Published online by Cambridge University Press: 10 June 2004
The ‘Muguga cocktail’ live vaccine comprises three Theileria parva stocks (Muguga, Kiambu 5 and the buffalo-derived Serengeti-transformed) and has been used extensively in Eastern, Central and Southern Africa with an infection and treatment protocol to protect cattle against East Coast fever. We report the characterization of the three component vaccine stocks using a panel of polymorphic micro-satellite and mini-satellite markers and the development of a stock-derived PCR method that distinguishes two of the vaccine stocks. These markers, with the use of a recently developed Reverse Line Blot assay, have enabled us to address four important questions in relation to vaccination. First, how closely related are the vaccine stocks, secondly do all three stocks persist post-vaccination and induce a carrier state, thirdly is there evidence for the transmission of the vaccine stocks and fourthly does vaccination prevent infection with local genotypes? The results show that Muguga and Serengeti-transformed stocks are highly related but very distinct from Kiambu 5 that persists in vaccinated cattle establishing a carrier state. No evidence was obtained for the transmission of vaccine stocks to co-grazed animals, although these animals were infected with up to 8 different T. parva genotypes showing there was a significant level of tick challenge. Some of the vaccinated animals become infected with a subset of local genotypes providing evidence for limited vaccine ‘breakthrough’.
East Coast fever (ECF), caused by the haemoparasite Theileria parva, is considered to be the most economically important tick-borne disease in eastern, central and southern Africa. The disease is associated with high levels of mortality, especially in exotic (Bos taurus) and crossbred (Bos taurus×Bos indicus) cattle, and is thus a major constraint to increasing livestock production through adoption of genetically improved taurine cattle. At present, apart from the regular use of acaricides to kill ticks, the only effective means of protecting cattle at risk from ECF is by the ‘infection and treatment’ method of immunization. This method of vaccination involves the inoculation of a live, potentially lethal dose of the parasite and simultaneous treatment with a long-acting oxytetracycline (reviewed by Radley, 1981). Since protection against ECF is stock specific, combinations or ‘cocktails’ of stocks that give significant protection throughout East and Central Africa have been developed. The most commonly used such mixture, the ‘Muguga cocktail’, was developed in the 1970s (Radley et al. 1975a,b). The ‘Muguga cocktail’ live vaccine has been administered in several African countries including Uganda and provides significant protection to immunized cattle (see Morzaria & Williamson (1999) for recent summaries). The ‘Muguga cocktail’ live vaccine is composed of three T. parva stocks (Muguga, Kiambu 5 and Serengeti-transformed), which were selected on the basis of cattle cross-immunity trials (Radley et al. 1975a,b). Previously it has been shown, by the use of a combination of anti-schizont monoclonal antibodies, Southern blotting using four T. parva repetitive DNA probes and PCR-based assays detecting polymorphism within four single copy loci encoding antigen genes, that the cattle-derived Muguga and the buffalo-derived Serengeti-transformed components of the cocktail are genetically very similar, while the cattle-derived Kiambu 5 stock was divergent (Bishop et al. 2001).
Live vaccination against ECF by infection with a sporozoite stabilate and simultaneous treatment with long-acting tetracycline induces a carrier state, defined as a persistent tick-transmissible infection, with some T. parva stocks (Kariuki et al. 1995). However, until this study an investigation of the carrier status induced by different components of the ‘Muguga cocktail’ vaccine in a field trial has not been performed. Determining the carrier status of the component stocks of the ‘Muguga cocktail’ vaccine is important since most T. parva stocks appear to induce immunity with a carrier status (Bishop et al. 1992; Kariuki et al. 1995). It is also possible, however, for an infected animal to clear a T. parva infection, suggesting that animals can remain immune in the absence of a carrier state, a condition known as sterile immunity. One of the stocks present in the ‘Muguga cocktail’ vaccine, the T. parva Muguga stock, has previously been demonstrated not to induce a long-term carrier state based on the inability to detect the parasite in the blood by PCR amplification, or through xenodiagnosis by experimental tick application (Bishop et al. 1992; Skilton et al. 2002). In the latter study the relative persistence of T. parva in cattle immunized with T. parva Muguga and T. parva Marikebuni was investigated and it was found that the Muguga stock could be detected by PCR for between 33 and 129 days post-infection; however, the Marikebuni stock could be detected for up to 487 days when the study was terminated.
