Introduction
Parasitic relationships vary in the degree of reliance on hosts for survival and reproduction (Renaud et al., Reference Renaud, Clayton and De Meeos1996; Schmid-Hempel, Reference Schmid-Hempel2013). Obligate parasites must infect a host to survive and reproduce, whereas facultative parasites can complete their life cycle with or without a host (Stasiuk et al., Reference Stasiuk, Scott and Grant2012). For example, the nematode Parastrongyloides trichosuri can express either a free-living or parasitic strategy depending on the environment (Stankiewicz, Reference Stankiewicz1996). Some have hypothesized that intermediate strategies, such as facultative parasitism and phoresy, may serve as important evolutionary stepping-stones in the evolution towards obligate parasitism from free-living ancestors (Rothschild and Clay, Reference Rothschild and Clay1953; Poulin, Reference Poulin2007; Schmid-Hempel, Reference Schmid-Hempel2013; Dowling, Reference Dowling, Morand, Krasnov and Littlewood2015). According to this stepping-stone hypothesis, there must be fitness benefits associated with parasitic activity (Darwin and Wallace, Reference Darwin and Wallace1858; Poulin, Reference Poulin2007). If parasitizing a host provides a fitness benefit, what prevents natural selection from driving parasitic strategies to fixation, (i.e. obligate parasitism) in facultative parasites?
One possible explanation is that the infection strategies of facultative parasites are phenotypically plastic. Thomas et al. (Reference Thomas, Brown, Sukhdeo and Renaud2002) argue that most parasites face stochastic environments and are capable of adaptively responding with various infection and life-history strategies. The trematode, Coitocaecum parvum, can utilize either a 2- or 3-host lifecycle depending on host availability (Poulin, Reference Poulin2001). Evolutionary constraints may also prevent the fixation of obligate infection strategies. In particular, evolutionary trade-offs arising from linkages between multiple traits may prevent the simultaneous evolution of all traits (Stearns, Reference Stearns1992; Garland, Reference Garland2014). Crossan et al. (Reference Crossan, Paterson and Fenton2007) found that Steinernema feltiae (an obligate parasite) selected for fast infection expressed reduced female fecundity, decreased lipid content, as well as increased pre-infection mortality compared to the nematodes that were selected for delayed infection (Crossan et al., Reference Crossan, Paterson and Fenton2007). Similarly, Paterson and Barber (Reference Paterson and Barber2007) tested for trade-offs in a facultative parasite (Strongyloides ratti) artificially selected for two different infection strategies. The authors reported a trade-off between infection strategy and fecundity, but it was context-dependent: ‘fast-infecting’ nematodes showed higher fecundity compared to the ‘slow-infecting’ nematodes when population densities were low. The relationship was reversed when parasite-population density was high (Paterson and Barber, Reference Paterson and Barber2007).
Here, we investigate the roles of evolutionary trade-offs and phenotypic plasticity in maintaining attachment variation in a facultative parasite. Macrocheles muscaedomesticae (Scopoli, 1772) mites typically inhabit decomposing environments, such as compost and dung, where they feed upon small invertebrates, mate and lay eggs (Wade, Reference Wade1961; Jalil and Rodriguez, Reference Jalil and Rodriguez1970; Krantz, Reference Krantz1998). As a means of dispersal, adult female mites attach to fly hosts. During mite attachment, flies suffer physical costs, but they experience physiological costs even after mite detachment (Luong et al., Reference Luong, Penoni, Horn and Polak2015). Furthermore, there is evidence that suggests these mites feed on fly hosts while attached, but it remains equivocal. Jalil and Rodriguez (Reference Jalil and Rodriguez1970) recorded weight loss in Musca domestica fly hosts after mite exposure and recent unpublished data from our lab show mites previously attached to a Drosophila hydei fly weighed significantly more than mites that did not attach to a fly. Finally, Abo-Taka et al. (Reference Abo-Taka, Heikal and Abd El-Raheem2014) provide evidence for M. muscaedomesticae predating on adult M. domestica, killing them after multiple days of exposure. The propensity to attach to a fly host varies widely among M. muscaedomesticae. Upon host exposure in the lab, some females attempt attachment within seconds, while other postpone or pass on the opportunity altogether (pers. observation). Their attachment behaviour is plastic and influenced by their own internal state (Jalil and Rodriguez, Reference Jalil and Rodriguez1970; Luong et al., Reference Luong, Brophy, Stolz and Chan2017), the state of their potential hosts (Farish and Axtell, Reference Farish and Axtell1971; Campbell and Luong, Reference Campbell and Luong2016; Luong et al., Reference Luong, Brophy, Stolz and Chan2017) as well as the external environment (Farish and Axtell, Reference Farish and Axtell1971; Durkin and Luong, Reference Durkin and Luong2018).
