Published online by Cambridge University Press: 18 November 2004
Plasmodium falciparum gametocytes grown in vitro were fed through membrane feeders to laboratory-reared Anopheles stephensi mosquitoes. Intact midguts, including entire bloodmeal contents, were removed between 24 and 48 h post-bloodfeeding. Giemsa-stained histological sections were prepared from the midguts and examined by light microscopy. Contrary to previous reports, ookinetes were clearly visible within midgut epithelial cells, demonstrating intracellular migration across the midgut wall. Ookinetes entered epithelial cells through the lateral apical membrane at sites where 3 adjacent cells converged. There was no evidence for the existence of a morphologically distinct group of epithelial cells preferentially invaded by ookinetes. However, ookinete penetration was associated with significant morphological changes to invaded cells, including differential staining, condensation and fragmentation of the nucleus, vacuolization, loss of microvilli and various degrees of extrusion into the midgut lumen. Epithelial cells completely separated from the midgut wall were found within the midgut lumen. These cells were associated with invading parasites suggesting that ookinete penetration resulted in complete ejection of invaded cells from the midgut wall. Small clusters of morphologically altered midgut cells and invading parasites spanning the membranes of adjacent abnormal epithelial cells were observed, consistent with intracellular movement of ookinetes between neighbouring midgut cells. Extruded epithelial cells were also observed rarely in uninfected midguts. Epithelial cell extrusion, therefore, may be a general mechanism of tissue repair through which damaged cells are removed from the midgut wall rather than a parasite-specific response. These observations demonstrate that human malaria parasite infection of mosquitoes is consistent with, and provides further support for, the Time Bomb model of ookinete invasion of the mosquito midgut epithelium previously proposed for rodent malaria parasites.
Malaria parasites must invade and traverse the midgut wall of invertebrate vectors to be transmitted between vertebrate hosts. Motile invasive parasite stages, known as ookinetes, migrate through the bolus of ingested blood, penetrate the surrounding peritrophic matrix and invade the midgut wall. The latter consists of a monolayer of polarized epithelial cells possessing an apical (inner) surface covered with microvilli and a basal (outer) surface (Billingsley, 1990). Intercellular junctions in the apical midgut wall region connect the lateral membranes of adjacent epithelial cells forming a contiguous barrier to parasite invasion. The nature of ookinete midgut wall penetration, and the effects this invasion has upon the mosquito host, have been the subject of much debate (Maier, Becker-Feldman & Seitz, 1987; Sinden & Billingsley, 2001).
Initial controversies, unresolved for the human malaria Plasmodium falciparum, concerned the route of ookinete migration. Numerous light and electron microscopy studies investigated ookinete invasion of various avian, rodent and primate malaria species in diverse mosquito hosts (Reichenow, 1932; Huff, 1934; Indacochea, 1935; Stohler, 1957; Garnham, Bird & Baker, 1962; Omar, 1968; Garnham et al. 1969; Maier, 1973; Canning & Sinden, 1973; Davies, 1974; Mehlhorn & Peters, 1980; Becker-Feldman, Maier & Seitz, 1985; Meis & Ponnudurai, 1987a; Meis et al. 1989; Syafruddin et al. 1991). Some authors reported intercellular and others intracellular migration across the midgut wall. Different studies using the same parasite and mosquito species reported contradictory observations. The route of ookinete penetration was proposed to depend upon the parasite–vector combination, with ‘well-adapted’ parasites invading between cells to reduce the harm caused to mosquito vectors (Maier, 1987; Meis et al. 1989). The issue seemed resolved when ookinetes of the avian malaria Plasmodium gallinaceum were found in both intra- and intercellular locations within the midgut of Aedes aegypti (Torii et al. 1992). Intra- and intercellular ookinetes were observed in apical and basal regions of the midgut epithelium respectively, implying that ookinetes initially enter midgut cells and subsequently exit into the basolateral space between adjacent cells.
Recently, 2 conflicting models of ookinete invasion have also been proposed (Shahabuddin, 2002). The first, based upon an in vitro system using cultured P. gallinaceum ookinetes and isolated Ae. aegypti midguts, proposes that ookinetes preferentially invade a subpopulation of morphologically and biochemically distinct epithelial cells called Ross cells (Shahabuddin & Pimenta, 1998; Cociancich et al. 1999). Ross cells are characterized by several features including light staining by toluidine blue, vacuolated cytoplasm and an apical surface lacking or relatively denuded of microvilli. The second model, based upon in vitro and ex vivo systems using the rodent malaria, Plasmodium berghei, and the mosquito, Anopheles stephensi, contends that ookinetes do not invade a specific cell type but induce pathological changes in invaded epithelial cells similar to the morphological and biochemical characteristics of Ross cells (Han et al. 2000; Zieler & Dvorak, 2000). This is termed the Time Bomb model as parasite invasion triggers a cascade of physical and chemical events (the ‘time bomb’) presumed to lead to the death and extrusion of invaded cells from the midgut wall (Han & Barillas-Mury, 2002). Consequently, ookinetes have limited time to traverse invaded cells before being killed by invasion-induced host responses or ejected into the midgut lumen with the extruding cell. Ookinetes may also move laterally through adjacent cells and basally away from the initial site of entry to avoid deleterious host responses triggered by invasion. Observations consistent with this model have been made using the in vitro P. gallinaceum–Ae. aegypti system, suggesting a mechanism of ookinete invasion conserved across evolutionarily distant parasite and mosquito species (Zieler & Dvorak, 2000).
