INTRODUCTION
The larval stage (metacestode) of the taeniid cestode Echinococcus granulosus causes cystic echinococcosis, also called hydatid disease, in a variety of livestock species as well as in humans (reviewed by Thompson (Reference Thompson, Thompson and Lymbery1995)). The E. granulosus metacestode is a bladder-like structure (hydatid) that dwells in the parenchymas of internal organs, most commonly liver and lungs, and can reach up to tens of cm in diameter. The hydatid is defined by the hydatid wall (HW), a structure comprising a thin inner layer of cells (germinal layer, GL) and the massive outer laminated layer (LL). The LL (reviewed by Díaz et al. (Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a, Reference Díaz, Casaravilla, Irigoín, Lin, Previato and Ferreirab)) is a peculiar extracellular structure formed by a meshwork of mucins bearing galactose-rich glycans. Additionally, in E. granulosus but not other species of the genus, it contains nano-deposits of calcium inositol hexakisphosphate (InsP 6) (Irigoín et al. Reference Irigoín, Casaravilla, Iborra, Sim, Ferreira and Díaz2004). Being up to 3 mm thick, and permeable to macromolecules, the LL represents a very large area for the adsorption of diffusible proteins (Coltorti and Varela-Díaz, Reference Coltorti and Varela-Díaz1974; Casaravilla et al. Reference Casaravilla, Brearley, Soule, Fontana, Veiga, Bessio, Ferreira, Kremer and Diaz2006).
Whereas establishing larval E. granulosus elicits local inflammatory responses, these responses normally resolve upon parasite deployment of the LL. Thus established hydatids normally grow surrounded by a non-infiltrated or minimally infiltrated host-derived collagen capsule (reviewed by Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). This is readily seen in both sheep and human infections (Yamashita et al. Reference Yamashita, Ohbayashi and Sakamoto1961; Mufarrij et al. Reference Mufarrij, Arnaut, Meshefedjian and Matossian1990). Although certainly a key aspect of this parasite's survival strategy, inflammatory resolution does not always take place. Chronic inflammation can often be seen, even in host species (sheep, humans) considered suitable for the G1 strain of the parasite, the most prevalent worldwide (Jenkins et al. Reference Jenkins, Romig and Thompson2005). The prototypical case of lack of inflammatory control is infection of cattle by this parasite strain. As a result of an undetermined host-parasite mismatch, chronic local inflammation is the rule, and parasite vitality is accordingly compromised (Rao and Mohiyuddin, Reference Rao and Mohiyuddin1974; Bortoletti and Ferretti, Reference Bortoletti and Ferretti1978; Sakamoto and Cabrera, Reference Sakamoto and Cabrera2003; Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). Intermediate and variable degrees of inflammation are observed in pig infections (Slais and Vanek, Reference Slais and Vanek1980).
When present, the chronic inflammatory response to E. granulosus is typically granulomatous. A layer of palisading epithelioid and multinucleated giant macrophages is directly apposed to the parasite's LL (Nieberle and Cohrs, Reference Nieberle and Cohrs1967; Slais and Vanek, Reference Slais and Vanek1980). Behind this first layer is a mononuclear cell infiltrate, featuring lymphocytes, plasmocytes, conventional macrophages and some eosinophils. More externally and/or intermixed with the mononuclear cell infiltrate is a layer of fibroblasts and collagen. A similar granulomatous response is (invariably) elicited by the highly invasive larval stage of Echinococcus multilocularis. In this context it has been demonstrated that the granuloma is T-cell dependent, and that is it damaging to the parasite (Gottstein and Hemphill, Reference Gottstein and Hemphill1997; Dai et al. Reference Dai, Waldvogel, Siles-Lucas and Gottstein2004).
The large capacity of the parasite's LL to adsorb proteins implies that the secreted products of the local host reaction tend to accumulate in the HW. Additional host proteins in HW extracts can derive from remnants of epithelioid cells that adhere tightly to the LL. By far the major parasite-derived macromolecules in HW extracts are the LL structural mucins (since the LL is quantitatively very dominant over the GL). As these mucins are highly insoluble, conventionally prepared HW extracts are dominated by host proteins (Casaravilla and Díaz, Reference Casaravilla and Díaz2010; Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). When HW derive from a non-resolutive (granulomatous) context, these proteins include prominently the products of the epithelioid and multinucleated giant cells (MGC) (Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a, Reference Díaz, Willis and Simb; Marco et al. Reference Marco, Baz, Fernández, Gonzalez, Hellman, Salinas and Nieto2006). This has led to observations of general interest for granuloma biology being made initially in the E. granulosus sytem (Díaz et al. Reference Díaz, Willis and Sim2000b).