Although plasticity contributes to the observed variation in M. muscaedomesticae's propensity to attach; it does not preclude the importance of genotypic variation. Furthermore, phenotypically plastic traits can evolve to become more or less plastic over time (Crispo, Reference Crispo2007; Gilbert and Epel, Reference Gilbert and Epel2015). Genetic assimilation describes events in which the range of expression in an originally phenotypically plastic trait is reduced or eliminated so that it no longer responds to environmental stimuli (Waddington, Reference Waddington1942; Pigliucci, Reference Pigliucci2006; Crispo, Reference Crispo2007; Gilbert and Epel, Reference Gilbert and Epel2015). Suzuki and Nijhout (Reference Suzuki and Nijhout2006) observed evidence for genetic assimilation in their selection experiments with hornworms (Manduca sexta). Black hornworm morphs typically occur in response to heat shock, but after seven generations of selection, the black larval coloration became fixed: the larvae expressed black coloration without being exposed to heat-shock (Suzuki and Nijhout, Reference Suzuki and Nijhout2006). The Baldwin effect describes a phenomenon whereby plastic organisms are better able to survive novel environments and thus, natural selection favors phenotypic plasticity; the result is an increase in or maintenance of phenotypic plasticity over time (Baldwin, Reference Baldwin1896; Crispo, Reference Crispo2007). As an example, Nussey et al. (Reference Nussey, Postma, Gienapp and Visser2005) observed a positive relationship between the plasticity in egg-laying time and fitness in a wild population of Great-Tits (Parus major). They hypothesized that the plastic females were better able to synchronize their egg-laying time with prey availability, which increased their overall fitness and thus plasticity in egg-laying was under positive selection (Nussey et al., Reference Nussey, Postma, Gienapp and Visser2005).
In a previous study, we experimentally evolved M. muscaedomesticae populations to exhibit an increased propensity to attach to fly hosts using artificial selection (Durkin and Luong, Reference Durkin and Luong2018). In this study, we determined whether evolutionary trade-offs, phenotypic plasticity or a combination of both might play a role in maintaining attachment variation in M. muscaedomesticae. We compared the fecundity, longevity, attachment plasticity and morphology of M. muscaedomesticae mites selected for increased propensity for host attachment relative to unselected control mites. Because evolutionary trade-offs are often context-dependent, we measured the fecundity and longevity of the mites in two different scenarios, with and without access to a fly host. In the presence of a host, selected mites can reap the benefits of increased attachment behaviour. However, in the absence of hosts, the benefits of high attachment propensity cannot be realized, and the cost (if any) of maintaining this trait should manifest. Compared to control mites (regardless of host availability), we expected selected mites to exhibit higher fecundity and longevity following fly attachment. Conversely, when selected mites were denied the opportunity to infect a host, we expected lower fecundity and longevity compared to control mites overall.
We also hypothesized that direct selection for a single phenotype (host attachment) may result in a loss in attachment plasticity (i.e. via genetic assimilation) and predicted that selected populations would exhibit high attachment prevalence regardless of environmental conditions. We predicted the attachment prevalences of control populations to respond to environmental conditions. If attachment plasticity is adaptive for organisms living in ephemeral habitats, a single infection strategy and concomitant loss of plasticity could be detrimental to their fitness, which would constrain the evolution of increased attachment behaviour in M. muscaedomesticae.
Selection for increased propensity for host attachment may also result in correlated changes in other traits such as body and chelicera size, as well as chelicera strength. Poulin and Morand (Reference Poulin and Morand1997) reported that among ticks, the distribution of scutum size was log right-skewed, suggesting a trend towards smaller body sizes in these obligately parasitic members of Acari. They hypothesized that this pattern was a result of host grooming acting as a selective pressure to drive down body size. Given that fly hosts respond to the presence of ectoparasitic mites by grooming (Luong and Polak, Reference Luong and Polak2007), we predict a negative correlation between increased propensity for attachment and mite body size. Lastly, because M. muscaedomesticae use their chelicerae for host attachment (Farish and Axtell, Reference Farish and Axtell1971; Dowling, Reference Dowling, Morand, Krasnov and Littlewood2015), we expected that selection for increased attachment will lead to positive selection on chelicerae size and strength.
Methods
Fly and mite culture
The M. muscaedomesticae and D. hydei cultures were established from mites and flies collected from compost in Edmonton, Alberta, Canada in the fall of 2013. The cultures were maintained at 24 °C, 70% relative humidity and a 12D: 12L photoperiod. Please see Durkin and Luong (Reference Durkin and Luong2018) for detailed fly and mite culture methods.
Artificial selection
Artificial selection was conducted on M. muscaedomesticae in a previous experiment (Durkin and Luong, Reference Durkin and Luong2018); here we report on the correlated changes in life history and morphometric traits as well as attachment plasticity. The selection protocol was constrained by logistical challenges associated with the selection regime, limiting the number of concurrent replicate lines we could maintain. Hence, we repeated the selection experiment on the same source population of mites yielding a total of four replicate ‘increased propensity for attachment’ and four control lines. The first experiment was performed in the fall of 2015 (experiment A) and the second in the spring of 2016 (experiment B). Both experiments consisted of two selection regimes or treatments: ‘increased propensity for attachment’ and control (see Durkin and Luong, Reference Durkin and Luong2018 for detailed selection methods). We generated two replicate lines per selection regime in each. We refer to the replicate lines in experiment A as A1 and A2 and those in experiment B as B1 and B2. In both experiments A and B, the ‘increased propensity for attachment’ lines responded positively to selection; the mean realized heritability for attachment behaviour was estimated to be 0.166 ± 0.058 s.e. (Durkin and Luong, Reference Durkin and Luong2018). We refer to the ‘increased propensity for attachment’ line as the ‘selected’ line from here on. We measured the fecundity and longevity of the selected and control mites generated in experiment A. Attachment plasticity and morphological measurements were performed on the mites generated from experiment B. Life history measurements were not repeated on the mites generated in experiment B because the assays were highly time consuming, limiting the number of concurrent experiments we were able to conduct. The restricted number of experimental replicates limited our ability to distinguish between the effects of genetic drift and selection.