Interpretation of these recent studies is complicated by the use of in vitro/ex vivo systems and unnatural parasite–vector combinations; the observations could be artefacts of the laboratory models used (Han et al. 2000; Zieler & Dvorak, 2000). Furthermore, the relevance of these studies for human malarias is unknown (Shahabuddin & Pimenta, 1998; Cociancich et al. 1999; Han & Barillas-Mury, 2002). Here we report observations using P. falciparum in the natural vector An. stephensi. Mosquito midguts infected in vivo were processed with minimal manipulation to produce histological sections examined by light microscopy. We found no evidence that ookinetes preferentially invaded a subset of midgut epithelial cells but there was clear evidence that ookinete invasion was intracellular and caused significant morphological changes to invaded cells similar to those described by the Time Bomb model. Furthermore, our observations provide direct evidence of complete ejection of invaded cells from the midgut wall as hypothesized by this model.
P. falciparum clone 3D7A was cultured in vitro under conditions permissive for the development of mature gametocytes infective to mosquitoes as described elsewhere (Ifediba & Vanderberg, 1981; Ponnudurai et al. 1982; Carter, Ranford-Cartwright & Alano, 1993).
An. stephensi Dutch strain mosquitoes were maintained in an insectary at 26±1 °C and 70–80% relative humidity in a 12[ratio ]12 h light/dark cycle. Young larvae were fed daily with Liquifry whilst older larvae were given ground Tetramin®. Adult mosquitoes were provided ad libitum, both before and after bloodfeeding, with a solution of 5% glucose/0·05% para-amino-benzoic acid, except for the 24 h prior to bloodfeeding when distilled water only was given to encourage engorgement.
Five to seven days after emergence from pupae, adult female An. stephensi mosquitoes were membrane-fed either uninfected human blood or uninfected blood mixed with cultured asexual and gametocyte stages of P. falciparum according to standard procedures (Ponnudurai et al. 1982; Carter et al. 1993). Two separate experiments using infectious gametocytes were undertaken, the first giving a marginally higher level of oocyst infection at day 10 post-infection than the second feed.
At various times post-bloodfeeding (p.b.f.; 24, 28, 32, 36, 40, 44 and 48 h), mosquitoes were anaesthetized with chloroform and kept on ice until dissection. Intact midguts, including bloodmeal contents, were dissected into phosphate-buffered saline at pH 7·2 and immediately fixed in 2·5% glutaraldehyde in 0·1 M phosphate buffer (Na2HPO4/KH2PO4) at pH 7·2 for at least 24 h. Subsequently, midguts were washed 3 times for 10 min each in phosphate buffer and dehydrated through 10 min rinses in 30, 50, 70 and 90% ethanol in phosphate buffer and then absolute ethanol for 30 min. Midguts were then embedded in Technovit 7100 (Heraeus Kulzer, Germany), a glycol methacrylate-based resin, according to the manufacturer's instructions. Longitudinal serial sections 2 μm thick were dry-cut on an LKB-2 ultramicrotome using either a glass or diamond knife, stretched on water and attached to glass slides over a hot plate at 60 °C for 10 min. Sections were stained with 5% Giemsa's solution for 15–20 min, washed with water, air dried and mounted with a cover-slip using DePex mounting medium (BDH Laboratory Supplies, Poole, UK). Each midgut was completely sectioned and all resulting sections were examined using light microscopy. Images were captured using a Photometrics CoolSnap™ digital camera attached to a Zeiss Axioskop light microscope and Improvision® Openlab™ version 2 software. Further processing of the images was performed using Adobe® Photoshop® version 5.5.
The number of extruding epithelial cell events in the midguts of mosquitoes fed blood with or without parasites were initially compared using the Kruskal–Wallis test of the NPAR1WAY procedure of the statistical package SAS version 8.2 (SAS Institute Incorporated, Cary, NC, USA 1999–2001). Multiple pairwise comparisons were subsequently performed manually, using the Dunn test for samples of unequal size, to identify which individual groups differed significantly (Zar, 1984). Significance values were appropriately adjusted using the method of Dunn-šidák to control the experimental error rate (Sokal & Rohlf, 1995). The number of extruding epithelial cells and the number of invading parasites within each midgut were compared using correlation and simple linear regression in the ANALYST feature of the SAS software package.
Three distinct cell types could be distinguished in the posterior midgut wall of adult female An. stephensi mosquitoes fed either uninfected or infected blood. The predominant cell type was normal columnar epithelial cells (Fig. 1A). These cells were morphologically indistinguishable, staining with the same intensity, and possessing granular cytoplasm, a large centrally located nucleus containing a single prominent darkly staining nucleolus and dense tufts of microvilli. The microvilli typically extended vertically, in an ordered array, from the apical surface of epithelial cells towards the midgut lumen. Consequently, partings were apparent within the microvillar brush border beneath the lateral membranes of overlying epithelial cells (Figs 1B and 3H). There was no evidence of a subset of normal epithelial cells that were highly vacuolated, that lacked microvilli, or that stained differentially with Giemsa, with the exception of the extruding cells described below.

Fig. 1. (A) Normal columnar epithelial cell (EC) showing nucleus (N) containing a single nucleolus and microvilli (MV). Peritrophic matrix (PM) can be seen beneath, surrounding the bloodmeal (BM) and creating the ectoperitrophic space (EPS) between the latter and the midgut wall. 28 h p.b.f. (B) Section through the plane of the microvillar brush border looking down into the midgut lumen. The ectoperitrophic space can be seen between the microvilli (MV). Arrowheads indicate clear channels beneath the borders of adjacent overlying epithelial cells. Note honeycomb arrangement of overlying cells and convergence of three cells at corners of the latter. 28 h p.b.f. (C) Triangular dark cell (white arrow) in the basal region of the midgut wall surrounded by two adjacent epithelial cells (EC). Muscle fibre (MF) lies above, adjacent to the exterior surface of the midgut wall. 28 h p.b.f. (D) Pale triangular cell (white arrow) with apical extension opening into the midgut lumen, flanked by two epithelial cells. 28 h p.b.f. (E) Unusual epithelial cell (white arrow) possessing basal nucleus (N) and apical cavity (AC) surrounded on sides (1, 2) and underneath (3) by three normal epithelial cells. Arrowheads indicate apical surface of the unusual epithelial cell. 28 h p.b.f. (F) Ookinete (yellow arrow) within the peritrophic matrix (PM) which is not clearly distinct from the ectoperitrophic space (EPS). 24 h p.b.f. (G–I) Consecutive serial sections showing a single ookinete (yellow arrow) entering midgut wall where three adjacent epithelial cells (1, 2 and 3) converge. The ookinete appears to be entering cell 2 or 3 through the lateral apical plasma membrane. Arrowheads indicate boundaries between adjacent cells. Disruption of the peritrophic matrix and bloodmeal material within the ectoperitrophic space is also apparent beneath the ookinete (G). 24 h p.b.f.
