were inoculated orally by stomach tubes. One week before the first 50000 egg inoculation, all pigs were weighed and blood samples were taken. After infection, weighing and blood sampling were conducted at necropsy. For the negative controls, blood sampling and weighing were carried out weekly. Clinical signs or abnormal behaviour were recorded during daily normal management.
Faecal samples were collected on days 1 and 2 p.i. and examined to detect eggs that were excreted without hatching, using a concentration McMaster technique as described by Roepstorff & Nansen (1998). Pigs were stunned with a captive bolt pistol, exsanguinated and eviscerated immediately. Exsanguinated blood was sampled for haematological and serological studies. The entire liver without the gallbladder, lungs, brain, kidneys, diaphragm, masseter, tongue, heart and eyes, and all mesenteric lymph nodes and all colic lymph nodes were removed separately and weighed. One meter of the small intestine in the middle of the jejunum, a section of muscles comprising 100 g of front limb (brachial triceps) and 100 g of hind limb (femoral biceps), were also removed for the larval recovery. The number of the granulation-tissue type white spots (GT-WS) and the lymphonodular type white spots (LN-WS) (Roneus, 1966) on the surface of the liver were recorded. White foci on the kidneys were also counted.
Sampled organs or tissues were blended into pieces of less than approximately 5 mm3 by use of a kitchen blender, and a maximum of 100 g subsamples were digested. All samples were digested in a solution containing 1% HCl (37%) and 1% pepsin (1[ratio ]10000 N. F.) in tap water for 2 h at 46 °C under constant stirring. The ratio between tissue (g) and fluid (ml) was approximately 1[ratio ]10. Following digestion, fluid was poured through a tea sieve into a conical beaker, and allowed to stand for 40 min for sedimentation of larvae. Subsequently, supernatants were removed, beakers were filled with water and allowed to settle for another 40 min. This procedure was repeated at least 3 times until the suspension became translucent. The sediment was then placed in a plastic tube and fixed in an iodine solution (6·25% iodine, 31·25% potassium and 62·5% distilled water). Before counting in a dissecting microscope, the sample was decolorized using 3% sodium thiosulphate. The total number of larvae in each organ or tissue was estimated by multiplying the number of larvae counted in a gram of tissue by the total weight of the organ or tissue.
For haematological studies, total red blood cell counts (109/l), the haemoglobin concentration (mmol/l), the haematocrit (l/l), and the total white blood cell counts (109/l) were measured in an Abbott CellDyn3500. The differential white blood cell percentages were determined manually following the counting of 200 cells in a Wright's stained blood smear, and the actual number of cells was estimated by multiplying total white blood cell counts by cell percentages. For blood biochemistry, albumin (g/l), alanin aminotransferase (U/l), total protein (g/l), alkaline phosphatase (U/l), creatinin (umol/l), aspartate aminotransferase (U/l), and creatin kinase (U/l) were measured by use of an automated spectrophotometer Cobas Fara II (Roche).
Swine sera were tested in duplicate against T. canis larval ES antigens by use of an indirect enzyme-linked immunosorbent assay (ELISA) for 3rd-stage T. canis-specific IgG antibodies. T. canis ES antigens obtained from in vitro cultivation of the larvae were provided from the Institute for Parasitology, University of Zurich, Switzerland. The ES antigens were diluted 1[ratio ]1000, sera were diluted 1[ratio ]200, and the secondary antibody (alkaline phosphatase-conjugated goat-anti-swine IgG (H+L); SIGMA) was diluted 1[ratio ]1000. After coating the microwell plate (NUNC, maxisorp) with antigen dilution, the plates were incubated overnight at 4 °C, 100% humidity. Plates were washed 3 times (NaCl 0·9%, Tween20 0·3%) and blocked with PBS/Tween20 (0·3%) for 30 min at 37 °C. Following removal of the blocking solution, the serum dilutions were added to the microwells and left for 1 h at room temperature with slight shaking. After washing, goat-anti-swine IgG was added and left for 30 min at room temperature. Following washing, substrate solution (p-nitrophenyl phosphate, 1 mg/ml) was added and left for 30 min at room temperature in the dark. A stop solution (3 M NaOH) was added thereafter, and optical density (OD) values were read by Multiscan EX (Labsystems) with a 405 nm filter. A dilution of T. canis-positive sera and a negative control (Ascaris suum positive sera) were applied to all plates. The cut off level was determined at 0·188, which is defined as the upper 95 percentile of OD values from 125 serum samples of T. canis-negative pigs.
The arithmetic mean and standard deviation are used in presenting the data. Before the analysis, total larval recovery, liver white spot counts, eosinophil counts and ELISA OD values were transformed by log10(x+10) to normalize the data. Total larval recovery (L), liver white spot counts (W), eosinophil counts (S), ELISA OD values (O), or body weight gains (B) were separately analysed using a generalized linear model (GLM) with explanatory factors; group main effect (αi), time main effect (βj), host sex main effect (γk), and these interactions (δ). Starting with full factorial models, terms were deleted in turn to allow evaluation of significance. Finally, minimum significant models were fitted incorporating only significant terms and their interaction.
Thus, models were defined for analysis of the single infection (group A):
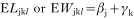