Experiment A: fecundity and longevity
We measured the lifetime fecundity and longevity of selected and control mites from experiment A following 15 and 17 generations of selection. To determine if the nature of the trade-off was context-dependent (i.e. access to a suitable host), half of the experimental mites from each of the control and selection treatments were allowed to attach to a fly, while the other half had access to nematodes as the only food source. Detailed methods for the measurements of mite longevity and lifetime fecundity can be found in the Supplemental material.
Experiment B: phenotypic plasticity
To determine whether selected lines experienced genetic assimilation we measured the plasticity of host attachment in selected and control lines after 10 and 12 generations of selection. The prevalence of fly attachment in selected and control lines was recorded over three environments, each with varying levels of food (no food, low food and high food). Populations of a closely related mite species, Macrocheles subbadius, exhibit increased attachment prevalence when starved (Luong et al., Reference Luong, Brophy, Stolz and Chan2017). We assumed food availability to be a key component of habitat quality and that habitat quality diminishes with lower food availability.
Fifty females from control and selected lines were randomly selected from the final generations of selection in experiment B. Ten females (without exposure to a fly host) were placed in five replicate containers filled with 50 mL of organic medium. The mites laid eggs in the media for 3 days before being removed. The F1 females from these mites were then used in the plasticity experiment.
The ‘no food’ treatment group was maintained on 14.8 cm3 of aspen wood chips moistened with distilled water. A batch of ‘low food’ media was made by diluting 29.6 cm3 of organic nematode media containing nematodes with 118.3 cm3 of moistened aspen wood chips. The ‘high food’ treatment consisted of 14.8 cm3 of undiluted organic media containing nematodes. The final volume of food media in all replicates and treatments was 14.8 cm3 per container. Nematode density of the low and high food treatments was estimated by extracting nematodes from 14.8 cm3 of media using a Baermann funnel. The extracted nematodes were then counted under a stereomicroscope. Replicate line 1 had 1.7 ± 0.27 s.d. nematodes cm−3 in the low food treatments and 12.4 ± 0.66 s.d. nematodes cm−3 in the high food treatments. Replicate line two had 0.5 ± 0.15 s.d. nematodes/1 cm3 in the low food treatments and 25 ± 1.66 s.d. nematodes cm−3 in the high food treatments.
The food treatments were replicated across five containers (90 mL) per food level. Ten female deutonymphs and five adult males were added to each of the containers. The mites were left to mature and mate in their treatment condition for 5 days. Individual mites (now matured) were then removed and exposed to a female fly host in an infection chamber, constructed from 200 µL pipette tips reduced to half their length (~1.5 cm) and stoppered with cotton, for 60 min. Each mite was then scored as ‘attached’ or ‘unattached’ and attachment prevalence was calculated.
Experiment B: morphological measurements
To determine whether selection for increased attachment propensity had a correlated effect on body size, we measured the length of the dorsal shield, ventrianal shield and the moveable chelicera digit and estimated cheliceral strength of adult female mites from selection experiment B. Detailed morphological methods can be found in the Supplemental material. We assayed mites from the selected and control groups ten generations after selection ceased. Mites from the selected lines continued to exhibit significantly higher attachment prevalence 20 generations after selection was relaxed (Durkin and Luong, Reference Durkin and Luong2018).
Data analyses
We used generalized linear modeling (GLM) to analyze the data with R statistical software (R Core Team, 2017). The minimal model was determined using backwards model selection; significant variables (χ 2 test, P < 0.05) were retained in the models. The model selection criterion (P < 0.05) was based on an F-test for models that required a quasi-likelihood error distribution to account for over-dispersion. Final models were validated by checking the homogeneity, normality and independence of the residuals. We report the deviance (~sums of squares) and P value of variables.
Generalized linear models with quasi-poisson (log link) error distributions were used to determine the effects of selection treatment and host availability on lifetime fecundity (total number of nymphs produced during a single mite's lifetime). The full model contained replicate line, selection treatment and host availability as well as their interactions. Mite longevity was included in the model as a covariate because lifetime fecundity and longevity were positively correlated (slope = 3.00 ± 0.38, P < 0.001).
A GLM with a gamma (inverse link) error distribution was used to determine the effects of selection treatment and fly attachment on mite longevity. Replication line, selection treatment and fly attachment were included in the full model.
We used a GLM with a quasibinomial error distribution (logit link) to determine whether selected and control lines exhibited significantly different reaction norms. The prevalence of attachment was the response variable, and food treatment, selection treatment and replicate line were the fixed explanatory variables. Along with the model selection results, we also report an estimation of phenotypic plasticity of infection for the selected and control mites. Plasticity was calculated by dividing the standard deviation of the mean infection prevalence for the selected or control mites by the mean attachment prevalence of all (selected and control) lines (Valladares et al., Reference Valladares, Sanchez-Gomez and Zavala2006).
GLM models with a gaussian (identity link) error distribution were used to determine whether selection treatment affected the size of the dorsal shield, ventrianal shield, chelicerae and cheliceral strength. For each measurement, the full model contained selection treatment, replicate line, and their interactions.
Results
Lifetime fecundity
We predicted that selected mites would exhibit lower fecundity compared to control mites in the absence of flies. Conversely, we predicted that selected mites with access to a host would show higher fecundity compared to control mites in general (with or without hosts). Replicate line significantly interacted with selection treatment (deviance = −33.69, P = 0.011) and fly attachment (deviance = −30.02, P = 0.016). For this reason, we analyzed the replicate lines separately.