In this study, we investigated the association of phagocyte-specific S100 proteins with the granulomatous response to the E. granulosus metacestode. S100 is a large family of cytosolic calcium-binding proteins thought to regulate cytoskeletal function and other calcium-dependent cellular responses. Three S100 proteins, namely S100A8, S100A9 and S100A12 are expressed prominently by myeloid cells, and therefore referred to as the phagocyte-specific S100 protein subfamily (reviewed by Ehrchen et al. Reference Ehrchen, Sunderkotter, Foell, Vogl and Roth2009 and Pietzsch and Hoppmann, Reference Pietzsch and Hoppmann2009). They form Ca2+ and Zn2+-dependent dimers and higher oligomers; while S100A9 and S100A8 most commonly heterodimerize (oligomerize), S100A12 associates only with itself. These proteins are actively secreted by a non-conventional mechanism and fulfil extracellular functions. The clearest of these is as amplifiers of the inflammatory response, i.e. as endogenous danger-associated signals (DAMPs). S100A8 and also the S100A9/S100A8 heterodimer are agonists of TLR4, and this interaction has a strong impact in inflammatory disorders (Vogl et al. Reference Vogl, Tenbrock, Ludwig, Leukert, Ehrhardt, Van Zoelen, Nacken, Foell, Van Der Poll, Sorg and Roth2007; Loser et al. Reference Loser, Vogl, Voskort, Lueken, Kupas, Nacken, Klenner, Kuhn, Foell, Sorokin, Luger, Roth and Beissert2010). S100A12 is thought to be an agonist of the receptor for advanced glycation end-products, RAGE (Hofmann et al. Reference Hofmann, Drury, Fu, Qu, Taguchi, Lu, Avila, Kambham, Bierhaus, Nawroth, Neurath, Slattery, Beach, McClary, Nagashima, Morser, Stern and Schmidt1999). Functions are not necessarily well conserved across mammalian species: S100A12 is absent in rodents, and rodent S100A8 has been proposed to be the functional homologue of human S100A12 (Pietzsch and Hoppmann, Reference Pietzsch and Hoppmann2009). We reasoned that the analysis of the phagocyte-specific S100 proteins across the inflammation-resolution spectrum in hydatid disease may contribute a valuable element towards understanding the regulation of local inflammation in this infection.
MATERIALS AND METHODS
Parasite materials
Hydatids from mouse experimental infections were retrieved 8–12 months after intraperitoneal inoculation of protoscoleces obtained from natural bovine infections. Human hydatid surgical samples (fresh and/or paraffin-embedded) were obtained from the Clínica de Cirugía Pediátrica, Hospital Pereira-Rossel (Dr G. Giannini), and the Laboratorio de Anatomía Patológica, Hospital Maciel (Dr M. Roldán), both in Montevideo, Uruguay. Bovine, sheep and pig hydatid material was from natural livestock infections in Uruguay. For the bovine host, a panel of paired fresh (for protein extracts) and paraffin-embedded (for inflammatory scoring and immunohistochemistry) samples was set up.
Hydatid wall protein extracts
Hydatid walls (HW; comprising LL and GL) were retrieved from fresh samples as described by (Irigoín et al. Reference Irigoín, Ferreira, Fernández, Sim and Díaz2002). HW were washed with PBS containing 0·5 mm CaCl2 to remove loosely bound proteins. They were then extracted using PBS containing the calcium chelators EGTA or EDTA (in excess of the molar amount needed to solubilize the calcium InsP 6 deposits (Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a), with 2 m NaCl, or sequentially with both agents, as indicated. The protease inhibitors PMSF (2 mm), iodoacetamide (2 mm), pepstatin A (2 μg/ml), and E-64 (100 μ m) were added in each step. Extracts were concentrated prior to analysis by precipitation with 10% (w/v) trichloroacetic acid.
Antibodies
Rabbit polyclonal antibodies against human and mouse S100A9 and S100A8, and human S100A12 were raised as previously described (Zwadlo et al. Reference Zwadlo, Bruggen, Gerhards, Schlegel and Sorg1988; Roth et al. Reference Roth, Burwinkel, Van Den Bos, Goebeler, Vollmer and Sorg1993; Vogl et al. Reference Vogl, Propper, Hartmann, Strey, Strupat, Van Den Bos, Sorg and Roth1999a). A monoclonal antibody against human S100A9 (clone S36·48) was purchased in biotinylated form from BMA Biomedicals AG (Switzerland); this antibody works in paraffin-embedded sections but does not react with the denatured protein in Western blotting. A rabbit polyclonal antibody was raised against bovine S100A12 recombinantly expressed in E. coli. Total RNA was obtained from bovine peripheral blood leukocytes (buffy coat), reverse-transcribed using an oligodT primer, and the coding sequence for S100A12 amplified by nested PCR using the primers: tctcctgaaggtgaacgtagt (outer forward), cggatccatgactaagctggaagat (inner forward), cgtcgacaagcttctactctttgtggatatct (inner reverse) and cgtcgaccgggtaaggcagcctcaggg (outer reverse). The amplicon was ligated into pGEM-T Easy (Promega), and the insert then excised using BamHI and SalI and introduced into the pET28a vector (Novagen) for expression in E. coli. The fusion protein was purified by nickel affinity chromatography from the soluble fraction of bacterial lysates. The recombinant protein was also bound to CNBr-activated Sepharose (Sigma) for affinity purification of the rabbit antibodies obtained.
SDS-PAGE and Western blotting
HW extracts were run under reducing conditions, either in conventional SDS-PAGE (10% (w/v) acrylamide) or in the Tris-tricine system (16·5% (w/v) acrylamide), and Coomassie blue- or silver-stained as indicated. Alternatively, proteins were transferred to nitrocellulose membranes for Western blotting. Membranes were probed with the specific rabbit antisera, followed by alkaline phosphatase goat anti-rabbit IgG (Calbiochem), and developed with nitro-blue tetrazolium/ 5-bromo 4-chloro 3-indolyl phosphate (NBT/BCIP; Sigma) substrate. Control membranes in which normal rabbit serum was used in the first probing step gave no staining.