The 2 other cell types were sparsely scattered throughout the midgut epithelium. Firstly, small flat triangular cells of variable size, which stained darkly and homogeneously, were found between adjacent epithelial cells in the basal region of the midgut epithelium (Fig. 1C). The triangular cells did not extend to the apical surface of the midgut epithelium but terminated within the basal half of the midgut wall. Occasionally, these cells were observed in adjacent pairs or, very rarely, triplets. Secondly, lightly staining triangular cells similar in size to the dark cells just described, but which possessed long narrow apical extensions that opened through the microvillar brush border into the midgut lumen, were also observed (Fig. 1D). The light cells were constant in size and were not observed in adjacent pairs. Both triangular cell types had relatively large nuclei that were basally located. A single dark triangular cell was observed that contained 2 conjoined nuclei.
A fourth distinct cell morphology was also observed at a low frequency – typically fewer than 20 cells per midgut, although the number varied appreciably between midguts (Fig. 1E). These cells resembled normal epithelial cells except for basally located nuclei and large apical cavities, lined by microvilli-like structures, which sometimes contained refractory material. The cells were basally located, immediately adjacent to dark triangular cells, and varied in size and degree of extension into the apical midgut wall region. The apical surface did not reach the midgut lumen but terminated within the midgut epithelium. The luminal microvilli present beneath the cells appeared to derive from adjacent epithelial cells. Although the cells were sometimes associated with extruding epithelial cells, there was no obvious correlation between this cell type and extruded cells.
All stages of parasite invasion of the midgut wall were observed in the midguts of mosquitoes given an infectious bloodmeal containing gametocytes (Fig. 4). Ookinetes were seen within the bolus of ingested blood, penetrating the peritrophic matrix (Fig. 1F), within the ectoperitrophic space, adjacent to the microvillar brush border, within the midgut epithelium and on the basal midgut wall surface. Parasites were most frequently observed within the peritrophic matrix and in the basal region of the midgut wall, and least frequently within the ectoperitrophic space.
Several ookinetes were observed before entry into the midgut wall within the microvillar brush border, aligned with the partings present beneath the lateral membranes of the overlying midgut cells. The midgut epithelium was morphologically normal in these regions, exhibiting no signs of invagination. No ookinetes were observed within the mounds of microvilli covering the central apical surface of epithelial cells.
The initial moment of ookinete penetration of the apical surface of the midgut epithelium was observed on 5 occasions. Ookinetes entered the midgut wall at sites where the lateral membranes of adjacent epithelial cells converged (Figs 1G–I and 2A). In 3 instances, ookinetes clearly entered where 3 midgut cells converged. Two other ookinetes were observed apparently having just entered epithelial cells, also at sites where 3 midgut cells intersected (Fig. 2C). In all but 1 of these 7 instances (Fig. 2B), the midgut epithelial cells appeared morphologically normal, possessing dense tufts of microvilli and centrally located nuclei similar to those of uninfected cells. However, the midgut epithelium exhibited significant local invagination at the site of ookinete invasion (Figs 1G–I and 2C). Similar invaginations were occasionally found beneath other parasites further in the process of invasion.

Fig. 2. (A–B) (A) Extracellular ookinete (yellow arrow) entering the midgut wall between adjacent epithelial cells (1, 2 and 3). Midgut cell 2 is only partly apparent. (B) Consecutive section showing fuller view of epithelial cell 2 which exhibits abnormal, significantly darker, staining. 28 h p.b.f. (C) Intracellular ookinete (yellow arrow) adjacent to the lateral corner of an epithelial cell. Three adjacent converging epithelial cells (1, 2, and 3) are visible. The middle epithelial cell (2), in which the ookinete resides, is barely apparent. 24 h p.b.f. (D) Intracellular ookinete (yellow arrow) surrounded by unstained halo in basal region of a marginally swollen epithelial cell (EC). 24 h p.b.f. (E) Intracellular ookinete (yellow arrow) within dark slightly protruding flaccid epithelial cell (EC). 28 h p.b.f. (F) Intracellular ookinete (yellow arrow) in the basal region of protruding pale epithelial cell (1). Note second, partially apparent, adjacent protruding epithelial cell (2). 28 h p.b.f. (G) Intracellular ookinete (yellow arrow) within pale epithelial cell (EC) protruding from the overlying midgut wall (MGW). 28 h p.b.f. (H) Ookinete (yellow arrow) emerging from the basal surface of an invaded epithelial cell (EC) extruding into the midgut lumen. 28 h p.b.f. (I) Epithelial cell (EC) extruding form the midgut wall. 28 h p.b.f. (J) Epithelial cell extruded from the midgut wall (MGW) possessing vacuoles (V) and several condensed bodies within the nucleus (N). 32 h p.b.f.
Ookinetes (n=54) were visible within midgut epithelial cells (Fig. 2C–G) but were never seen in any other cell type, including the unusual epithelial cells with microvilli-lined apical cavities. Most intracellular ookinetes appeared to be in direct contact with the host cell cytoplasm (Fig. 2C,E–G), although an unstained region was apparent around 6 parasites. Two of these were probably extracellular in the basolateral space between adjacent epithelial cells but the others were intracellular (Fig. 1D). Several intracellular ookinetes were observed lying parallel and adjacent to the lateral membrane of the invaded cell (Fig. 2C,F) whilst other parasites occupied a central location (Fig. 2D,G). Most intracellular ookinetes were observed in the basal region of the host cell (Fig. 2D,F,H).