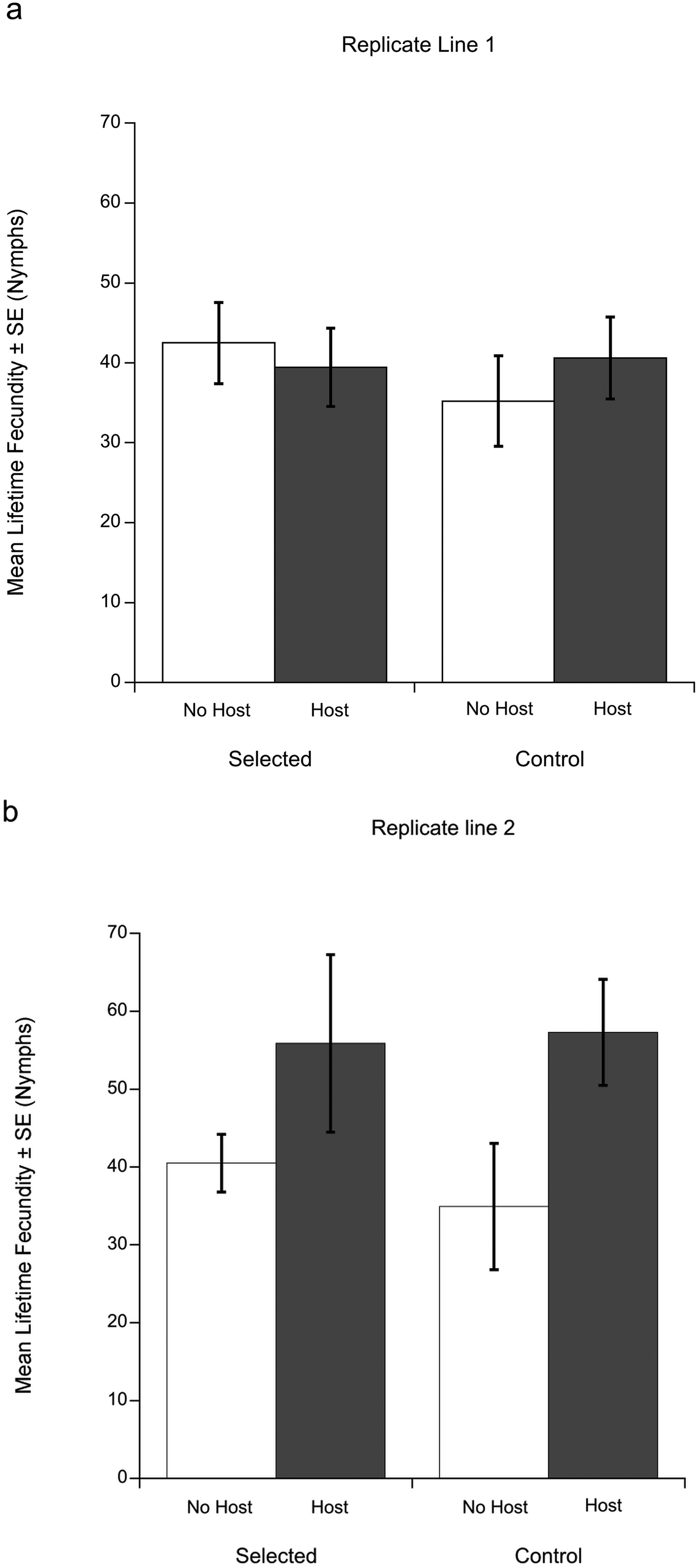
In replicate line 1, there was no significant difference in lifetime fecundity between the control and selected line (Fig. 1a). Neither fly attachment (deviance = −0.37, P = 0.75) or selection treatment (deviance = −4.63, P = 0.26) were significant predictors of lifetime fecundity.
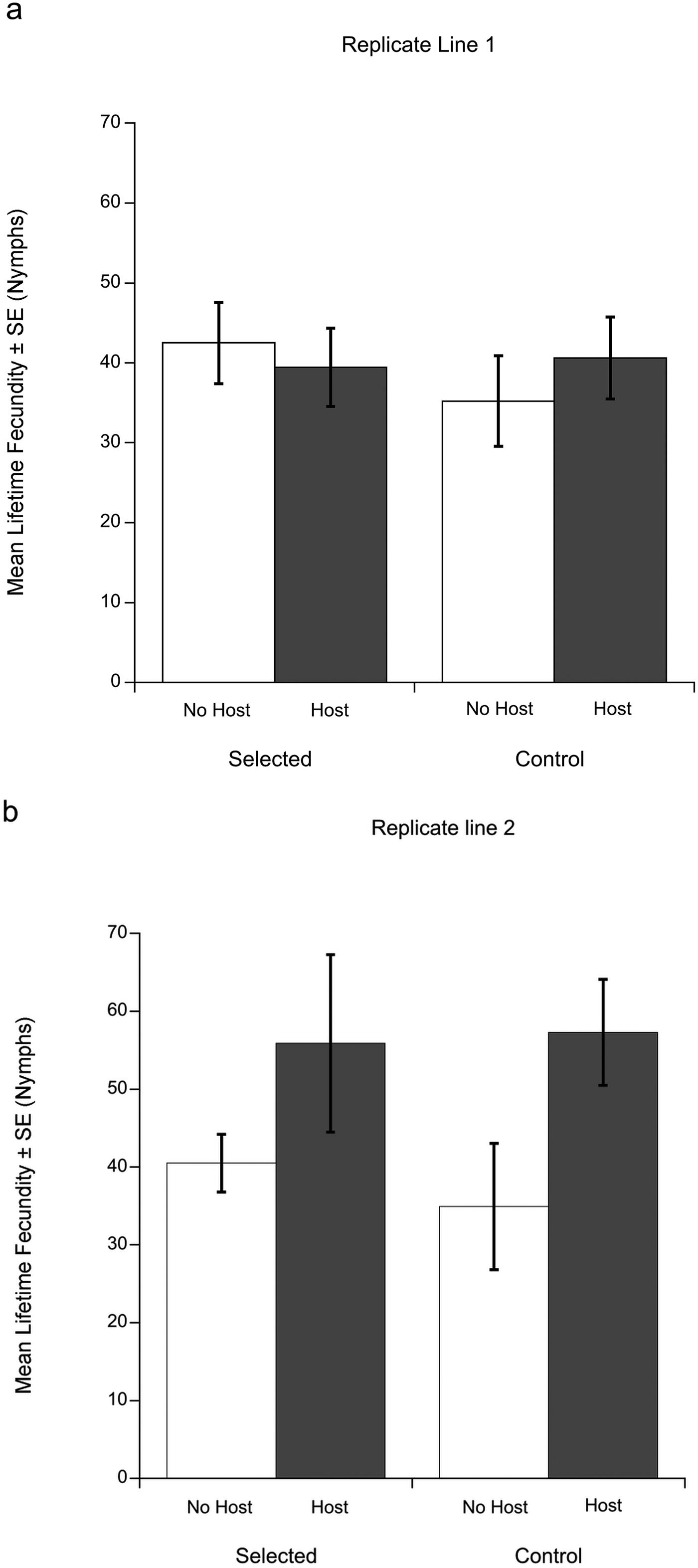
Fig. 1. Mean lifetime fecundities (± s.e.) of Macrocheles muscaedomesticae mites selected for increased propensity for host-attachment compared to unselected control mites in replicate lines 1(a) and 2 (b). Mites were without a host (white bars) or allowed to attach to a fly host, Drosophila hydei (gray bars). In replicate line 1 (Fig. 1a) selected and control mites exhibited similar lifetime fecundities regardless of host attachment. However, in replicate line 2 (Fig. 1b), mites that attached to hosts produced significantly more offspring in their lifetimes compared to those that did not, regardless of their selection treatment.
In replicate line 2, both selection treatment (deviance = −35.79, P = 0.023) and fly attachment (deviance = −77.60, P = 0.001) had a significant affect on lifetime fecundity; their interaction