One dimensional SDS-PAGE and MALDI-TOF-based proteomics
Selected Coomassie blue-stained protein bands were digested with sequencing-grade trypsin, and peptides analysed by MS and MS/MS in the Applied Biosystems 4800 Analyzer. Proteins were then identified by searching the NCBI nr database (2010) using the MASCOT program in the ‘sequence query’ mode. The following search parameters were employed: monoisotopic mass tolerance 0·08–0·10 Da; fragment mass tolerance 0·2–0·6 Da; methionine oxidation, and in cases also propionamide addition to cysteine residues and/or acetylated protein N-terminus, as variable modifications; 1 missed tryptic cleavage allowed. Significant peptide and/or protein scores (P<0·05) were used as criteria for positive protein identification. In initial experiments using equipment without MS/MS capabilities (Voyager DE-Pro also from Applied Biosystems), a tryptic peptide fingerprinting approach was taken, leading to presumptive identifications that were later confirmed by MS/MS as above and/or by Western blotting using specific antibodies.
Nano-LC-MS-based proteomics
HW 2 m NaCl extracts were dialysed using 3500 kDa cut-off membranes against 10 mm ammonium bicarbonate, 0·5 mm EDTA and protein content in them measured by absorbance at 280 nm. Proteins in the extracts were reduced, carbamidomethylated, and digested with sequencing-grade trypsin (1:10 enzyme to total protein ratio, 24 h at 37°C) in the presence of 1 m guanidine hydrochloride. Samples were then injected into a nano-HPLC system (Proxeon easy-nLC, Thermo Scientific) fitted with a reverse-phase column (easy C18 column, 3 μm; 75 μm ID×10 cm; Proxeon, Thermo Scientific) and separated using a 0·1% (v/v) formic acid in water – 0·1% (v/v) formic acid in acetonitrile gradient (0–60% acetonitrile in 60 min; flow 400 nl/min). Online MS detection/analysis was carried out in the LTQ Velos nano-ESI-linear ion trap instrument (Thermo Scientific) in the data-dependent triple play MS2 mode (full scan followed by zoom scan and MS/MS of the top 5 peaks in each segment). Proteins were identified by searching the IPI database (bovine, 2010) using the following parameters in the Mascot software in the MS/MS ion search mode: peptide tolerance 400 ppm, MS/MS tolerance 0·8 Da, and cysteine carbamidomethylation, protein N-terminus acetylation, methionine oxidation and asparagine/glutamine deamidation as these allowed variable modifications. The significance limit for protein identification was set at P<0·05.
Immunohistochemistry
Microtome sections (0·5 mm thick) were dewaxed, treated with proteinase K as an antigen retrieval procedure and with 1% H2O2 in methanol to inhibit endogenous peroxidase activity, and blocked using 10% (v/v) goat serum. Bovine S100A12 was detected using affinity-purified rabbit antibodies; the purification flow-through was used as negative control, and gave no staining. Human S100A9 was detected with the biotinylated S36·48 monoclonal antibody followed by streptavidin-peroxidase (Sigma). Human S100A8 was detected with the rabbit polyclonal antiserum; normal rabbit serum used at the same concentration as control gave no staining. Sections probed with the rabbit primary antibodies were then incubated with peroxidase-conjugated goat IgG against rabbit IgG (Calbiochem). All sections were finally developed using diaminobenzidine substrate and counterstained with Mayer's haematoxylin. In parallel, sections from each sample were stained only with haematoxylin-eosin and scored for inflammatory status on an arbitrary scale from ‘−’ (no inflammation, only collagen present) to ‘+++++’ (intense granulomatous inflammation featuring a full rim of epithelioid cells surrounding the parasite).
RESULTS
Phagocyte-specific S100 proteins are associated with the E. granulosus hydatid in different host species
Treating E. granulosus HW with calcium chelators dissolves the calcium InsP 6 deposits (Irigoín et al. Reference Irigoín, Ferreira, Fernández, Sim and Díaz2002, Reference Irigoín, Casaravilla, Iborra, Sim, Ferreira and Díaz2004). Being initially interested in proteins associated with these deposits, we prepared extracts using EGTA- or EDTA-containing buffer from intact hydatids obtained by experimental infection of mice. The extracts featured 3 major protein bands (Fig. 1 A). For the bands with apparent molecular masses 14 and 8 KDa, tryptic peptide fingerprinting suggested that they corresponded to host-derived S100A9 and S100A8. This was confirmed by MS/MS (Table S1 online version only) and by Western blotting (Fig. 1 B). Immunoblotting also showed that the association of mouse S100A8 with the HW was strictly Ca2+-dependent, while that of S100A9 was less strictly so. Both proteins could be extracted even in the presence of Ca2+, by high ionic strength.

Fig. 1. Association of S100A8, S100A9 and S100A12 with the Echinococcus granulosus metacestode, in different hosts. (A) Intact mouse peritoneal hydatids were extracted externally with buffers without or with calcium chelators as indicated, and solubilized proteins were run on SDS-PAGE and selected Coomassie blue-stained bands studied by tryptic peptide fingerprinting; identifications were confirmed on similar samples by MS/MS-based proteomics (Table S1, online version only). (B) Mouse hydatids were extracted externally with buffer with or without calcium chelator as indicated and, in a second step, the proteins still associated with the hydatids in each case were extracted by use of 2 m NaCl. Extracted proteins were analysed by Western blotting with antibodies to mouse S100A9 or S100A8. (C) HW from natural infections in different hosts were extracted with buffers containing EDTA, solubilized proteins run on SDS-PAGE, and selected Coomassie blue-stained bands identified proteomically, as detailed in Table S1 (online version only). Bands marked as 1 and 2 correspond respectively to bovine cathepsin K pro-enzyme and truncated bovine annexin A2, on the basis of previously reported observations (Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a, Reference Díaz, Willis and Simb). The band marked ‘not S100A12’ could not be identified, but the possibility that it was human S100A12 was ruled out. (D) The extract of human host origin was analysed by Western blotting with antibodies to human S100A8 and S100A8 or to human S100A12. Native human S100A9/A8 dimer (100 ng) or recombinant human S100A12 (20 ng) were run as positive controls.