With the exception of 5 parasites within morphologically normal epithelial cells, 91% of infected midgut cells exhibited a range of atypical morphological characteristics. All atypical infected cells exhibited some degree of extrusion from the midgut epithelium into the midgut lumen, ranging from minor swelling and bulging to complete separation from the midgut wall (Fig. 2D–H). Complete separation of epithelial cells from the midgut wall was demonstrated by following relevant cells through multiple consecutive serial sections. Other extruding cells appeared separated from the midgut wall in some sections but were connected via thin prominences in others (Fig. 2I). The density of microvilli on the luminal surface of extruded cells varied according to the degree of extrusion; cells exhibiting marked extrusion had few or no discernable microvilli. Some partially extruded cells containing parasites appeared morphologically normal in some sections but abnormal in other sections. In several instances, ookinetes were present within extruded cells nearly or completely separated from the midgut wall. Extracellular ookinetes, within the ectoperitrophic space, were rarely observed adjacent to extruded cells where no other invading parasites were apparent. Extruded cells were also observed that were not obviously associated with invading parasites.
Extruding epithelial cells exhibited 3 types of distinct morphology: ‘turgid’, ‘flaccid’ and ‘dissolving’. Turgid cells, the most common type of abnormal midgut cell, had apically situated nuclei (Fig. 2I,J) that contained a single, or sometimes several discrete, intensely staining condensed areas (Figs 2J and 3C), and had cytoplasm exhibiting light (Fig. 2F,G), normal (Fig. 2D,I) or dark staining (Fig. 3C,D). Individual turgid cells also varied in intensity, staining normally in some areas and lighter and/or darker in others (Fig. 2H). These cells depressed the underlying peritrophic matrix pushing it towards the bloodmeal (Fig. 2G) and were found completely separated from the midgut epithelium (Fig. 2J). Occasionally, turgid cells contained numerous, regularly-shaped vacuoles (Fig. 2J). Flaccid cells invariably stained darkly, were predominantly found within the midgut epithelium, and appeared to have collapsed inwardly (Fig. 2E). Dissolving cells did not have a defined cell boundary, were often difficult to discriminate, and appeared to be lysing into the ectoperitrophic space (Fig. 3A,B). Their nuclei appeared morphologically normal (Fig. 3B) and the cytoplasm uniquely stained pale blue (Fig. 3A,B). No depression of the peritrophic matrix was apparent beneath dissolving cells.

Fig. 3. (A) Invading ookinete (yellow arrow) emerging from a dissolving epithelial cell (LC) lying within the ectoperitrophic space and entering an overlying normal epithelial cell (EC). 28 h p.b.f. (B) Another dissolving epithelial cell (LC) pouring from the midgut wall into the ectoperitrophic space. The nucleus (white arrow) is apparent in the lysing cell. 28 h p.b.f. (C) Ookinete (yellow arrow) emerging from the basal surface of a dark abnormal epithelial cell (DC) and entering the intercellular space immediately in front of a normal epithelial cell (2). Note condensed nucleus (white arrow) of the invaded cell. 28 h p.b.f. (D) Intracellular ookinete (yellow arrow) moving between two adjacent dark extruding epithelial cells (1, 2). 28 h p.b.f. (E) Trail of eight dark epithelial cells (1 to 8) left behind an invading ookinete (not visible) surrounded by healthy epithelium. Note lower magnification. 24 h p.b.f. (F–G) Consecutive sections showing a single ìstalk-formî ookinete (yellow arrow) within a pale extruding epithelial cell (EC). Note narrow elongated portion of the parasite converging with the basal contracted surface of the extruding epithelial cell (white arrowhead). 28 h p.b.f. (H) Extracellular ookinete (yellow arrow) in basal region of midgut wall within and above the intercellular region (arrowheads) between adjacent morphologically normal epithelial cells. Note parting in microvillar brush border beneath site where the two epithelial cells meet. 28 h p.b.f. (I) Parasite (yellow arrow) on the basal surface of the midgut wall. Directly beneath a dark epithelial cell (white arrow) extruding into the midgut lumen can be partly seen emerging from between two overlying epithelial cells. 28 h p.b.f. (J) Early oocyst (yellow arrow) lying above a morphologically normal midgut epithelium. Note parasite resides immediately above the lateral membranes (arrowheads) of the underlying epithelial cells. 36 h p.b.f.
Adjacent epithelial cells exhibiting various degrees of altered morphology were frequently observed at sites of parasite penetration (Figs 2F and 3D,E). Thirty-four% (n=203) of parasites found in morphologically altered midgut wall regions were associated with more than one abnormal epithelial cell. Typically, clusters of 2 or 3 epithelial cells were found but greater numbers of extruded cells were occasionally seen. In one instance, 8 adjacent altered cells were observed (Fig. 3E). Epithelial cells were extruded as a single mass or sequentially forming a trail of partially and completely ejected cells. Ookinetes were also observed spanning the membranes of adjacent morphologically abnormal epithelial cells (Fig. 3D) and emerging from the basal region of invaded cells (n=31) (Figs 2H and 3A,C). It was not always clear which host cell membranes (lateral and/or basal) parasites were crossing (Fig. 3D) or whether ookinetes were exiting epithelial cells into adjacent midgut cells or the intercellular space between them (Fig. 3C). No ookinetes were observed crossing the lateral membranes of adjacent cells. In several instances, ookinetes were observed emerging from the basal surface of extruded cells and entering the apicolateral membrane of overlying midgut cells (Fig. 3A).