was not significant (deviance = −14.77, P = 0.12). Selection treatment had a positive effect on mean lifetime fecundity for mites regardless of host availability. Overall, the lifetime fecundity of selected mites (mean = 55.58 ± 7.04 s.e.) was significantly higher than the control mites' (mean = 46.10 ± 5.85 s.e.). Host availability also had a positive effect on lifetime fecundity for both selected and control mites (Fig. 1b). Mites that attached to flies produced significantly more offspring over their lifetime (mean = 64.42 ± 6.94 s.e.) than mites without access to hosts (mean = 37.70 ± 4.51 s.e.) regardless of the selection treatment. In other words, mites that attached to a fly had higher reproductive success.
Longevity
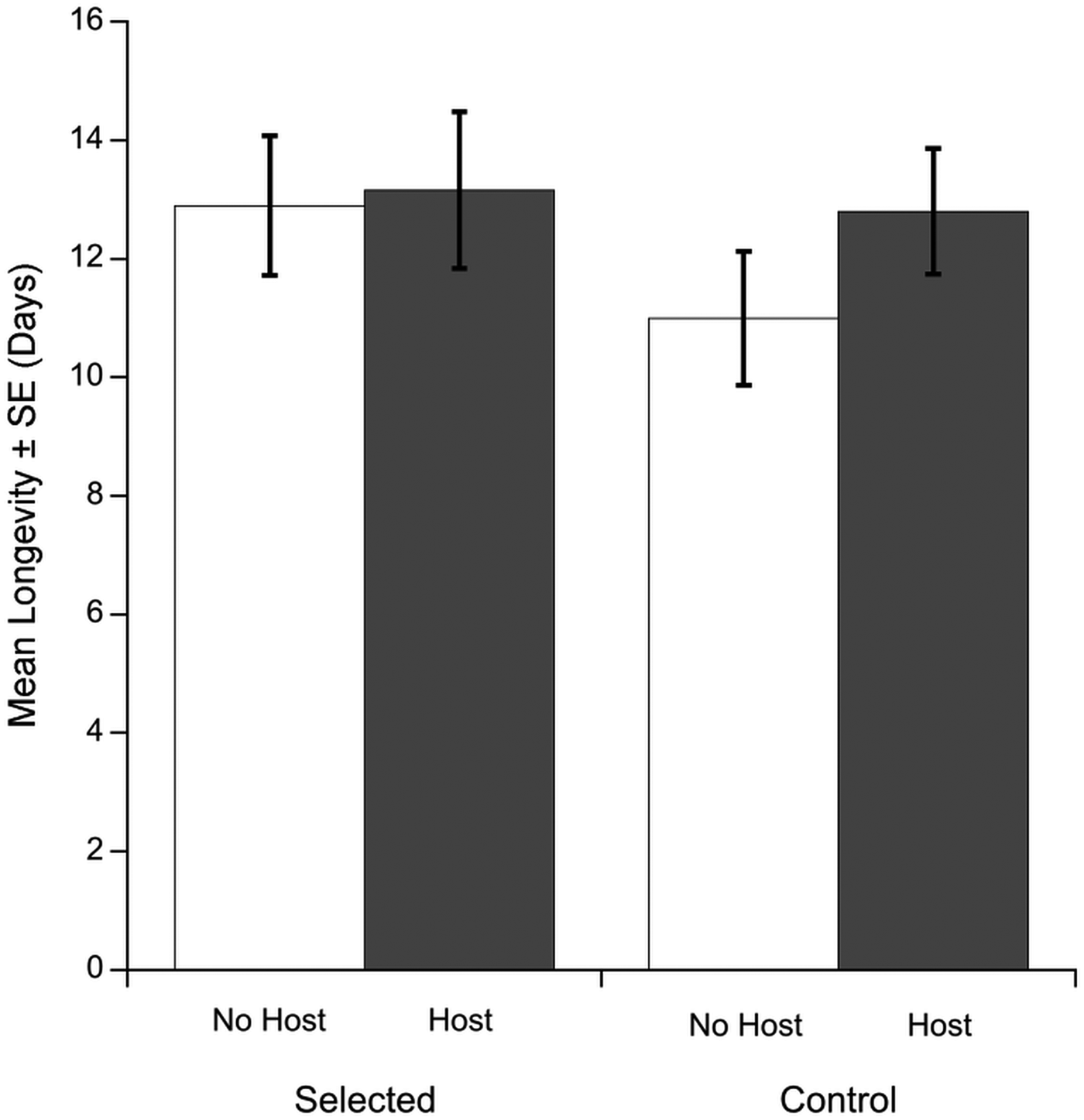
We predicted that selected mites would exhibit greater longevity compared to control mites when given access to a host and vice versa when hosts were not available. However, the mean longevity among the treatment groups was not significantly different (Fig. 2). Selection treatment (deviance = −0.16, P = 0.36) and host attachment (deviance = 0.13, P = 0.41)-were not significant predictors of mite longevity.