In this study we address the following questions. (1) How genetically distinct are the three component stocks of the ‘Muguga Cocktail’ vaccine, as assessed using a genome-wide panel of selectively neutral markers, and can these markers be used to distinguish between the 3 vaccine stocks? (2) Do animals remain infected with all the components of the vaccine following immunization with the ‘Muguga Cocktail’? (3) Is there evidence that the vaccine stocks are transmitted to cattle at the vaccine sites? (4) Is the ‘Muguga Cocktail’ vaccine protective against challenge with local parasites or are there parasite genotypes in the field that are able to infect vaccinated cattle?
The T. parva stocks used in this study were obtained as stabilates from the FAO Tick-borne Diseases Vaccine Production Centre in Malawi. The Muguga stabilate 73 (CVL, 1.10.91) was derived from a cattle/tick passage of stabilate 57 (CVL, 9/11.84), which in turn was derived from a tick/cattle passage of stabilate 147 (KARI, 17.9.76). The Kiambu 5 stabilate 68 (CVL, 20.9.89) was derived from a tick/cattle passage of an unspecified stabilate obtained from KARI in May 1987. The Serengeti-transformed stabilate 69 (CVL, 14.9.89) was derived from stabilate 17 (CVL, 23.10.81) that was prepared from ticks obtained from KARI in October 1980. Cell lines were established as described by Bishop et al. (2001) and DNA was isolated from cultured T. parva schizont-infected lymphoblasts as described by Conrad et al. (1987). The T. parva Marikebuni DNA was prepared from a cloned cell line (Morzaria et al. 1995). The ‘Muguga cocktail’ vaccine used in this study was obtained from the FAO Tick-borne Diseases Vaccine Production Centre in Malawi via the ECF Immunisation Project in Entebbe, Uganda.
All the cattle sampled were from the same farm in the Iganga district of central Uganda. The trial was performed between June 2002 and May 2003. The cattle on the farm were cross-bred (African Shorthorn×Friesian) and all the cattle on the farm shared common grazing. The cattle were generally in good condition, were visibly free from ticks and were in good health. The adult cattle were dipped weekly and the calves were sprayed weekly in acaricide (Supona Extra). There were 3 groups of cattle sampled in the study. Group (1): adult unvaccinated cattle. These cattle were more than 4 years old, most were born on the farm and all had been on the farm for at least 4 years. Group (2): cattle vaccinated in previous years. Of the 10 cattle in this group 8 were 2–3 years old and 2 were approximately 18 months old. All the calves in this group had been on the farm since birth. Cattle numbers 2, 3, 5, 6, 8 and 9 were vaccinated with the ‘Muguga Cocktail’ vaccine 24 months prior to sampling, cattle numbers 7 and 10 were vaccinated 18 months prior to sampling and cattle numbers 1 and 4 were vaccinated 12 months prior to sampling. Group (3): calves awaiting vaccination. These calves were 2–8 months old and had been on the farm since birth. The calves had not shown any signs of ECF since birth.
Ten unvaccinated cattle in Group 1 and 10 vaccinated cattle in Group 2 together with 15 calves from Group 3 were bled on the day of vaccination. On the day of vaccination 15 calves in Group 3 were vaccinated with the ‘Muguga cocktail’ vaccine subcutaneously just behind the ear near the parotid lymph node. These calves were simultaneously treated with long-acting oxytetracycline (1 mg/kg) by intramuscular injection. Further blood samples, in EDTA, were taken from the 15 vaccinated calves at 17, 48, 87, 122, 164, 241 and 303 days post-vaccination. Serum samples were taken from the calves both pre-vaccination and 48 days post-vaccination.
Serum samples were assayed for antibodies to T. parva by ELISA using the recombinant polymorphic immunodominant molecule (PIM) as the antigen with percentage positivity (PP) values >15 indicating that an animal had been previously infected with T. parva (Katende et al. 1998).
The reverse line blot (RLB) assay was carried out according to the protocol described by Gubbels et al. (1999) with the modifications described by Oura et al. (2004).
In order to differentiate between the Muguga, Kiambu 5, Serengeti-transformed and Marikebuni stocks PCR amplifications were performed using 50 ng of schizont-infected lymphocyte DNA, Taq polymerase (0·5 Unit) (Amersham), PCR buffer (45 mM Tris–HCl, pH 8·8, 11 mM ammonium sulphate, 4·5 mM magnesium chloride, 4·4 mM EDTA, pH 8, dATP, dCTP, dGTP, dTTP (each at 1 mM), 113 μg/ml BSA), and primers (50 ng) designed from the conserved flanking regions of a panel of mini-and micro-satellite. The panel used included 8 microsatellite (ms 1, 2, 3, 4, 7, 8, 9 and 10) and 23 minisatellite (MS 1, 2, 3, 5, 7, 8, 9, 11, 12, 14 15, 16, 17, 19, 21, 22, 24, 25, 30, 37, 43, 45 and 221). The primer sequences have been described by Oura et al. (2003). The total reaction volume was 10 μl. Thirty cycles were carried out under the following conditions: denaturation at 94 °C for 60 s, annealing at 60 °C for 60 s and extension at 65 °C for 60 s. Products (5 μl of PCR volume) were separated on 1·5% agarose gels in TAE buffer, gels were stained with ethidium bromide and the DNA bands were visualized on a UV light box and photographed. The products were then separated on Spreadex gels to define different alleles at high resolution (Oura et al. 2003).