Intracellular ookinetes appeared intact and morphologically normal, possessing malaria pigment and a nucleus (Fig. 2C,E–H). There was no evidence of parasite lysis or other forms of degenerative change, with the possible exception of some indistinct intracellular forms observed within extruded epithelial cells. However, 3 unusual ‘stalk-form’ ookinetes were observed with extremely narrow elongated regions (Fig. 3F,G). In 2 instances, these parasites were emerging from the basal surface of extruding cells; the constricted basal cell surface converging with the narrow portion of the parasites. In the third instance, an extruding cell was present beneath an intercellular parasite, located in the central region of the midgut wall, but the origin of the elongated parasite region was unclear.

Fig. 4. Temporal pattern of parasite migration across the mosquito midgut wall. ([squf ]) Ookinetes found within the peritrophic matrix, ectoperitrophic space or microvillar brush border. () Ookinetes found within the midgut epithelium. () Parasites found on the basal surface of the midgut wall. Data from both infectious (gametocyte) feeds were pooled.
Ookinetes were also observed in intercellular locations within the central and basal regions of the midgut epithelium (Fig. 3H). These parasites were situated between morphologically normal epithelial cells. However, 86% (n=35) of intercellular parasites were associated with epithelial cells exhibiting altered morphology. The altered cells, displaying marked extrusion from the midgut wall, were either in the immediate vicinity or, occasionally, some distance from the parasites. Frequently, intercellular parasites and extruding cells were not present within the same section.
Parasites reached the basal surface of the midgut wall and assumed the rounded oocyst form as early as 24 h p.b.f. No parasites on the basal midgut wall surface, entirely outside the midgut epithelium, possessed the elongated ookinete form. Frequently, basal parasites were located above the lateral membranes of underlying epithelial cells (Fig. 3H–J). Epithelial cells were observed extruding from beneath the lateral cell membranes over which basal parasites were positioned (Fig. 3I). Sometimes nothing abnormal was observed in the section containing the parasite but protruding and/or ejected epithelial cells could be found in adjacent sections. Epithelial cells surrounding basal parasites appeared morphologically normal and, at later time-points, there was no evidence of any disruption to the midgut epithelium (Fig. 3J).
Epithelial cells exhibiting various degrees of morphological alteration were observed only rarely in midguts from mosquitoes fed uninfected blood or blood containing non-infective asexual stage parasites (Table 1). The morphology of these cells was similar to ookinete-invaded cells, although clusters of multiple altered cells were not observed. The number of morphologically abnormal cells was not significantly different between mosquitoes given uninfected blood and asexual stage parasites alone. However, the number of these cells was significantly higher in mosquitoes given an infectious bloodmeal containing gametocytes (Table 1). (As asexual parasites are present in gametocyte cultures, mosquitoes were fed asexual parasites alone to demonstrate that extruding epithelial cells were not a consequence of the presence of these parasites within the infectious bloodmeal.) The number of extruding cells within each midgut also showed a highly significant positive correlation with the number of invading parasites (r2=0·81, P<0·001) (Fig. 5).

Fig. 5. Relationship between the number of parasites invading the midgut wall and the occurrence of morphologically abnormal epithelial cells within each midgut. ([bull ]) Mosquitoes fed either uninfected blood or non-infectious asexual stage parasites. (○) Mosquitoes fed infectious sexual gametocyte stage parasites. Data from all feeds were pooled and only mosquitoes examined between 24 and 36 h p.b.f. were included in the analysis.

Overall, 92% (n=123) of ookinetes observed within the midgut epithelium were associated with epithelial cells exhibiting altered morphology. The remaining ookinetes were either intracellular (4%) within, or intercellular (4%) between, morphologically normal epithelial cells. Only 47% (n=192) of parasites observed on the basal midgut wall surface were associated with morphologically altered midgut epithelium. Forty% (n=508) of midgut cells displaying altered morphology were associated with invading parasites. If parasite invasion is assumed always to be intracellular and to cause epithelial damage, this would account for 60% of observed abnormal cells. Extrapolating from uninfected mosquitoes, 13% of altered cells in infected mosquitoes are background, parasite-independent events, leaving 27% of the abnormal cells unexplained.
Our observations demonstrate intracellular migration of P. falciparum ookinetes across the An. stephensi midgut epithelium and provide further support for the Time Bomb model of ookinete invasion previously proposed for rodent malaria parasites (Han et al. 2000).
Ookinetes, including those of P. falciparum, have previously been observed in fixed material entering the midgut wall where the lateral membranes of adjacent epithelial cells meet (Stohler, 1957; Meis & Ponnudurai, 1987a; Syafruddin et al. 1991). Although we observed ookinete entry where lateral cell membranes converged, we also found intracellular parasites, which questions the previous assumption that ookinete entry where adjacent epithelial cells meet implies intercellular migration (Meis & Ponnudurai, 1987a; Syafruddin et al. 1991). Such ookinetes could be entering into epithelial cells through the lateral apical membrane as reported for P. gallinaceum (Zieler & Dvorak, 2000). If so, the site of ookinete entry appears to be conserved across diverse parasite–vector combinations.
We observed ookinetes entering the midgut wall where the lateral membranes of three adjacent epithelial cells converged. Reinterpretation of previous observations also suggests that ookinetes invade the midgut wall at such sites (Meis & Ponnudurai, 1987a; Syafruddin et al. 1991; Zieler & Dvorak, 2000). As the microvillar brush border is parted where adjacent midgut epithelial cells converge, ookinetes might gain unimpeded access to host cell membranes at these sites. Alternatively, the mechanical and structural properties of the midgut wall in these regions might be of importance for invading parasites.