Hydatids, developing after intraperitoneal infection of mice, grow loose in the peritoneal cavity, while those arising from natural infections are embedded within organ parenchymas. Also, the spectrum of inflammatory conditions observed in hydatid disease is not reproduced in this model, in which resolution is always observed (Richards et al. Reference Richards, Arme and Bridges1983; Breijo et al. Reference Breijo, Spinelli, Sim and Ferreira1998). In addition, as mentioned, rodents do not encode S100A12 in their genomes. We therefore analysed the presence of host S100 proteins in parasite samples from natural infections in cattle, pig, sheep, and humans. A limited proteomic screening was carried out, using one-dimensional gels and focusing on the apparent molecular mass of the monomeric S100 proteins (6–14 kDa) and of the S100A9/S100A8 dimer (24 kDa), which for unknown reasons can run as such even after denaturation and reduction (see for example Fig. 1 D, left-hand panel). This analysis showed that prominent 8 kDa bands in the extracts studied from cattle, pig and sheep origins corresponded to S100A12 (Fig. 1 C). Part of the S100A12 molecules from all 3 species appeared to be N-terminally acetylated (Tables S1 and S2, online version only), a modification previously observed for human S100A9 but not reported for S100A12 proteins (Ilg et al. Reference Ilg, Troxler, Burgisser, Kuster, Markert, Guignard, Hunziker, Birchler and Heizmann1996; Vogl et al. Reference Vogl, Roth, Sorg, Hillenkamp and Strupat1999b; McMorran et al. Reference McMorran, Patat, Carlin, Grimwood, Jones, Armstrong, Galati, Cooper, Byrnes, Francis, Robertson, Hume, Borchers, Wainwright and Wainwright2007). S100A12 was undetectable both by proteomic methods and by more sensitive Western blotting in the human sample analysed which, however, did contain immunochemically detectable S100A9 and S100A8 (Fig. 1 D). An immunochemical assessment of the presence of S100A9 and S100A8 in the non-human samples was precluded by the lack of suitable antibodies. In sum, the initial analysis suggested that association of host phagocyte-specific S100 proteins with the HW may be a general phenomenon but differences probably exist in terms of individual S100 proteins across different host species and/or inflammatory status of individual hydatids.
Host proteins unrelated to S100 that were identified in the limited proteomic studies described above included the pentraxin-family acute-phase proteins serum amyloid P (in mouse-derived samples; Fig. 1A; GeneBank Accession no. P12246) and C-reactive protein (in sheep-derived samples; Fig. 1C; GeneBank Accession no. EE780797, identified by TBLASTN search of mammalian ESTs). Non-S100 host proteins identified in the bovine sample will be discussed in the context of similar findings by nano-LC-MS-based proteomics described in the next section.
S100A12 is consistently associated with the E. granulosus larva in the bovine host
The cross-host species studies described in the previous section were hampered by the limited number of samples available and the lack of a histological assessment of the local inflammatory status for each sample. We therefore chose to focus on (readily available to us) bovine-derived samples, scoring individual samples for local inflammatory status. Although complete inflammatory resolution was never found, cattle hydatid samples displayed a wide range of intensities in inflammation, thus allowing the assessment of S100 proteins across different biological conditions.
In an analysis based on 1-D SDS-PAGE, a prominent 8 kDa band was identified proteomically as S100A12 in each of 9 independent samples studied (Fig. 2 and Table 2). The presence of S100A12 across all bovine-derived hydatid samples was confirmed by Western blotting (Fig. 2 and data not shown). S100A12 appeared to be more abundant in extracts from samples with middle to high inflammation scores than in those samples with middle to low scores (Fig. 2). S100A9 and S100A8 were not detected, either as obvious separate bands or contaminating the S100A12 band.

Fig. 2. Association of S100A12 with the reaction to the Echinococcus granulosus metacestode in representative samples from the bovine host. HW were extracted with 2 m NaCl, solubilized proteins run on SDS-PAGE and either silver stained (A), or transferred to nitrocellulose and probed with antibodies to bovine S100A12 (B). Portions from the same HW were fixed, paraffin-embedded, sectioned, haematoxylin-eosin stained, and scored from the intensity of inflammation surrounding the parasite, as indicated; in some cases 2 scores were given because inflammatory status varied between regions of each sample. The anatomical locations of the different hydatids are also indicated. The identity of the S100A12 band was confirmed proteomically after Coomassie blue staining of gels similar to that shown, as detailed in Table S2 (online version only).