Forward and reverse outer primers were designed in the conserved 5′ and 3′ regions on the PIM gene (Toye et al. 1995a,b). Muguga forward and reverse as well as Kiambu forward and reverse inner nested primers were designed in the central variable region of the PIM gene. The sequences of these primers are listed below. PIM Forward outer nested primer: ccactggttcttccgatgtaacac, PIM Reverse outer nested primer: attgcccacaaccgtggaatggcg, Muguga forward inner nested primer: ctggacaaggacctgttgaacccg, Muguga reverse inner nested primer: tcggtggttcctgttgctgatcta, Kiambu forward inner nested primer: agatggtcaagattcacaaggaac, Kiambu reverse inner nested primer: ggttgatactgtaatacttgttg.
Both outer and inner nested primers were designed in the flanking region of the mini-satellite repeat. The sequences of the inner nested primers are described in Oura et al. (2003) and the sequences of the outer nested primers are listed below. MS 3 forward outer nested primer: cccgatctcactcacatacaacc. MS 3 reverse outer nested primer: cagcaaatccaactcgtcgtcctg. MS 7 forward outer nested primer: ctcctcagcatcctgctgctcattg. MS 7 reverse outer nested primer: gcgcatgactgcttttacattaaccc. MS 8 forward outer nested primer: ggcgtgacggtaatacaccttcc. MS 8 reverse outer nested primer: cctcctagacactcccgaagatg. MS 16 forward outer nested primer: catggcattcctaggcatcacatc. MS 16 reverse outer nested primer: ccaagggaattaatactgttggag.
DNA was purified from blood samples spotted on to FTA filter paper (Whatman BioScience) following the manufacturer's protocol. A single punch (1·2 mm) from the dried blood sample was placed into a thin-walled PCR tube and the punch was washed 3 times in FTA Purification Reagent and then twice in 10 mM Tris–HCl, pH 8: 0·1 mM EDTA, pH 8 (TE) according to the Whatman FTA single-punch purification for PCR protocol (Whatman Bioscience). The punch was dried for 2 h at room temperature.
PCR amplifications were performed by adding the mastermix described above containing the outer nested primers (50 ng), to the dried punch. Thirty cycles were carried out under the following conditions: denaturation at 94 °C for 60 s, annealing at 60 °C for 60 s and extension at 65 °C for 60 s. Dilutions (1[ratio ]100) of the first round reaction were made and 1 μl of the 1[ratio ]100 dilution was added to the second round PCR reaction along with 10 μl of PCR mastermix described above containing the inner nested primers. Twenty-five cycles were performed under the same PCR conditions as the outer nested PCR. Products (5 μl of PCR volume) were separated on 1·5% agarose gels in TAE buffer, gels were stained with ethidium bromide and the DNA bands were visualized on a UV light box and photographed.
The use of Spreadex gels to define different mini- and micro-satellite alleles of T. parva at high resolution has been described by Oura et al. (2003). Under optimal conditions these gels provide a resolution of 3 base pairs. The running buffer was composed of 30 mM TAE. Following electrophoresis gels were stained in ethidium bromide (0·4 μg/ml) for 40 min and then destained in distilled water for 30 min. Gels were then viewed under UV light (254 nm) and photographed. Precise estimation of the size of the alleles was carried out by direct comparison with the M3 marker (Elchrom Scientific), which contains over 50 DNA fragments between the sizes of 75 and 622 base pairs.