for analysis of the challenge infection (with data after 35 days of primary infection):

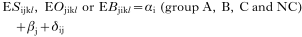
where index l represents the pig. To evaluate the sex main effect on eosinophil counts and ELISA OD values, group as a explanatory factor was omitted from model (2), and also, data from group NC were omitted from model (4) in the analysis for ELISA OD values. Bonferroni correction was made for pairwise test between groups (Altman, 1991). Spearman's rank correlations coefficient was used to evaluate the relationship between total larval recoveries, liver white spot counts, eosinophil counts and ELISA OD values by necropsy date for each group. All statistical analyses were performed as two-tailed tests with a significance level of 5%, using the SAS Release 8.2 software package.
No T. canis eggs from the inoculated dose were detected in the faeces of pigs up to day 2 p.i. At no time in the experiment did pigs show abnormal behaviour or clinical signs that could be related to the experimental infection. Also, there were no significant differences in body weight gains between groups (data not presented). No significant correlations were seen between total larval recoveries, liver white spot counts, eosinophil counts and ELISA OD values, with the exception of total larvae and liver white spots on day 14 p.i. (see below).
Results of the larval recoveries from pigs receiving only a primary infection are shown in Table 1, group A. Total larval recoveries varied widely in 5 pigs of each necropsy date (Fig. 3). The highest percentage of recovery (total larvae recovered/inoculated eggs) was 1·42% on day 7 p.i. The total larval recovery significantly reduced over time (F6,27=7·12, P<0·001), and only a few larvae were recovered on day 49 p.i. Host sex had no significant effect on larval counts (F1,27=0·29, P=0·592).
Table 1. Mean number of larvae in the intestinal lymph nodes, liver, lungs, and other tissues and mean white spots on the liver and mean white foci on kidneys of pigs experimentally infected with Toxocara canis eggs (Group A were given only a primary inoculation, group B were given both primary and challenge inoculations, group C were given only a challenge inoculation and group NC served as negative control.)

Overall, the majority of the larvae were recovered from the intestinal lymph nodes, liver and lungs throughout the experiment. The relative distribution of larvae in the intestinal (mesenteric and colic) lymph nodes, liver, lungs, and total larvae in other examined organs and tissues is shown in Fig. 2. The largest proportion of larvae was found in the intestinal lymph nodes on day 7 p.i., but thereafter larvae predominated in the lungs until the end of the experiment (day 49 p.i.). Severe petechiae on the surface of the lungs were associated with high numbers of larvae recovered. A few larvae were found in the brain, eyes, kidneys, diaphragm, masseter, tongue, heart, small intestinal wall, and the muscle of front and hind limb, thus the sum of the number of larvae from these organs and tissues are presented in Table 1. The total larval recovery in the brain were 2, 4, 10, 1, 2, and 0 on days 7, 14, 21, 35, 42 and 49 p.i., respectively. Two larvae were found in the eyes of 2 pigs on day 35 p.i.

Fig. 2. The relative distribution of Toxocara canis larvae in the intestinal lymph nodes (mesenteric and colic), liver, lungs, and other selected tissues or organs (kidneys, brain, eyes, heart, diaphragm, masseter, tongue, a part of jejunum, a part of front and hind limb muscles). Five pigs were necropsied weekly after a single inoculation with 50000 T. canis eggs (Group A).

Fig. 3. Variable susceptibility of individual pigs to Toxocara canis larval infestation. Host sex had no effect on larval infestation (P=0·592). Five pigs were necropsied weekly after a single inoculation with 50000 T. canis eggs (Group A).
The result of liver white spot counts are presented in Table 1, group A. Large variations were seen in counts for 5 pigs of each necropsy, especially on days 7 and 14 p.i. (Fig. 4A). Liver white spot counts significantly fell with time (F6,27=11·85, P<0·001). There was no effect of host sex on counts (F1,27=0·13, P<0·722). A positive correlation between the liver white spot counts and the total larval recovery was seen on day 14 p.i. (rs=1·00, n=5, P<0·001).