P. falciparum ookinetes in An. stephensi were previously only found in extracellular positions between morphologically normal epithelial cells suggesting intercellular migration (Meis & Ponnudurai, 1987a; Meis et al. 1989). Ookinetes, sought between 29 and 36 h pbf, were exclusively observed in basal midgut wall regions (Meis & Ponnudurai, 1987a; Meis et al. 1989). Han et al. (2000) suggested that parasite invasion could initially be intracellular at 24 h p.b.f. but subsequently become intercellular over the following hours. Our observations of both intra- and intercellular ookinetes support this interpretation. Furthermore, we found that morphologically abnormal epithelial cells were associated with most intercellular ookinetes providing evidence that midgut wall entry was initially intracellular. However, the previous failure to find intracellular ookinetes is unlikely to be due to the time invading parasites were sought (Han et al. 2000), as others observed ookinete midgut wall entry between 28 and 36 h p.b.f. (Meis & Ponnudurai, 1987a) and we observed intracellular ookinetes during this time. Our data demonstrate the asynchrony of ookinete invasion, as previously noted (Omar, 1968). Invasion takes place over a period of at least 12 h; some parasites reach the basal midgut wall surface within 24 hours p.b.f. whilst others only begin invasion after 32 h p.b.f. Asynchrony is presumably related to differences in the rate of ookinete development and parasite location within the midgut lumen, with parasites on the bloodmeal periphery reaching the midgut wall before those in its centre (Omar, 1968).
As previously reported (Omar, 1968; Meis et al. 1989; Syafruddin et al. 1991), we also found basal parasites above the lateral membranes of underlying epithelial cells consistent with intercellular parasite migration. However, basal parasites were often associated with abnormal epithelial cells although approximately half were not. Increasing physical distance between basal parasites and invaded cells as the latter are extruded makes it difficult to discern spatial associations in sectioned material. Some basal parasites are distantly located from abnormal epithelial cells suggesting movement away from the initial invasion site (Han et al. 2000). Such movement was apparent in invasion events we observed and could contribute to the relatively weak association between basal parasites and extruded cells.
Indacochea (1935) reported P. falciparum within the midgut cells of Anopheles maculipennis, noting a “colourless hyaline capsule” around intracellular ookinetes. We observed an unstained region, possibly analogous to Indacochea's capsule, surrounding several intracellular parasites. It is not clear if this is an artefact of fixation or a genuine phenomenon. A parasitophorous vacuole is not believed to surround ookinetes of other malaria species during migration through midgut cells (Garnham et al. 1962; Mehlhorn & Peters, 1980; Meis et al. 1989; Torii et al. 1992) and does not appear to surround most ookinetes we observed. However, vacuoles of unknown origin are found around some invading parasites (Syafruddin et al. 1991).
We found no evidence that P. falciparum ookinetes preferentially invade a subset of epithelial cells in the An. stephensi midgut wall. Ookinetes appeared to invade morphologically normal epithelial cells possessing abundant microvilli, as previously shown (Paskewitz et al. 1988; Torii et al. 1992; Vernick, Fujioka & Aikawa, 1999; Han et al. 2000; Zieler & Dvorak, 2000). Although there was no morphological evidence that midgut cells invaded by ookinetes differ from other epithelial cells, it remains possible invaded cells possess distinct biochemical and/or molecular characteristics recognized by ookinetes (Zieler & Dvorak, 2000).
Parasite invasion induced morphological changes in invaded epithelial cells suggestive of significant pathology. We observed loss of microvilli from the cell surface, translocation of the nucleus from a central to an apical position and partial extrusion of invaded cells from the midgut wall as has been previously reported (Han et al. 2000; Zieler & Dvorak, 2000). Furthermore, some epithelial cells were completely extruded into the midgut lumen formally demonstrating what had only previously been inferred (Han et al. 2000; Shahabuddin, 2002). Our observations suggest that P. falciparum ookinetes move intracellularly through multiple adjacent epithelial cells leaving a trail of cellular destruction, as reported for other malaria species (Vernick et al. 1999; Han et al. 2000; Zieler & Dvorak, 2000). The narrow elongated ookinetes associated with the basal surface of extruding epithelial cells also demonstrates the occurrence of the ‘stalk-form’ morphology in P. falciparum (Vernick et al. 1999; Han et al. 2000).
The three distinct types of abnormal cell morphology observed may represent distinct modes of cell death, different stages of a common process, or simply reflect differences in the initial physiological state of invaded cells. Whether parasite-invaded epithelial cells undergo necrotic or programmed cell death following parasite penetration is unclear (Zieler & Dvorak, 2000; Han & Barillas-Mury, 2002; Shahabuddin, 2002). Some invaded cells explode upon penetration, releasing their cytoplasmic contents (Zieler and Dvorak, 2000). Such cells might be equivalent to flaccid and/or dissolving epithelial cells. Invaded cells also undergo molecular and morphological changes indicative of apoptosis (Han et al. 2000; Zieler & Dvorak, 2000). Similar changes were observed in the nuclei of turgid extruded cells.
The correlation we observed between invading parasites and abnormal epithelial cells strongly suggests that the former events cause the latter. However, an appreciable proportion of extruded cells observed in mosquitoes given an infectious bloodmeal did not have an obvious causal explanation. Technical limitations inherent with non-specific staining of sectioned material could account for these cells. Firstly, extruding cells not associated with intact parasites have been found in infected mosquito midguts, although a parasite-specific molecule was detected in these cells suggesting intracellular lysis of invading ookinetes (Han et al. 2000). However, we found no clear evidence of parasite destruction. Secondly, the number of invading parasites is likely to be underestimated relative to the number of extruding cells; the smaller size and similar coloration of parasites compared to midgut cells makes the former harder to detect. Furthermore, some sections are inevitably lost or damaged during midgut sample processing, and as parasites typically occur in only a few sections whilst epithelial cells can persist through many, counts of parasites are likely to be disproportionately affected.
Although extruded epithelial cells were not previously observed in uninfected An. stephensi (Han et al. 2000), such cells have been reported in uninfected and virus-infected midguts of other mosquito species (Houk et al. 1985; Weaver et al. 1988; Weaver & Scott, 1990a; Weaver, Lorenz & Scott, 1992; Okuda et al. 2002). Epithelial cell extrusion may be a general mechanism of tissue repair, rather than a parasite-specific response, utilized in the mosquito midgut in response to cell damage induced by senescence, stress or infection (Okuda et al. 2002). Consequently, morphological and/or molecular changes occurring in midgut cells after ookinete invasion are not necessarily a specific response to malaria parasites.