Alleles were sized and designated a letter. The number of loci that differed between each pair of stocks was counted and then entered in the input window in a lower triangular distance matrix format of the Clustering calculator programme (www.biology.uaalberta.ca/jbrzusto/cluster.html). The output in a Phylip readable file was viewed as a dendrogram using Treeview (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
The three component stocks of the ‘Muguga cocktail’ vaccine (Muguga, Kiambu 5 and Serengeti-transformed) were genotyped with 31 mini- and micro-satellite markers previously described by Oura et al. (2003). The cloned Marikebuni stock, which has been evaluated as a live vaccine in Kenya, was also analysed. To differentiate 3–6 base pair size differences between the alleles, the PCR products from the vaccine stocks were run on Spreadex gels and results from a representative selection of markers are shown in Fig. 1A. Mini-satellites MS 7, 25 and 43 identified different-sized alleles between the Muguga and the Serengeti-transformed stocks, whereas mini-satellites MS 30, 37, 45 and micro-satellite ms 9 revealed identically sizes alleles in these two vaccine stocks. Out of the 31 satellite markers 27 revealed identical allele sizes between the Muguga and Serengeti-transformed stocks while 4 mini-satellites (MS 7, MS 19, MS 25 and MS 43) exhibited different-sized alleles. The allele sizes for the Kiambu 5 and the Marikebuni stocks were distinct from each other, with only 3 out of the 31 markers amplifying identical-sized alleles, and also distinct from the Muguga and Serengeti-transformed stocks with only 1 out of the 31 markers amplifying an identically sized allele. A multilocus genotype for each parasite stock was generated by combining the data for each allele at the 31 loci. Each allele was designated a letter corresponding to its size. The number of loci that differed between each pair of stocks was determined and then analysed using the clustering calculator program (see the Materials and Methods section) to produce a dendrogram showing genetic relationships among the 3 stocks present in the ‘Muguga cocktail’ vaccine and also the Marikebuni stock (Fig. 1B). The dendrogram confirms that the Muguga and Serengeti-transformed stocks are genetically similar but not identical and that the Kiambu 5 and Marikebuni stocks are very distinct both from each other and also from the Muguga and Serengeti-transformed stocks.

Fig. 1. (A) Spreadex gels showing PCR products generated using a selection of micro- and mini-satellite primers to amplify DNA from the vaccine stock used in Kenya, Theileria parva Marikebuni (Lane 1), and the 3 component stocks in the ‘Muguga cocktail’ live vaccine, T. parva Muguga (Lane 2), T. parva Serengeti-transformed (lane 3) and T. parva Kiambu (lane 4). (B) Phenogram showing genetic relationships of 4 vaccine stocks. Alleles were sized on Spreadex gels and the number of loci that differed between each pair was entered into a lower triangular distance matrix format of the clustering calculator programme and viewed as a phenogram using Treeview.
Fifteen cross-bred calves were vaccinated with the ‘Muguga Cocktail’ live vaccine as part of a vaccination programme on a farm in Uganda. The antibody titres both on the day of vaccination and on day 48 post-vaccination are shown in Table 1. The vaccinated calves were all negative on the day of vaccination indicating that they had not previously been exposed to T. parva infection. On day 48 post-vaccination 13 out of the 15 vaccinated calves had sero-converted (calves 2 and 12 remained seronegative). All the vaccinated calves were T. parva negative by an RLB assay on the day of vaccination and 12 out of 14 calves sampled (calf number 9 was not presented for sampling at this time-point) were T. parva positive by the same assay on day 17 post-vaccination (calves 2 and 12 remained negative) (Table 1). These data indicate that the 15 vaccinated calves had not been challenged with T. parva prior to vaccination and that 13 of the calves (87%) were successfully vaccinated. Two of the vaccinated calves (numbers 2 and 12) did not sero-convert post-vaccination and remained both seronegative and RLB negative throughout the course of the study. This indicates that these two calves were probably not effectively vaccinated. Before the vaccination programme was introduced on the farm, under a similar weekly acaricide dipping regime, calves frequently showed symptoms of acute ECF indicating that there was sufficient tick challenge to cause clinical symptoms of ECF in the calves. After vaccination the calves remained healthy throughout the course of the trial showing no signs of ECF indicating that the vaccine was inducing protection against local stocks.
Table 1. Summary of sero-conversion of calves (at 48 days post-vaccination), carrier status of Theileria parva by a reverse line blot (RLB) assay and carrier status of the Muguga and Kiambu 5 ‘Muguga cocktail’ vaccine stock components pre-vaccination and at 17, 48, 87, 164 and 303 days post-vaccination (+, Positive signal; −, negative signal; N.T., sample not taken; Mug, Muguga stock; Ki, Kiambu 5 stock; RLB, Reverse line blot.)