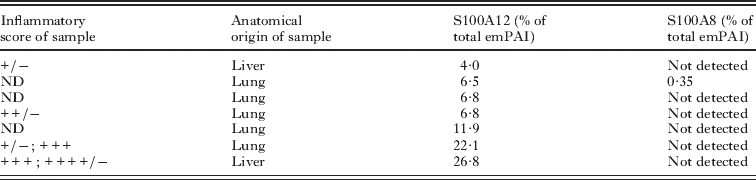
In order to analyse more carefully the apparent selective abundance of S100A12 as compared to S100A9 and S100A8, we subjected several bovine host-derived samples to proteomic analysis by nano-LC-MS. In this approach, the total protein content of each sample is subjected to tryptic digestion and the resulting peptides separated and analysed, thus avoiding the bias inherent to picking bands or spots in electrophoresis gels. Also, protein abundances in the samples can be roughly estimated by emPAI (Ishihama et al. Reference Ishihama, Oda, Tabata, Sato, Nagasu, Rappsilber and Mann2005). S100A12 was detected in all 7 samples analysed by this method (Table 1); as mentioned previously, some of the molecules were N-terminally acetylated (Table S3, online version only). The estimated abundance of S100A12 ranged between 4 and 27% of total host protein, and again samples with lower inflammatory scores tended to display lower relative abundances of S100A12 than those with higher scores. In relation to total tissue dry mass (of which most corresponds to the LL mucins (Díaz et al. Reference Díaz, Casaravilla, Irigoín, Lin, Previato and Ferreira2011b)), the emPAI data and total protein contents of extracts allowed us to estimate that S100A12 is present in the range of 0·1–1 mg per g of tissue dry mass. S100A9 was not detected in any of the samples, while S100A8 was detected in a single sample, with an emPAI that was 18-fold lower than that of S100A12 (Table 1). The emPAI method is based on the ratio between experimentally observed signals and theoretically observable peptides for each protein (Ishihama et al. Reference Ishihama, Oda, Tabata, Sato, Nagasu, Rappsilber and Mann2005). The Mascot software estimates this second figure on the basis of protein molecular mass. As the molecular masses of bovine S100A8 and S100A9 are similar to and larger than those of S10012 respectively, the number of observable peptides for S100A8 and S1009 is at least as high as that of S100A12. Using the emPAI formula and the fact that a single peptide can allow significant protein identification, we estimate that the abundances of S100A9 and S100A8 in the bovine-origin samples must be at least an order of magnitude lower than that of S100A12. In sum, in the host species in which local inflammation against the parasite is strongest and most maintained, S100A12 is a major product at the host-parasite interface, while S100A8 and S100A9 are not present in similarly high amounts.
Table 1. Semi-quantitation of S100 proteins in a panel of bovine host samples by LC-MS and emPAI
Non-S100 host proteins detected in the LC-MS-based proteomics search across over half the individual samples analysed (and in cases also in the SDS-PAGE-based experiments shown in Fig. 1) are listed in Table S4, online version only. These include 2 proteins previously known to be abundant in this system (Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a, Reference Díaz, Willis and Simb), namely the cysteine protease cathepsin K and the cortical (non-conventionally secreted) protein annexin A2. Abundant host proteins newly identified included cystatin C, an inhibitor of papain-family cysteine proteinases including cathepsin K (reviewed by Turk et al. Reference Turk, Stoka and Turk2008), and regakine-1, a CC chemokine present at high concentrations in bovine plasma (Struyf et al. Reference Struyf, Proost, Lenaerts, Stoops, Wuyts and Van Damme2001). They also included galectin-1, a non-conventionally secreted anti-inflammatory mediator (reviewed by Rabinovich and Ilarregui, Reference Rabinovich and Ilarregui2009) previously observed in hydatid fluid of the same host origin (Monteiro et al. Reference Monteiro, De Carvalho, Zaha and Ferreira2010). Other proteins found worth mentioning were the calcification inhibitor α-2-HS-glycoprotein (reviewed by Lee et al. Reference Lee, Bongcam-Rudloff, Sollner, Jahnen-Dechent and Claesson-Welsh2009) and the cytotoxic T cell and NK cell cytolytic protein granulysin (reviewed by Krensky and Clayberger, Reference Krensky and Clayberger2009). The proteins mentioned, including S100A12, can be considered representative of the subset of abundant (bovine) host-derived LL-associated proteins that can be solubilized by high ionic strength and/or EDTA. Other abundant host proteins exist in this system that require more drastic treatments for extraction, namely immunoglobulins and terminal complement components (our unpublished results).
Epithelioid cells and MGC from the host granuloma adjacent to the parasite are the main source of S100A12 in the bovine host
We analysed the distribution of S100A12 in the host-parasite interface of hydatid infection in cattle by immunohistochemistry. The protein was strongly expressed in the epithelioid and MGC and present in the necrotic remnants adhering to the surface of the LL (Fig. 3 B, C, D, E). Although the strongest and most consistent staining was in the epithelioid cell and MGC rim, S100A12 was also expressed by other cells of the host reaction, namely morphologically conventional macrophages present in the mononuclear cell infiltrate (Fig. 3 A, C, F), and some fibroblasts (Fig. 3 A, C, G). S100A12 was also observed to be expressed by alveolar macrophages, but this appeared to be independent of the presence of the parasite as it was also observed in samples taken from tens of cm from the infection site (not shown). As expected, when the local inflammatory reaction was weak, S100A12 staining was also weak, restricted to some macrophages infiltrating the collagenous capsule (Fig. 3 A). As for the parasite structures, we did not find clear-cut evidence of the presence of S100A12 within the LL itself, but since it is generally very difficult to stain proteins within the LL in immunohistochemistry (Stadelmann et al. Reference Stadelmann, Spiliotis, Muller, Scholl, Muller, Gottstein and Hemphill2010; Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a), we do not take this as evidence against the protein being also present in the interior of the LL. In contrast, the S100A12 antibodies did stain the GL (not shown). The GL is known to take up host proteins, as observed by immunohistochemistry and by proteomics (Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a; Monteiro et al. Reference Monteiro, De Carvalho, Zaha and Ferreira2010). The immunohistochemical findings on S100A12 are summarized in Table 2. The strong expression of S100A12 by the epithelioid cells and MGC directly apposed to the HW explains the consistent presence of this protein in the extracts from bovine-origin HW samples (Fig. 2 and Table 1).