The Ross Cell hypothesis has placed renewed emphasis on characterizing cells comprising the midgut wall and the possible involvement of these cells in ookinete invasion. Previous studies of intact midguts from anopheline and aedine mosquitoes concluded epithelial cells were morphologically indistinguishable, but the existence of distinct epithelial cell types was not precluded (Bertram & Bird, 1961; Freyvogel & Stäubli, 1965; Hecker, 1977). Although we observed several distinct midgut cell types in An. stephensi, we found no normal epithelial cells possessing the morphological characteristics ascribed to Ross cells (Shahabuddin & Pimenta, 1998).
The dark triangular cells observed in the basal midgut wall region are probably regenerative cells (Hecker, 1977). These cells probably correspond to the small flat triangular cells overexpressing vesicular ATPase described in An. stephensi (Han et al. 2000). Although we occasionally observed parasites adjacent to these cells, their basal location makes them improbable initial invasion sites for ookinetes (Shahabuddin & Pimenta, 1998; Han et al. 2000).
The pale triangular cells with narrow apical extensions have not been reported in anopheline mosquitoes. These cells resemble the “open” endocrine cells described in Ae. aegypti (Brown et al. 1985, 1986). Endocrine cells have been reported in anopheline midguts (Hecker, 1977; Glättli, Rudin & Hecker, 1987) but the overall morphology of these cells was not described and, compared to aedine mosquitoes, they are poorly characterized. Although these cells are potentially accessible to ookinetes as initial invasion sites, no penetrating parasites were associated with them, as reported for P. gallinaceum (Shahabuddin & Pimenta, 1998).
The epithelial cells containing microvilli-lined apical cavities are similar to midgut cells of unknown significance previously described in several culicine mosquito species (Houk, 1977; Weaver & Scott, 1990b). They may represent a distinct cell type with specialized functions or normal cells undergoing some particular transformational event. We propose that these cells represent nascent normal epithelial cells differentiating from neighbouring dark triangular cells. Our observations suggest a model where basal regenerative cells divide and subsequently grow in an apical direction, pushing between adjacent midgut cells. An anterior microvilli-lined cavity develops that could open and evert into the midgut lumen to become the apical brush border surface of newly formed epithelial cells. If these unusual midgut cells are newly differentiating epithelial cells, the midgut wall presumably possesses the capacity to replace lost cells. This could explain the ability of mosquitoes to tolerate high levels of parasite-induced pathology and the absence of increased mortality in heavily infected mosquitoes (unpublished observations; Meis & Ponnudurai, 1987b; Han et al. 2000).
What is the relevance of our findings to the debate regarding P. gallinaceum ookinete invasion and the existence of Ross cells in Ae. aegypti? Our observations concur with those in P. gallinaceum–Ae. aegypti which support the Time Bomb model (Torii et al. 1992; Vernick et al. 1999; Zieler & Dvorak, 2000) and extruding cells in An. stephensi do possess characteristics reminiscent of Ross cells including differential staining, vacuolisation and few or no microvilli (Shahabuddin & Pimenta, 1998). Rather than being a specific cell type, Ross cells might be normal but damaged epithelial cells exhibiting morphological changes as a result of parasite invasion or in vitro manipulation of the midgut (Han et al. 2000; Zieler & Dvorak, 2000). Alternatively, ookinete invasion may proceed differently in P. gallinaceum–Ae. aegypti (Han & Barillas-Mury, 2002; Shahabuddin, 2002).
If the Time Bomb model is generally applicable, why have few investigators fully described the same phenomena (Shahabuddin, 2002)? The small number of observations made in previous studies has probably contributed to contradictory reports of ookinete invasion (Torii et al. 1992). In addition, most previous studies have used conventionally stained sectioned material, in which it can be difficult to observe spatial associations between invading parasites and epithelial cells exhibiting altered morphology. Many previous observations are, however, consistent with the Time Bomb model (Omar, 1968; Maier, 1973; Meis & Ponnudurai, 1987a; Paskewitz et al. 1988; Meis et al. 1989; Syafruddin et al. 1991; Torii et al. 1992; Shahabuddin & Pimenta, 1998; Vernick et al. 1999). For example, Maier and colleagues reported extrusion of parasite-invaded midgut cells, although they did not publish detailed supporting evidence (Becker-Feldman et al. 1985; Maier et al. 1987). Our work on P. falciparum in An. stephensi adds to the weight of evidence in support of the Time Bomb model.
We would like to thank Fiona McMonagle, Elizabeth Peat and Keith Scott for assistance with maintaining the mosquito colony and preparation of media required for parasite culture, Dr Laurence Tetley and Eoin Robertson for advice and assistance with preparation of the sectioned material, and Professor Tim Mitchell and Dr Gill Douce for use of their imaging equipment. We would also like to thank Dr Peterkin and staff at the Glasgow and West of Scotland Blood Transfusion service, Scottish National Blood Transfusion Service, for the provision of human blood and serum. This research was funded by a grant from the Royal Society (Ref. No. 22065) and L.A.B. is supported by a scholarship from the University of Glasgow.