An RLB assay was carried out on DNA prepared from blood collected from the 15 vaccinated calves at selected time-points throughout the trial. Results are summarized in Table 1, and Fig. 2 shows a representative selection of the primary RLB blot data used to generate the table. All the calves sampled were T. parva negative prior to vaccination and, on day 17 post-vaccination, 12 out of the 14 calves sampled (86%) were positive for T. parva. The majority of the vaccinated calves remained carriers of T. parva throughout the course of the study with 80% of calves being T. parva RLB positive on day 48 post-vaccination and 77% being positive on day 303 post-vaccination. Two of the calves (number 8 and 9) were RLB positive for T. taurotragi prior to vaccination and remained positive for this species throughout the trial. Calf number 9 was negative for T. taurotragi on day 122 post-vaccination. However, it was positive at all the other time-points throughout the trial (Fig. 2). By day 303 post-vaccination a further 2 calves (numbers 5 and 14) had become positive for T. taurotragi. Throughout the trial none of the calves were infected with T. mutans or T. velifera.

Fig. 2. A reverse line blot (RLB) assay to measure the carrier status of 15 cross-bred calves both pre- and post-vaccination with the ‘Muguga Cocktail’ live vaccine against Theileria parva. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). All the PCR products from DNA corresponding to the oligonucleotides, when added in the control panel on the left, gave positive reactions indicating that the oligonucleotides were correctly hybridized to the blot. The PCR products from the 15 calves were added to lanes 1–15. Time-points pre-vaccination and on day 17, day 48, day 122, day 164 and day 303 post-vaccination are shown. On day 17 post-vaccination calf number 9 was not sampled and on day 303 post-vaccination calf numbers 8 and 15 were not sampled.
The RLB assay revealed the carrier status of the vaccinated cattle for T. parva but was unable to distinguish whether the cattle were carrying the T. parva vaccine stocks or local stocks resulting from tick challenge. In order to detect the carrier status for the vaccine stocks, Muguga and Kiambu 5-derived primers were designed from the variable central region of the PIM gene (see Materials and Methods section). The primers were tested against a panel of T. parva stocks and were found to be specific for Muguga and Kiambu 5 respectively (data not shown). Due to the complicated repeats structure present in the central variable region of the PIM gene it was only possible to design Kiambu 5-derived primers that amplified 3 products differing in size by 129 base pairs. This resulted in the generation of a ladder of amplification products in the Kiambu 5-derived samples resulting in products of 80, 209 and 338 base pairs in size. The PIM gene in the Muguga and the Serengeti-transformed stocks is identical in nucleotide sequence (data not shown) so it was impossible to differentiate between these two stocks and both generated identical products of 300 base pairs. DNA was prepared from blood samples taken from the calves at different time-points post-vaccination and the primers derived from either the Muguga/Serengeti or the Kiambu 5 vaccine stocks were used to PCR amplify the PIM gene in order to define the presence or absence of these stocks. A sample of the results is shown in Fig. 3, demonstrating the strain-specific products separated by agarose gel electrophoresis and all the results are summarized in Table 1. The 15 vaccinated calves were negative for both vaccine stocks and calf numbers 2 and 12 remained negative throughout the study, in agreement with the RLB and ELISA results for these animals. On day 17 post-vaccination, 11 of the calves were positive for the Muguga/Serengeti stock (79%) and 5 were positive for the Kiambu stock (36%). At 48 days post-vaccination 3 (20%) of the calves were positive for Muguga/Serengeti, whereas 8 (53%) were positive for Kiambu 5. At 87 days post-vaccination all the calves were negative for the Muguga/Serengeti stock (data not shown) and, apart from the Muguga/Serengeti stock appearing in calf number 10 at day 241 and day 303 post-vaccination, the other calves remained free of these stocks for the rest of the trial period. The two PCR products seen with the Muguga/Serengeti derived primers at day 122 post-vaccination in calf number 11 (weak bands) and at day 303 post-vaccination in calf numbers 11 and 14 represent a local genotype. These ‘breakthrough’ genotypes are also seen at these time-points in Fig. 6. By day 303 post-vaccination 8 (62%) of the remaining calves were positive for the Kiambu 5 stock. All of the calves that were positive for Kiambu 5 at 48 days post-infection remained positive for Kiambu 5 until the end of the trial (303 days post-vaccination). Interestingly, calf number 1 showed a delayed response to infection with the Kiambu 5 stock that was first seen at 122 days post-vaccination, was present 164 and 241 days post-vaccination (data not shown) but was negative at the 303 days time-point.