Fig. 2. Mean longevities (± s.e.) of Macrocheles muscaedomesticae mites selected for increased propensity for host-attachment and unselected control mites. White bars represent mites that did not have access to a host, gray bars represent mites that successfully attached to a fly host. Data from both replicate lines were pooled. Selected and control mites exhibited similar longevities regardless of host attachment.
Phenotypic plasticity
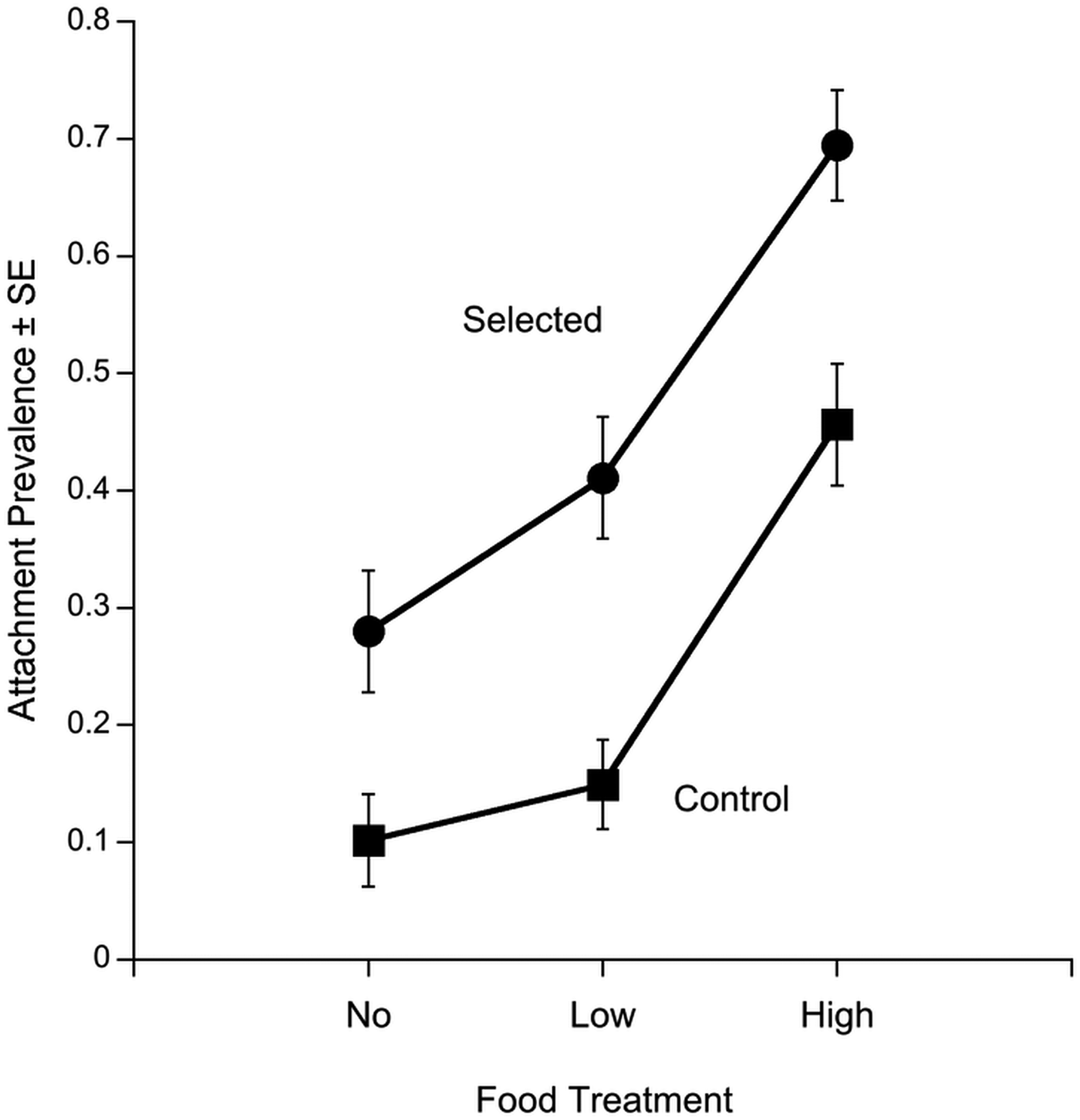
Selection treatment (deviance = −32.68, P < 0.001) and food treatment (deviance = −63.37, P < 0.001) were both significant predictors of attachment prevalence. Neither replicate line nor any of the possible interactions were significant (P > 0.05). In other words, the genetics of the mite (selection regime) and the environment (food treatment) had a significant effect on mite infection prevalence. However, there was no evidence for a genotype-by-environment interaction (deviance = 0.74, P = 0.84). The estimate of phenotypic plasticity in attachment was similar between the selected (0.78) and control mites (0.62). The similarity in attachment plasticities of the selected and control mites is illustrated by the similar reaction norms across the different environments (Fig. 3).