Fig. 1. (A) Normal columnar epithelial cell (EC) showing nucleus (N) containing a single nucleolus and microvilli (MV). Peritrophic matrix (PM) can be seen beneath, surrounding the bloodmeal (BM) and creating the ectoperitrophic space (EPS) between the latter and the midgut wall. 28 h p.b.f. (B) Section through the plane of the microvillar brush border looking down into the midgut lumen. The ectoperitrophic space can be seen between the microvilli (MV). Arrowheads indicate clear channels beneath the borders of adjacent overlying epithelial cells. Note honeycomb arrangement of overlying cells and convergence of three cells at corners of the latter. 28 h p.b.f. (C) Triangular dark cell (white arrow) in the basal region of the midgut wall surrounded by two adjacent epithelial cells (EC). Muscle fibre (MF) lies above, adjacent to the exterior surface of the midgut wall. 28 h p.b.f. (D) Pale triangular cell (white arrow) with apical extension opening into the midgut lumen, flanked by two epithelial cells. 28 h p.b.f. (E) Unusual epithelial cell (white arrow) possessing basal nucleus (N) and apical cavity (AC) surrounded on sides (1, 2) and underneath (3) by three normal epithelial cells. Arrowheads indicate apical surface of the unusual epithelial cell. 28 h p.b.f. (F) Ookinete (yellow arrow) within the peritrophic matrix (PM) which is not clearly distinct from the ectoperitrophic space (EPS). 24 h p.b.f. (G–I) Consecutive serial sections showing a single ookinete (yellow arrow) entering midgut wall where three adjacent epithelial cells (1, 2 and 3) converge. The ookinete appears to be entering cell 2 or 3 through the lateral apical plasma membrane. Arrowheads indicate boundaries between adjacent cells. Disruption of the peritrophic matrix and bloodmeal material within the ectoperitrophic space is also apparent beneath the ookinete (G). 24 h p.b.f.

Fig. 2. (A–B) (A) Extracellular ookinete (yellow arrow) entering the midgut wall between adjacent epithelial cells (1, 2 and 3). Midgut cell 2 is only partly apparent. (B) Consecutive section showing fuller view of epithelial cell 2 which exhibits abnormal, significantly darker, staining. 28 h p.b.f. (C) Intracellular ookinete (yellow arrow) adjacent to the lateral corner of an epithelial cell. Three adjacent converging epithelial cells (1, 2, and 3) are visible. The middle epithelial cell (2), in which the ookinete resides, is barely apparent. 24 h p.b.f. (D) Intracellular ookinete (yellow arrow) surrounded by unstained halo in basal region of a marginally swollen epithelial cell (EC). 24 h p.b.f. (E) Intracellular ookinete (yellow arrow) within dark slightly protruding flaccid epithelial cell (EC). 28 h p.b.f. (F) Intracellular ookinete (yellow arrow) in the basal region of protruding pale epithelial cell (1). Note second, partially apparent, adjacent protruding epithelial cell (2). 28 h p.b.f. (G) Intracellular ookinete (yellow arrow) within pale epithelial cell (EC) protruding from the overlying midgut wall (MGW). 28 h p.b.f. (H) Ookinete (yellow arrow) emerging from the basal surface of an invaded epithelial cell (EC) extruding into the midgut lumen. 28 h p.b.f. (I) Epithelial cell (EC) extruding form the midgut wall. 28 h p.b.f. (J) Epithelial cell extruded from the midgut wall (MGW) possessing vacuoles (V) and several condensed bodies within the nucleus (N). 32 h p.b.f.

Fig. 3. (A) Invading ookinete (yellow arrow) emerging from a dissolving epithelial cell (LC) lying within the ectoperitrophic space and entering an overlying normal epithelial cell (EC). 28 h p.b.f. (B) Another dissolving epithelial cell (LC) pouring from the midgut wall into the ectoperitrophic space. The nucleus (white arrow) is apparent in the lysing cell. 28 h p.b.f. (C) Ookinete (yellow arrow) emerging from the basal surface of a dark abnormal epithelial cell (DC) and entering the intercellular space immediately in front of a normal epithelial cell (2). Note condensed nucleus (white arrow) of the invaded cell. 28 h p.b.f. (D) Intracellular ookinete (yellow arrow) moving between two adjacent dark extruding epithelial cells (1, 2). 28 h p.b.f. (E) Trail of eight dark epithelial cells (1 to 8) left behind an invading ookinete (not visible) surrounded by healthy epithelium. Note lower magnification. 24 h p.b.f. (F–G) Consecutive sections showing a single ìstalk-formî ookinete (yellow arrow) within a pale extruding epithelial cell (EC). Note narrow elongated portion of the parasite converging with the basal contracted surface of the extruding epithelial cell (white arrowhead). 28 h p.b.f. (H) Extracellular ookinete (yellow arrow) in basal region of midgut wall within and above the intercellular region (arrowheads) between adjacent morphologically normal epithelial cells. Note parting in microvillar brush border beneath site where the two epithelial cells meet. 28 h p.b.f. (I) Parasite (yellow arrow) on the basal surface of the midgut wall. Directly beneath a dark epithelial cell (white arrow) extruding into the midgut lumen can be partly seen emerging from between two overlying epithelial cells. 28 h p.b.f. (J) Early oocyst (yellow arrow) lying above a morphologically normal midgut epithelium. Note parasite resides immediately above the lateral membranes (arrowheads) of the underlying epithelial cells. 36 h p.b.f.
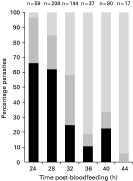
Fig. 4. Temporal pattern of parasite migration across the mosquito midgut wall. ([squf ]) Ookinetes found within the peritrophic matrix, ectoperitrophic space or microvillar brush border. () Ookinetes found within the midgut epithelium. () Parasites found on the basal surface of the midgut wall. Data from both infectious (gametocyte) feeds were pooled.

Fig. 5. Relationship between the number of parasites invading the midgut wall and the occurrence of morphologically abnormal epithelial cells within each midgut. ([bull ]) Mosquitoes fed either uninfected blood or non-infectious asexual stage parasites. (○) Mosquitoes fed infectious sexual gametocyte stage parasites. Data from all feeds were pooled and only mosquitoes examined between 24 and 36 h p.b.f. were included in the analysis.

Table 1. Summary of sectioned mosquito midguts examined and comparison of the number of morphologically abnormal epithelial cells observed in mosquitoes given bloodmeals with or without parasites