Fig. 3. Agarose gels (1%) showing PCR products amplified from DNA extracted from the blood of 15 calves (lanes 1–15) both pre-vaccination and on day 17, day 48, day 122 and day 303 post-vaccination. On day 17 post-vaccination calf number 9 was not sampled and on day 303 post-vaccination calf numbers 8 and 15 were not sampled. PCR products amplified with Muguga-derived primers (left panel) and Kiambu 5-derived primers (right panel) are shown. Negative controls (−) using no DNA and positive controls (+) using either Kiambu 5 or Muguga DNA are run on the right-hand lanes of the gels.
In summary, of the 13 calves that were successfully vaccinated (the original 15 vaccinated calves minus calf numbers 2 and 12 that did not sero-convert). All, apart from calf number 10, were positive for the Muguga/Serengeti stock at 17 days post-vaccination but this stock could not be detected in any of the calves beyond 87 days post-vaccination. In contrast, 70% of these calves were infected with the Kiambu 5 stock at 122 days post-vaccination and 90% of these calves remained carriers of this stock for at least 303 days post-vaccination when the trial was terminated. These data suggest that the persistence of the carrier state differs markedly between the different T. parva stocks that make up the ‘Muguga Cocktail’ vaccine.
An RLB assay was carried out on DNA purified from blood taken from 10 cattle vaccinated in previous years with the ‘Muguga cocktail’ live vaccine. These cattle shared grazing and were from the same farm as the calves sampled in the vaccine trial. Cattle numbers 2, 3, 5, 6, 8 and 9 were vaccinated 24 months prior to sampling, cattle 7 and 10 were vaccinated 18 months prior to sampling and cattle 1 and 4 were vaccinated 12 months prior to sampling. Of the previously vaccinated cattle 6 were RLB positive for T. parva and 4 were negative (Fig. 4A). To investigate the carrier status of these cattle further, FTA-purified DNA from blood taken from the 10 vaccinated cattle was PCR-amplified using the Kiambu 5 and Muguga/Serengeti-derived primers (Fig. 4B). No products of a similar size to those from the Muguga were observed with the Muguga/Serengeti-derived primers (left panel) indicating that the Muguga stock is no longer present in these cattle. PCR amplification with the Kiambu 5-derived primers showed that samples 2, 4, 7 and 10 produced a 3-band amplification pattern consistent with the presence of Kiambu 5 (right panel). Sample 6 revealed bands of different size to that expected for Kiambu 5, indicating that a local genotype was present in this animal. This indicates that 4 of the cattle vaccinated up to 2 years previously remained carriers of the Kiambu 5 stock. In order to confirm the presence of the Kiambu 5 stock in long-term vaccinated cattle, FTA-purified DNA was PCR amplified with primers derived from 6 polymorphic mini- and micro-satellite markers. Fig. 4C shows the separation of PCR products amplified from DNA purified from blood samples taken from 5 cattle that were RLB positive. Animal number 8 was positive on the RLB assay but amplified a PCR product with only 2 out of the 6 mini- and micro-satellite primers, indicating that the concentration of parasite DNA in this sample was at the threshold of PCR detection. The 4 remaining cattle that were RLB negative were also negative on PCR amplification with the mini- and micro-satellite markers (data not shown). A PCR product of an identical size to the Kiambu 5 vaccine stock was seen in samples 2, 4, 7 and 10 (the Kiambu-specific bands were sometimes weak in sample 2) and no PCR products of similar size to the Muguga stock were seen. Sample 2 was infected with at least 2 local genotypes in addition to Kiambu 5.

Fig. 4. Carrier status of 10 cattle vaccinated in previous years. (A) Reverse line blot (RLB) assay. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). The PCR products from the 10 calves were added to lanes 1–10. (B) Agarose gel (1%) showing PCR products amplified from DNA extracted from the blood of the 10 cows (lanes 1–10) with Muguga/Serengeti-derived primers (left panel) and Kiambu 5-derived primers (right panel). Positive controls using either Kiambu 5 or Muguga DNA are run on the righthand lanes of the gels (labelled +), M, 50 bp marker. (C) Spreadex gels showing PCR products amplified using 5 mini- and micro-satellite primers to amplify DNA extracted from the blood of the 5 vaccinated cattle that were RLB positive (not including sample 8) for T. parva (Cows 2, 4, 6, 7, and 10). PCR amplifications with Kiambu 5 (K), and Muguga (M) DNA are run on the right-hand lanes.
In summary, 4 out of 10 of the cattle are apparently not infected with either vaccine stocks nor additional genotypes, 4 of the cattle are carriers of Kiambu 5 and 8 out of 10 of the cattle are not infected by local genotypes of T. parva. Two of the cattle (numbers 2 and 6) were carrying local genotypes of T. parva that possessed a very different multilocus genotype to the vaccine stocks. Animal number 2 was carrying at least 2 local genotypes and animal number 6 was carrying a third non-vaccine genotype.