Fig. 3. Immunohistochemical detection of S100A12 in the host-hydatid interface in the bovine host. Paraffin-embedded sections of the local reaction to hydatids, including whenever possible the HW itself, were stained with haematoxylin-eosin (H/E), or subjected to immunohistochemical detection using affinity-purified antibodies to bovine S100A12 or control antibodies (corresponding to the flow-through of the affinity purification). (A–C) Panoramic views of 3 representative samples with different degrees of inflammation; inflammatory scores are given besides the hydatid localization in each case. In (C), the LL and attached host epithelioid cells have separated from the remainder of the host tissue: the 2 components are shown in separate micrographs. (D–G) Higher resolution views of the main elements showing positive reaction for S100A12: epithelioid cells (and MGC), necrotic area found between host reaction and LL, morphologically conventional inflammatory macrophages, activated fibroblasts and lung parenchyma. In each H/E stain in (A–C), the approximate orientation of the host (H)-parasite (P) interface has been indicated. LL, laminated layer; E, epithelioid and MGC; I, mononuclear cell-dominated infiltrate; C, collagen (and fibroblasts); F, fibroblast; AM, alveolar macrophage. Scale bars represent 100 μm throughout.
Table 2. Summary of immunohistochemical findings

Eosinophils distal to the parasite can express S100A9 and S100A8 in the human host
Human hydatid samples are, in most cases, characterized by the lack of inflammation, in particular in the tissue directly apposed to the parasite. Thus, this type of sample could complement the bovine hydatid samples, which are characterized by continuing inflammation. The non-infiltrated collagenous capsule of human hydatid samples generally did not stain for S100A9 or S100A8 (Fig. 4 A, B); however, some regions of collagen did give positive, extracellular, staining for both proteins (Fig. 4 D). Necrotic remnants adhered to the HW also stained for both proteins (Fig. 4 C). Some human samples feature inflammatory infiltrates that are distal to the parasite, so that the host structure in contact with the larva is still the collagenous capsule. In some cases, these infiltrates are dominated by lymphocytes/plasmocytes (Fig. 4 C); in this situation, macrophages interspersed in the lymphoplasmocytic infiltrate stained for S100A9 and S100A8 (not shown). Other human samples have infiltrates dominated by eosinophils, which expressed S100A9 and S100A8 strongly (Fig. 4 B, E). These observations are summarized in Table 2.

Fig. 4. Immunohistochemical detection of S100A9 and S100A8 in the host-hydatid interface in the human host. Paraffin-embedded sections of the local reaction to Echinococcus granulosus metacestodes were stained with haematoxylin-eosin (H/E), or subjected to immunohistochemical detection using antibodies to human S100A9 or S100A8, or control antibodies. (A–C) Panoramic views of 3 representative samples with different degrees of inflammation; inflammatory scores are given besides the hydatid localization in each case. The control stains shown correspond to normal rabbit serum. (D and E) Higher resolution views of the main elements showing positive reaction for the proteins under study, namely the collagenous capsule and an eosinophil infiltrate respectively. In (E) an H/E stain has been included in an inset to show that the infiltrating cells are eosinophils. In each H/E stain in (A–C), the approximate orientation of the host (H)-parasite (P) interface has been indicated. I, mononuclear cell-dominated infiltrate; C, collagen (and fibroblasts); HW, hydatid wall. Scale bars represent 100 μm throughout.
Overall, S100A9 and S100A8 are only weakly expressed at the host-parasite interface in conditions of inflammatory resolution or low inflammation in human hydatid disease. In the situation of existing (low) inflammation, they are most prominently expressed by eosinophils, and also macrophages, neither directly apposed to the parasite. These results are broadly consistent with the presence of small amounts of S100A9 and S100A8, detectable by Western blotting but not readily detected by SDS-PAGE, in an LL extract of human hydatid origin (Fig. 1 C, D).
DISCUSSION
In this work, we detected the phagocyte-specific S100 proteins associated with the HW of E. granulosus developed in different host species. In experimental infection in mice (a species in which S100A12 is absent), S100A9 and S100A8 were among the major host proteins associated with the HW. In cattle, a host species whose genome does encode S100A12, this protein was instead the dominant subfamily member associated with the parasite.
We did not determine what proportion of the S100 proteins in our extracts arose from necrotic deposits and/or epithelioid cells attaching to the LL vs from the interior of the LL itself. The lack of staining of the LL observed by immunohistochemistry (for bovine S100A12 in particular) is not informative, as the LL is remarkably refractory to immunostaining (Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). It should be noted that, in common with other host proteins previously found to be associated with the HW (Díaz et al. Reference Díaz, Ferreira and Sim1997; Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a,Reference Díaz, Willis and Simb), S100A12 and the S100A9/S100A8 dimer are known to bind heparin and related glycosaminoglycans (Robinson et al. Reference Robinson, Tessier, Poulsom and Hogg2002; Liu et al. Reference Liu, Mori, Wake, Zhang, Liu, Izushi, Takahashi, Peng and Nishibori2009). This is also the case of serum amyloid P (Hamazaki, Reference Hamazaki1987; Heegaard et al. Reference Heegaard, He and Blomberg2006), identified in this study as a further major HW-associated protein in mouse infections. Calcium InsP 6 may contribute anionic sites for adsorption of host proteins (Irigoín et al. Reference Irigoín, Laich, Ferreira, Fernández, Sim and Díaz2008), and the calcium-dependent extraction of S100 proteins is compatible with at least part of them being adsorbed onto the calcium InsP 6 deposits. We obtained preliminary evidence indicating adsorption of exogenously added purified human S100A9/S100A8 onto the native, but not the InsP 6-depleted, HW in vitro. However, this result was not robust with respect to changes in the experimental conditions. As complement factor H, a well-known heparin-binding protein, associates with the LL independently of InsP 6 (Irigoín et al. Reference Irigoín, Laich, Ferreira, Fernández, Sim and Díaz2008), we speculate that this structure may bear additional anionic sites independent of InsP 6, perhaps on mucin backbones (Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a).