Fig. 3. The reaction norms of Macrocheles muscaedomesticae mites selected for increased propensity for host-attachment (circles) and control mites, which did not experience selection (squares). Infection prevalence (± s.e.) of mites was measured in three different environments: without food (No), diluted organic medium (Low) and undiluted organic medium (High). The selected mites exhibited significantly greater attachment prevalence across all environments, but their reaction norm was similar to the control.
Although we predicted a negative relationship between food availability and attachment prevalence, we saw the opposite relationship. Both selected and control mites showed increased attachment with increased food availability. In the no food treatment, 10.2 ± 3.9% s.e. of the unselected control mites and 28.0 ± 5.2% s.e. of the selected mites attached to a fly host. Attachment increased in the low food treatment to 14.9 ± 3.8% s.e. in the unselected control mites and to 41.1 ± 5.2% s.e. in the selected mites. Attachment prevalence was highest in the high food treatment and increased to 45.7 ± 5.2% s.e. in the control mites and to 69.5 ± 4.7% s.e. in the selected mites.
Morphological measurements
The dorsal shield length of selected mites was not significantly different than the control mites' (deviance = −53.79, P = 0.81). The ventrianal shield length was analyzed separately for each replicate line due to a significant interaction with replicate line (deviance = −1098.98, P < 0.001). In line 1, the ventri-anal shield length of selected mites was significantly larger (deviance = −585.82, P = 0.039) than that of the control mites. Conversely, in line 2, the ventri-anal shields of the selected mites were marginally smaller (deviance = −513.71, P = 0.058) compared to the control mites. The size of the moveable chelicera digit was similar between the selected and control mites (deviance = −0.17, P = 0.83). Chelicera strength was comparable between selected and control mites (deviance = −7.19 × 10−06, P = 0.93).
Discussion
We hypothesized that evolutionary trade-offs between attachment behaviour and other life-history traits (including attachment plasticity) contribute to the maintenance of variation in attachment behaviour observed in M. muscaedomesticae. Because many trade-offs are context-dependent (Chamberlain et al., Reference Chamberlain, Bronstein and Rudgers2014), we predicted costs associated with attachment to manifest in the absence of hosts. However, our results did not indicate the presence of trade-offs between increased infectivity and fecundity, longevity, or attachment plasticity. Furthermore, we did not find evidence for correlated morphological traits associated with increased attachment.
It's possible that increased attachment is simply not costly. In our previous selection experiments, selected mites continued to exhibit significantly increased attachment prevalence 20 generations post-selection even without host access, which could suggest that increased attachment behaviour is maintained with little or no cost in M. muscaedomesticae (Durkin and Luong, Reference Durkin and Luong2018). Castagnone-Sereno et al. (Reference Castagnone-Sereno, Mulet and Iachia2015) also failed to detect trade-offs and suggested a lack of cost when they selected for increased virulence in nematodes. Alternatively, trade-offs may manifest in traits that we did not measure.
Trade-offs can also go undetected as a result of genotype × environment interactions (Stearns, Reference Stearns1992; Sgrò and Hoffmann, Reference Sgrò and Hoffmann2004). Environmental conditions can have a large effect on the direction and magnitude of a trade-off (Stearns, Reference Stearns1992). For example, nematodes selected for a fast-infection strategy exhibited increased fecundity, but only in low-density populations (Paterson and Barber, Reference Paterson and Barber2007). Furthermore, increased parasite resistance in a moth species, Plodia interpunctella, traded-off with growth rate, but the magnitude of the trade-off depended upon resource availability; the cost of parasite resistance was lower with unlimited resources (Boots, Reference Boots2011). In our experiments, selected and control mites were maintained with an abundant food source, low population densities and favourable abiotic conditions. Further research on trade-offs under stressful conditions, such as elevated temperatures, intraspecific competition and/or limited resources could exacerbate the evolutionary costs associated with increased infectivity.
It is worth noting that the mites selected for an increased propensity for host attachment in replicate line A2 produced significantly more offspring in their lifetimes than selected mites without access to flies (Fig. 1b). Luong and Subasinghe (Reference Luong and Subasinghe2017) reported similar results in their life history observations of a closely related species, Macrocheles subbadius. Females that had previously infected a fly host produced significantly more offspring compared to the females that did not (Luong and Subasinghe, Reference Luong and Subasinghe2017). Our data suggest that there are fitness benefits associated with attachment behaviour in M. muscaedomesticae. However, this was significant in only one replicate line; more comparisons of the fecundities of attaching and non-attaching mites are necessary to support this hypothesis.