An RLB assay was performed on DNA purified from blood taken from 10 adult unvaccinated cross-bred cattle that had shared grazing with cattle that had been vaccinated 12–24 months previously and therefore would have potentially been exposed to the ‘Muguga Cocktail’ vaccine stock components. Seven of the cattle were RLB positive for T. parva and 3 were negative (Fig. 5A). The unvaccinated adult cattle appeared to have a higher carrier prevalence of T. mutans (80%), T. taurotragi (50%) and T. velifera (50%) compared to the vaccinated group shown in Fig. 4A T. mutans (20%), T. taurotragi (20%) and T. velifera (20%) although, due to the low numbers of cattle involved, it is unclear whether the difference is significant. To address the question of whether vaccine stocks can be transmitted to unvaccinated cattle on the farm, PCR reactions using the Muguga/Serengeti and Kiambu 5-derived primers, were carried out on FTA-purified DNA from blood taken from the 10 unvaccinated cattle. PCR products were generated with both sets of primers, but these were not the same size as the Muguga/Serengeti-derived PCR product (Fig. 5B, right panel) and the Kiambu 5-derived PCR products (Fig. 5B, left panel). This indicates that local genotypes of T. parva are present in these cattle but the Muguga and Kiambu 5 vaccine stocks are absent. In order to confirm the presence of local genotypes of T. parva and the absence of the vaccine stocks in the 10 unvaccinated cattle, FTA-purified DNA from the 7 unvaccinated RLB-positive cattle (numbers 3, 4, 6, 7, 8, 9 and 10) along with the DNA from the 3 vaccine stocks was PCR amplified with primers from 4 polymorphic mini-satellite markers (MS 3, 7, 8, 16) and the resultant PCR products were separated on Spreadex gels (Fig. 5C). MS 16 amplified identically sized products with the Muguga and the Serengeti-transformed stocks. Multiple bands were generated in all the samples, indicating that the cattle are infected with many different genotypes of T. parva and that there is a high degree of diversity amongst these T. parva genotypes. The allele sizes of the vaccine stocks, run to the right of the gels, are different to the alleles amplified from the cattle indicating no evidence of vaccine stocks being passed to the unvaccinated cattle on the farm. The degree of multiplicity of infection seen in the unvaccinated cattle (Fig. 5C) was consistently higher than that observed in the vaccinated cattle (Fig. 4C).

Fig. 5. Carrier status of 10 unvaccinated cattle. (A) Reverse line blot (RLB) assay. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). The PCR products from the 10 cows were added to lanes 1–10. (B) Agarose gel (1%) showing PCR products amplified from DNA extracted from the blood of the 10 cows (lanes 1–10) with Kiambu 5-derived primers (left panel) and Muguga/Serengeti-derived primers (right panel). Positive controls using either Kiambu 5 or Muguga DNA are run on the right-hand lanes of the gels (labelled +), M, 50 bp marker. (C) Spreadex gels showing PCR products amplified using 4 mini-satellite primers to amplify DNA extracted from the blood of the 7 unvaccinated cattle that were RLB positive for Theileria parva (Cows 3, 4, 6, 7, 8, 9 and 10). PCR amplifications with Kiambu 5 (K), and Muguga (M) and Serengeti-transformed (S) DNA are run on the right-hand lanes (Serengeti-transformed stock is not run with MS 16 but amplifies an identically sized PCR product to the Muguga stock).
Allele size variation at the mini-satellite MS 7 locus allows differentiation between the Muguga and Serengeti-transformed vaccine component stocks, as well as discrimination from other T. parva genotypes. FTA-purified DNA was PCR amplified from blood taken from the vaccinated calves at all sample points throughout the trial, using MS 7 mini-satellite primers. The results obtained using the MS 7 primers (Fig. 6) correlated well with those produced using the Muguga/Serengeti and Kiambu derived primers (Fig. 3). At 17 days post-vaccination 9 out of the 12 calves that were RLB positive generated a product of similar size to the Serengeti-transformed stock and a fragment of similar size to the Muguga stock was also amplified from 4 of these samples. At 48 days post-vaccination blood samples from 5 of the calves amplified a product of similar size to both the Serengeti-transformed and the Muguga stocks, but beyond 87 days post-vaccination no PCR products of similar size to the Muguga or Serengeti transformed stocks were produced. Thus, although more calves were positive for the Serengeti-transformed stock than the Muguga stock at 17 days post-vaccination, the kinetics of the carrier state induced by these two stocks was similar in that neither was detectable by PCR amplification at 87 days post-vaccination and beyond. This was in marked contrast to the Kiambu 5 stock that was carried in the majority of cattle up until the end of the trial at 303 days post-vaccination.