The phagocyte-specific S100 proteins are amplifiers of inflammation (Ehrchen et al. Reference Ehrchen, Sunderkotter, Foell, Vogl and Roth2009; Pietzsch and Hoppmann, Reference Pietzsch and Hoppmann2009). We found them associated with a parasite characterized by controlling host inflammation (Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). This apparent paradox is reconciled by 2 important considerations. The first of these is that the establishing parasite does elicit inflammation, the cellular remnants of which may persist after inflammatory resolution (Richards et al. Reference Richards, Arme and Bridges1983). This applies to the observations on hydatids from mouse experimental infections. Although we did not study these samples by immunohistochemistry, this type of hydatid is known not to be surrounded by active inflammation (Richards et al. Reference Richards, Arme and Bridges1983). Therefore neutrophils from the reaction against the establishing parasite (Richards et al. Reference Richards, Arme and Bridges1983; Breijo et al. Reference Breijo, Spinelli, Sim and Ferreira1998) are the most likely cellular origin of S100A8 and S100A9. The second consideration is that the inflammatory control exerted by the parasite is not absolute, and a continuum of local response types is observed across natural host species, reaching chronic granulomatous inflammation in non-permissive host species such as cattle (Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). We thus found that S100A12, in particular, was a very abundant host protein in cattle infections, while S100A9 and S1008 were present at much lower levels if at all. S100A8, identified by us as being associated with the HW in low levels in a single sample, has previously been reported to be present in hydatid fluid, in material also from bovine host (Monteiro et al. Reference Monteiro, De Carvalho, Zaha and Ferreira2010).
In the context of granulomatous inflammation as studied in cattle hydatids, the major cell types expressing S100A12 were epithelioid and MGC immediately adjacent to the HW. This result, together with the proteomic data, suggests that the epithelioid and MGC express high levels of S100A12 selectively with respect to S100A9 and S100A8. The possibility that it is only the adsorption of S100A12 onto the HW that is selective is essentially ruled out by the results in the mouse system showing that S100A9 and S100A8 can indeed associate with the HW. In other systems, S100A12 is known to be expressed in granulocytes but not in monocytes or resident macrophages (Vogl et al. Reference Vogl, Propper, Hartmann, Strey, Strupat, Van Den Bos, Sorg and Roth1999a; Pietzsch and Hoppmann, Reference Pietzsch and Hoppmann2009). S100A12 expression in granuloma macrophages, including epithelioid cells and MGC specifically, has been reported, in human systems, in the last few years (Kim et al. Reference Kim, Choi, Choi, Myung and Cho2006; Morbini et al. Reference Morbini, Villa, Campo, Zorzetto, Inghilleri and Luisetti2006; Campo et al. Reference Campo, Morbini, Zorzetto, Tinelli, Brunetta, Villa, Bombieri, Cuccia, Agostini, Bozzi, Facoetti, Ferrarotti, Mazzola, Scabini, Semenzato, Pignatti, Pozzi and Luisetti2007); the expression of S100A9 and S100A8 was not analysed in these works. Other works have reported widely different observations on the expression of S100A9 and S100A8 in these cell types; these observations include co-expression of both, expression of only S100A9, and lack of expression of either (Zwadlo et al. Reference Zwadlo, Bruggen, Gerhards, Schlegel and Sorg1988; Delabie et al. Reference Delabie, De Wolf-Peeters, Van Den Oord and Desmet1990; Aguiar-Passeti et al. Reference Aguiar-Passeti, Postol, Sorg and Mariano1997; Arai et al. Reference Arai, Mizuno, Yamada and Nozawa1999; Sunderkotter et al. Reference Sunderkotter, Tomimori-Yamashita, Nix, Maeda, Sindrilaru, Mariano, Sorg and Roth2004; Kurata et al. Reference Kurata, Terado, Schulz, Fujioka and Franke2005; Terasaki et al. Reference Terasaki, Fujita, Shimomura, Tsukada, Otsuka, Otsuka, Katashima, Ikemoto and Kitaura2007). Also, in a helminth-induced granuloma model in mice (patent S. mansoni infection), S100A9 and S100A8 were found to be expressed by macrophages at the edge of the granuloma but not by the epithelioid and MGCs at the centre of the reaction (Yang et al. Reference Yang, Tzeng, Cheng, Burnett, Yoshizawa, Fukuyama, Lee and Epstein1997). Our results suggest that comparative analysis of all 3 phagocyte-specific S100 proteins in epithelioid and MGCs might show that, at least in some strongly inflammatory contexts, these cells express high levels of S100A12 selectively.
We also found S100A12 to be expressed by fibroblasts, although only in the samples displaying the most intense inflammatory reactions. S100A9 and S100A8 are now known to be expressed in certain non-haematopoetic cells (keratinocytes) in response to the Th17 cytokine IL-22 (Wolk et al. Reference Wolk, Witte, Wallace, Docke, Kunz, Asadullah, Volk, Sterry and Sabat2006), the receptor for which can also be expressed by fibroblasts (Sonnenberg et al. Reference Sonnenberg, Fouser and Artis2010). The possibility that S100A12 expression in our system may be an indication of a Th17 response is further discussed below.
In a human sample featuring an eosinophil infiltrate, we observed strong expression of S100A9 and S100A8 in this cell type. While eosinophil expression of S100A9 and/or S100A8 had not been reported before, S100A12 had been observed in asthma eosinophils, but not eosinophils from normal blood (Yang et al. Reference Yang, Yan, Cai, Tedla, Armishaw, Di Girolamo, Wang, Hampartzoumian, Simpson, Gibson, Hunt, Hart, Hughes, Perry, Alewood and Geczy2007). Therefore all 3 phagocyte-specific S100 proteins can be expressed by eosinophils in appropriate inflammatory contexts.