Fig. 4. The occurrence of liver white spots, kidney white foci, eosinophilia and antibody in pigs infected with Toxocara canis eggs: (A) mean liver white spots, (B) mean kidney white foci, (C) mean number of eosinophils, and (D) mean ELISA OD values for T. canis-specific IgG antibody. T bar indicates S.D.
Almost all of white spots observed were GT-WS in all groups. Only a few LN-WS (less than 3) were observed in 2 pigs on day 49 p.i. The majority of white spots on days 7 and 14 p.i. had a well-defined border; showing indistinguishable septal structures of hepatic lobules around the centre of these spots, and most spots showed haemorrhages at the centre. The maximal number of white spots was observed on day 14 p.i. Larger white spots (>5 mm in the diameter) were also seen at that interval. The livers with the higher number of white spot counts (>300 white spots) were more or less white in colour, therefore, counts above 200 were not always precise because large spots consisted of several confluent spots. On day 21 p.i. most of the white spots appeared to be in the process of resolving; the colour became fainter and septal structures of lobules became more visible. Afterwards, the number of white spots decreased but some (19% of total white spots) were well-defined white spots with haemorrhage at the centre similar to that seen on days 7 or 14 p.i. Fading of the white spots complicated precise counts, and very faint white spots were often observed without counting. Thus, on days 42 and 49 p.i., lower numbers of white spots were recorded; however, 6–10% of those counted were well-defined white spots having haemorrhagic centres.
White foci were observed in the kidneys although the numbers were not as high as liver white spots (Table 1, Fig. 4B).
Overall only non-significant differences were found in either the blood cell counts and the biochemistry studies, except in peripheral blood eosinophil counts. Eosinophil counts also varied in 5 pigs of each necropsy (Fig. 4C). Three and 2 blood samples on days 7 and 14 p.i., respectively, were coagulated, and could not be used for blood counts. Group and time as factors significantly affected counts (F1,50=25·48, P<0·001; F6,50=12·28, P<0·001, respectively), and there was a significant interaction between group and time (F6,50=10·01, P<0·001), due to the prominent peaking after 2 weeks of infection. Host sex had no significant effect on counts. A significant eosinophil increase was seen on day 14 p.i. compared with the negative control in pairwise comparison (t=8·27, D.F.=50, P<0·001).
Results of ELISA are presented in Fig. 4D. Group and time as factors significantly affected OD values (F1,55=129·20, P<0·001; F6,55=5·21, P<0·001, respectively) and there was a significant interaction of group and time (F6,55=4·98, P<0·001), due to the sero-conversion after 2 weeks of infection. Host sex had no effect on OD values. A clear sero-conversion were seen during days 7 to 14 p.i.; mean OD values significantly increased over those of negative controls from day 14 p.i. (t=4·43, D.F.=55, P=0·004) until the end of the experiment (Fig. 4D). There was slight cross-reaction with Ascaris suum-positive sera (OD=0·280) but well below the antibody levels of the experimental groups.
Time as a factor had a significant effect on larval recovery (F2,36=4·38, P=0·020), but group had no significant effect. There was no interaction of group and time. In a pairwise comparison, group A tended to show higher total larval recovery than group C (t=3·39, D.F.=36, P=0·061) on day 42 after the primary infection. The larval recovery in group C was very low (Table 1); the highest percentage of recovery (total larvae/inoculated eggs) was only 0·4% on day 7 p.i., and thereafter, only a few larvae were found.
Group as a factor had a significant effect on liver white spot counts (F2,36=4·75, P=0·015), but no significant effect of time, and no interactions were seen. No significant differences were seen between groups at each necropsy in pairwise comparisons.
Three blood samples of group B and 3 of group C on day 14 after challenge infection were not used in cell counts due to coagulation. On eosinophil counts, group as a factor had a significant effect (F3,24=3·23, P=0·032), and there was a significant interaction between group and time (F6,24=2·50, P=0·037), but time had no significant effect. No significant differences were seen between groups at each necropsy in pairwise comparisons. However, a single pig of group C (challenge control) showed marked eosinophilia on day 14 after challenge infection in contrast to group B (primary+challenge) (Fig. 4C). Further, in overall group pairwise comparisons, group C (n=12) tended to have higher eosinophil levels than group A (primary infection, n=15), (t=2·49, D.F.=30, P=0·056) and group B (n=12) (t=2·46, D.F.=30, P=0·059).
Group as a factor had a significant effect on ELISA OD values (F2,36=23·77, P<0·001), but no differences were seen between group A and B at each necropsy in pairwise comparisons. No effect of time or the interaction were seen. Separately, group A and B were compared by ANOVA after sero-conversion (day 14 p.i.), with explanatory factors; group, time and these interactions. There was no effect of time and the interaction, and when these were omitted from the model, group B (n=15) showed higher antibody levels than group A (n=30), (F1,43=4·42, P=0·041).
The results from the present study indicate that in T. canis infection in pigs there is little evidence of specific predilection sites for the larvae which can enhance survival over a long period; most of the larvae appear to be eliminated early in the infection, either before or after reaching the lungs, although a few larvae do appear to randomly migrate to other tissues such as muscles or brain. Thus, pigs may be less satisfactory as paratenic hosts for T. canis in contrast to mice or poultry in which larvae may persist for more than a year (Galvin, 1964; Nakamura et al. 1991; Bardon, Cuellar & Guillen, 1994). However, this inadequacy as a paratenic host and the resulting migration patterns may resemble more closely those in humans. The strong tendency for larval migration to the lungs of pigs may be relevant to clinical human toxocarosis, which often shows pulmonary involvements (Glickman & Schantz, 1981; Gillespie, 1993). Although only a few larvae migrate through the various organs and tissues in pigs, this appears comparable to clinical human toxocarosis, which shows diverse symptoms. Thus, pigs may be a useful and relevant model for human T. canis infection, for aspects of larval migration.