Contrary to our prediction, selected mites exhibited reaction norms similar to those of control mites, which does not support the hypothesis of genetic assimilation. Perhaps attachment plasticity (slope of reaction norm) is not correlated with the attachment mean (intercept) and/or lacks heritable variation. In this case, selection on the mean trait value (propensity to attach) may not affect the degree of plasticity. Similar to Scheiner and Lyman (Reference Scheiner and Lyman1991), our direct selection on the trait of interest did not produce a correlated response in the plasticity of the target trait. The lack of a correlated response between the mean and plasticity of attachment supports an epistatic model of plasticity, in which the mean and plasticity of a trait are determined by different genes (Lynch and Gabriel, Reference Lynch and Gabriel1987; Schlichting and Pigliucci, Reference Schlichting and Pigliucci1993). However, more research is required to tease apart whether genes for attachment mean and attachment plasticity act independently. The results of our plasticity experiment were consistent with the Baldwin effect, whereby plastic organisms are better able to survive novel environments and thus, natural selection favors phenotypic plasticity (Baldwin, Reference Baldwin1896; Crispo, Reference Crispo2007). Our selection for the ‘attaching’ mites may have concomitantly favored attachment plasticity, which could also explain the maintained reaction norm exhibited by the selected mites.
Our study revealed no differences in the morphologies between selected and control mites, except in the ventri-anal shield. However, there were some morphological differences between the replicate lines. Differences between replicate lines indicate a role of genetic drift in our replicate lines. Although we predicted to see a decrease in body size associated with parasitism in our mites, predicting changes in body size may be more complicated. Poulin (Reference Poulin1995, Reference Poulin2007) suggested that because there are so many selective pressures at play, it's difficult to accurately predict parasite body-size evolution. We also predicted to see an increase in chelicera size in the selected mites because of their utility in host-attachment. However, we did not find any differences in chelicera size associated with infection selection. According to Krantz (Reference Krantz1998) and Manning and Halliday (Reference Manning and Halliday1994), the size of the chelicerae themselves may not be critical for host-attachment. Instead, bidentate teeth on the chelicerae may be more useful for host attachment and thus a candidate trait for change associated with increased attachment propensity. Other morphological changes associated with parasitic lifestyles in Acari include a reduction in chelicera segment number and loss of the moveable digit, essentially creating a functional piercing mouthpart (Dowling, Reference Dowling, Morand, Krasnov and Littlewood2015). Due to their small size, investigation of cheliceral morphology would likely require scanning electron microscopy, which was beyond the scope of this project. The lengths of the first and second cheliceral segments (our proxy for strength) in the selected and control mites were also similar. Future investigation of cheliceral strength should focus on estimating the amount of muscle located in the cheliceral segments. Unfortunately, our method of slide mounting destroyed the muscle tissue.
If evolutionary trade-offs between attachment and other traits do not exist, how is the variation in attachment behaviour of M. muscaedomesticae maintained? Our data suggests that phenotypic plasticity in attachment behaviour is an important factor contributing to infection variation because attachment plasticity was maintained in spite of directional selection on a single trait (Fig. 3). Parasites often encounter variable and unpredictable environments in their lifetimes (Poulin, Reference Poulin2007). Adaptive plasticity in infection strategy may allow parasites (facultative or obligate) to deal with heterogenous environments. For example, Birget et al. (Reference Birget, Repton, O'Donnell, Schneider and Reece2017) showed that malaria parasites adjust gametocyte density in response to resource availability, allowing them to adaptively balance the costs and benefits of gametocyte production. Similarly, Lagrue and Poulin (Reference Lagrue and Poulin2009) demonstrated that the trematode Coitocaecum parvum was capable of sensing the absence of its definitive host and inducing progenesis and reproduction in the intermediate host (see also Thomas et al., Reference Thomas, Brown, Sukhdeo and Renaud2002; Kaltz and Koella, Reference Kaltz and Koella2003; Reece et al., Reference Reece, Ramiro and Nussey2009; Leggett et al., Reference Leggett, Benmayor, Hodgson and Buckling2013; Searle et al., Reference Searle, Ochs, Caceres, Chiang, Gerardo, Hall and Duffy2015 for more examples). However, it is unlikely that phenotypic plasticity acts alone in generating and maintaining infection variation in parasites.
Hosts also have the potential to alter the costs and benefits associated with parasitism, thus maintaining facultative parasitism. Huang et al. (Reference Huang, Traulsen, Werner, Hiltunen and Becks2017) hypothesized that antagonistic coevolution can cause dynamic trade-offs: an adaptation in one species might result in significant fitness gains initially but may decrease as antagonistic species counter-adapt. In nature, fly hosts can resist mites (Luong and Polak, Reference Luong and Polak2007) and potentially coevolve in response to increased infection. This type of antagonistic coevolution could drive down some of the benefits of increased infectivity, changing the cost-benefit ratio and the nature of the trade-off. In our experiments, flies were not allowed to coevolve with the mites. Future research that allows the evolutionary response of the host could uncover more of the potential costs of increased attachment behaviour experienced in nature.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019000507
Author ORCIDs
Emily S. Durkin, 0000-0002-5205-4460.
Acknowledgements
We thank H. Goraya, S. Chan, D. Yee and M. Mierzejewski for their assistance with fecundity data collection, S. Fang and P. Phiri for maintaining the fly cultures used in our experiments and especially I. Moghe for assistance with the morphological measurements. We also thank the two anonymous reviewers for strengthening a previous drafts of this manuscript.
Financial support
This research was funded by the Natural Sciences and Engineering Research Council of Canada [Discovery Grant #435245].
Conflict of interest
None
Ethical standards
Not applicable.