An early observation on S100A12 was its association with the inflammatory reaction to a tissue-dwelling helminth. In a study with strong parallels to ours, human S100A12 was identified in extracts of the adult stage of the nematode Onchocerca volvulus (Marti et al. Reference Marti, Erttmann and Gallin1996). Noteworthy in relation to our results, S100A8 was detected in this study but at only 20% the concentration of S100A12. As the starting material had been freed of host tissue, S100A12 and S100A8 were deduced to bind the parasite. In another study (previous to the discovery of S100A12), S100A9 and S100A8 were observed to be expressed by (morphologically conventional) inflammatory macrophages adjacent to adult O. volvulus, and apparently secreted onto the worms (Edgeworth et al. Reference Edgeworth, Abiose and Jones1993). In vitro, S100A12 has filariostatic and filaricidal activities, possibly because of binding to nematode paramyosin (Gottsch et al. Reference Gottsch, Eisinger, Liu and Scott1999; Akpek et al. Reference Akpek, Liu, Thompson and Gottsch2002). The possibility that S100A12 is one of the effectors through which the granulomatous response damages Echinococcus larvae deserves further investigation. Echinococcus possesses paramyosin (Muhlschlegel et al. Reference Muhlschlegel, Sygulla, Frosch, Massetti and Frosch1993), and therefore binding to this protein is a conceivable anti-parasite mechanism. An inflammatory response very similar to the chronic granulomatous version of the response to E. granulosus is normally elicited by E. multilocularis (Gottstein and Hemphill, Reference Gottstein and Hemphill1997; Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). It would be worth analysing the expression of S100A12 in the human host response against E. multilocularis.
Although immune responses to helminths have a dominant Th2 profile, other effector response arms, including the highly inflammatory Th17 arm, can contribute to the overall response (Díaz and Allen, Reference Díaz and Allen2007; Ritter et al. Reference Ritter, Gross, Kays, Ruland, Nimmerjahn, Saijo, Tschopp, Layland and Prazeres Da Costa2010). It has been proposed that the granulomatous response to larval Echinococcus has a Th17 component (Vuitton and Gottstein, Reference Vuitton and Gottstein2010; Díaz et al. Reference Díaz, Casaravilla, Allen, Sim and Ferreira2011a). There are strong links between granulomatous responses and Th17 immunity in other contexts (Coury et al. Reference Coury, Annels, Rivollier, Olsson, Santoro, Speziani, Azocar, Flacher, Djebali, Tebib, Brytting, Egeler, Rabourdin-Combe, Henter, Arico and Delprat2008; Rutitzky et al. Reference Rutitzky, Smith and Stadecker2009; Okamoto Yoshida et al. Reference Okamoto Yoshida, Umemura, Yahagi, O'brien, Ikuta, Kishihara, Hara, Nakae, Iwakura and Matsuzaki2010). Two previously known major products of the epithelioid and MGC elicited by E. granulosus, namely cathepsin K and MMP-9 (Díaz et al. Reference Díaz, Ibarguren, Breijo, Willis and Sim2000a; Marco et al. Reference Marco, Baz, Fernández, Gonzalez, Hellman, Salinas and Nieto2006), have reported associations with Th17 responses (Prause et al. Reference Prause, Bozinovski, Anderson and Linden2004; Koenders et al. Reference Koenders, Kolls, Oppers-Walgreen, Van Den Bersselaar, Joosten, Schurr, Schwarzenberger, Van Den Berg and Lubberts2005a,Reference Koenders, Lubberts, Oppers-Walgreen, Van Den Bersselaar, Helsen, Di Padova, Boots, Gram, Joosten and Van Den Bergb; Ivanov et al. Reference Ivanov, Bozinovski, Bossios, Valadi, Vlahos, Malmhall, Sjostrand, Kolls, Anderson and Linden2007). More specifically, S100A12, identified in this study as produced by same epithelioid cells and MGC, is also connected to Th17 responses, as are also S100A8 and S100A9 (Wolk et al. Reference Wolk, Witte, Wallace, Docke, Kunz, Asadullah, Volk, Sterry and Sabat2006; Haider et al. Reference Haider, Lowes, Suarez-Farinas, Zaba, Cardinale, Khatcherian, Novitskaya, Wittkowski and Krueger2008; Loser et al. Reference Loser, Vogl, Voskort, Lueken, Kupas, Nacken, Klenner, Kuhn, Foell, Sorokin, Luger, Roth and Beissert2010). It is therefore worth analysing Th17 responses in the larval Echinococcus system.
ACKNOWLEDGEMENTS
The authors are grateful to Madelón Portela (Unidad de Bioquímica y Proteómica Analíticas, Instituto Pasteur de Montevideo) for expert processing of protein bands for proteomics. They also acknowledge Dr Carolina Arredondo (Departamento de Patología, Facultad de Veterinaria, Uruguay) for assistance with microscopy and Dr Edgardo Berriel (Clínica Quirúrgica 1, Facultad de Medicina, Montevideo, Uruguay) for useful discussions. They are also grateful for Professor Robert B. Sim (Department of Pharmacology, University of Oxford, UK) for critical reading of the manuscript. This work was supported by the Government of Uruguay (A.D., PDT grant no. 54/078) and by the Third World Academy of Sciences (A.D., Research Grant 04-433 RG/BIO/LA).