The results of the present experiment revealed varied individual pig susceptibility to larval infection, i.e. most of the larvae were recovered from 1 or 2 pigs out of 5 from the same experimental group on all days of necropsy. Helwigh et al. (1999) also observed large variation in the larval recovery within a group of pigs. The variations in liver white spot counts in group A at the early stages of infection indicates high variability in host susceptibility, since white spots are evidence of the larval migration; the liver white spot counts correlated positively with the total larval recovery on day 14 p.i. No effect of host sex on larval recoveries or on ELISA OD values were seen in the present experiment. Holland et al. (1995) reported that boys have higher sero-prevalence values than girls, but suggested that this may relate to differences in playing activities. Since the pigs used in the present experiment were out bred, it is probable that the variability of the larval recoveries is a reflection of the host genetics which determine host resistance/susceptibility. Variable susceptibility to Toxocara infection has been observed between different strains of mice (Dunsmore, Thompson & Bates, 1983; Epe et al. 1994).
The larval migratory pattern observed in the present experiment accords generally with results from previous investigations (Helwigh et al. 1999). The present experiment demonstrated that larvae persist predominantly in the lungs after day 14 p.i. This contrasts with the report of Done et al. (1960) who observed larvae primarily in the liver until at least day 64 p.i. These authors used very large egg doses in relation to the host size (250000 eggs/5–7 kg), and it is possible that induced strong tissue responses impeded the progress of the larval migration through and out of the liver. Whether this impediment was due to non-specific mechanical effects caused by liver inflammation and fibrosis or to a difference in immune responses relating to the host age remains to be determined.
The fate of the larvae not remaining in the lungs is unknown, but several possibilities can be suggested. (1) Larvae were killed in host tissues and digested; (2) larvae migrated to unexamined tissues and (3) larvae migrated into the bronchae, moved up to the oral cavity and were swallowed back into the intestine and were eventually expelled (Oshima, 1961). Most likely, however, the larvae died and disintegrated early in the tissue due to host responses. Done et al. (1960) observed dead or dying larvae within granulomatous reactions with increasing frequency after day 8 p.i. Organs or tissues that were not examined in the present experiment, such as the spleen, glandular systems or other genito–urinary systems are probably not predilection sites for T. canis larvae since such organs or tissues did not show noticeable macroscopical changes at necropsy. There is a possibility that some larvae migrate back to the intestine via the trachea (Oshima, 1961). Further experimentation is needed to determine the fate of larvae in the pig.
The migration of larvae to the brain, muscles, kidneys, heart and eyes, although few in number, suggests the larvae migrate primarily via the blood circulatory system, although peritoneal migration cannot be ruled out. Done et al. (1960) suggested that larvae could migrate directly from the liver to the diaphragm in pigs, and extra-vascular larval migration through tissues and body cavities has also been suggested for mice (Sprent, 1952; Burren, 1968; Abo-Shehada, Al Zubaidy & Herbert, 1984).
The presence of some well-defined large liver white spots, even at the later stage of infection, indicates that some larvae circulate continuously. This may support the observation of Kayes (1997) that the encapsulated larvae can break out of the granuloma and elicit a reaction at a new site. The time-course of eosinophilia and antibody production observed in the present study is similar to that from previous reports (Helwigh et al. 1999; Sommerfelt et al. 2001). These authors observed that eosinophilia peaked on day 14 p.i. and decreased to normal by day 28 p.i. Further, antibody levels increased to day 14 p.i., and remained high until the end of the experiment (up to day 56) (Helwigh et al. 1999; Sommerfelt et al. 2001). Although a significant eosinophil increase occurred only on day 14 p.i. in the present experiment, the time-course was similar to previous reports. Eosinophilia is one infection response that may be different between humans and pigs. In pigs, eosinophilia subsides within 1 month after infection, probably because the majority of larvae are killed during this period. In contrast, in humans (Smith & Beaver, 1953), monkeys (Bisseru, 1969; Aljeboori & Ivey, 1970), and mice (Kayes & Oaks, 1980; Epe et al. 1994), eosinophilia continues for months, although, not all human cases are followed by eosinophilia (Magnaval et al. 1997). The chronic eosinophilia may be due to the prolonged larval migration seen in these hosts. In human cases, low dose (100–200 eggs) infection can cause chronic eosinophilia for up to 2 years (Smith & Beaver, 1953; Chaudhuri & Saha, 1959). It would be of interest to investigate the role of eosinophils, if any, in the killing of T. canis larvae in pig infections.
Due to low larval establishment in the challenge control, assessment of whether resistance to the establishment of challenge larvae occurred was not possible. Neither the larval recovery nor the liver white spot counts were significantly different between challenged groups. Further investigation is necessary to reach a firm conclusion on acquired resistance to T. canis in pigs.
Although there were no statistical differences in eosinophil responses between groups at each necropsy, responses were quite variable. Although none of the pigs of group B (primary+challenge) showed eosinophil proliferation, a single pig of group C (challenge control) had increased eosinophilia on day 14 after the challenge infection, and pigs in group C tended to have higher eosinophil counts than other groups. The immunogenicity of the T. canis larvae in pigs is apparent in the antibody responses; in spite of low larval recoveries in group C pigs, antibody responses increased above the cut-off level. Also group B showed elevated antibody levels compared to group A (primary infection). These observations lend support to Magnaval et al. (2001) who suggested that repeated infections might lead to antibody responses without eosinophilia.
In conclusion, the results demonstrated that larval migration in the pig was predominantly to the lungs; however, the majority of larvae were killed relatively early, with only a few larvae migrating to the other organs or tissues e.g., muscles or brain. This suggests that T. canis in pigs may be a more appropriate human model than mice for studies on larval migration. The fact that T. canis larvae migrate and persist in the tissues of pigs for more than 1 month suggests a zoonotic risk from infected pigs. To further evaluate the risk of a meat-borne zoonosis, it is important to determine if larvae recovered from pigs are infective to other hosts.
This work was supported by the Danish National Research Foundation under the auspices of the Danish Centre for Experimental Parasitology. We are grateful to the late Dr Peter Nansen for providing the idea for this project. We thank Dr Peter Deplazes for providing the ES antigen and Lone N. Møller for assisting during the serological work.

Fig. 1. Experimental design. Group A received only a primary 50000 Toxocara canis eggs, group B received both the primary and a challenge of 10000 eggs given 28 days after primary infection, and group C received only the challenge infection (10000 eggs). Group NC served as a parasite-free control. Arrows indicate inoculations. Crosses indicate necropsy intervals.

Table 1. Mean number of larvae in the intestinal lymph nodes, liver, lungs, and other tissues and mean white spots on the liver and mean white foci on kidneys of pigs experimentally infected with Toxocara canis eggs

Fig. 2. The relative distribution of Toxocara canis larvae in the intestinal lymph nodes (mesenteric and colic), liver, lungs, and other selected tissues or organs (kidneys, brain, eyes, heart, diaphragm, masseter, tongue, a part of jejunum, a part of front and hind limb muscles). Five pigs were necropsied weekly after a single inoculation with 50000 T. canis eggs (Group A).

Fig. 3. Variable susceptibility of individual pigs to Toxocara canis larval infestation. Host sex had no effect on larval infestation (P=0·592). Five pigs were necropsied weekly after a single inoculation with 50000 T. canis eggs (Group A).

Fig. 4. The occurrence of liver white spots, kidney white foci, eosinophilia and antibody in pigs infected with Toxocara canis eggs: (A) mean liver white spots, (B) mean kidney white foci, (C) mean number of eosinophils, and (D) mean ELISA OD values for T. canis-specific IgG antibody. T bar indicates S.D.