Fig. 6. Spreadex gels showing PCR products amplified using MS 7 mini-satellite primers to amplify DNA extracted from the blood of 15 calves (lanes 1–15) both pre-vaccination and on day 17, day 48, day 122, day 241 and day 303 post-vaccination. PCR amplifications with Kiambu 5 (K), Muguga (M) and Serengeti-transformed (S) DNA are run on the right of the gel.
Throughout the vaccine trial there was evidence for infection of vaccinated cattle by local genotypes of T. parva, since PCR amplification with mini-satellite markers showed alleles of different size to the vaccine stocks (Fig. 6). The emergence of possible ‘breakthrough’ local genotypes in the cattle is summarized in Table 2. Genotypes of T. parva with different-sized alleles to the vaccine stocks, that may have ‘broken through’ the immunity induced by vaccination were observed in 7 of the vaccinated calves (numbers 6, 8, 9 10, 11, 14 and 15) at varying time-points between 17 and 303 days post-vaccination (Table 2). Amplification products seen at day 17 post-vaccination may represent the ‘challenge’ genotypes infecting the cattle from tick challenge. These did not persist, possibly as a result of the immunity provided by vaccination. It is also possible that the unique genotypes seen on day 17 post-vaccination were present in the ‘Muguga Cocktail’ vaccine and that the vaccine stabilate contains more genotypes than previously known. Interestingly, there was evidence for a possible resurgence or reinfection of vaccine stocks at late time-points during the trial in calf number 10, that amplified a band of similar size to the Serengeti-transformed stock at 241 days post-vaccination, and calf number 5, that amplified bands of similar size to the Serengeti-transformed and the Muguga stocks on day 303 post-vaccination (Fig. 6). Although there is evidence of local genotypes breaking through the vaccine, these genotypes did not cause overt disease in the calves indicating that the vaccine is protective.
Table 2. ‘Breakthrough’ of local genotypes of Theileria parva (+, Local stock present; −, local stock absent; dpv, Days post-vaccination; N.T., Sample not taken.)

The results presented describe the application of a panel of mini- and micro-satellite markers to characterize stocks used in the immunization of cattle against ECF. We have characterized the 3 component stocks of the ‘Muguga Cocktail’ vaccine (Muguga, Kiambu 5 and Serengeti-transformed), which has been used extensively in vaccination programmes in Malawi, Tanzania and Uganda. We show, using a comprehensive genome-wide panel of markers that the Muguga and Serengeti-transformed stocks are genetically similar but not identical, with 4 out of 31 mini- and micro-satellite markers useful for differentiating between the two vaccine stocks. In addition we have shown that the kinetics of persistent infection are also similar with these two stocks. Our results are in agreement with a recent study in which anti-schizont monoclonal antibodies, Southern blotting using 4 T. parva repetitive DNA probes and PCR-based assays detecting polymorphism within 4 single-copy loci encoding antigen genes showed that the Muguga and Serengeti-transformed components of the cocktail are genetically and phenotypically very similar (Bishop et al. 2001). It is surprising that a cattle-derived stock (Muguga) and a buffalo-derived stock (Serengeti-transformed) appear to be so similar, as it has previously been shown that stocks directly isolated from buffalo are highly diverse, and that only a limited subset of these stocks can be transmitted by ticks between cattle (Conrad et al. 1987, 1989; Allsopp et al. 1989, reviewed by Bishop et al. 2002). In a recent study carried out with 17 mini- and micro-satellite markers, the diversity of T. parva stocks from different African countries, and from an individual farm in Kenya, were compared and these cattle-derived stocks were compared to 2 buffalo-derived stocks. The buffalo-derived stocks were found to be both distinct from each other and also distinct from the cattle-derived stocks (Oura et al. 2003). Many of the same mini- and micro-satellite markers were used in this current study in which we have found the cattle-derived Muguga stock and the buffalo-derived Serengeti-transformed stock to be genetically very similar. Therefore, it is possible that the Serengeti-transformed stock has been lost from the ‘Muguga cocktail’ vaccine and the vaccine probably now contains 2 different passages of the Muguga stock that are genetically very similar, as well as the Kiambu 5 stock. It would be interesting to carry out a mini/micro-satellite analysis of the original Serengeti-transformed stabilate component of the Muguga Cocktail vaccine in order to prove that this stock has been lost from the present ‘Muguga cocktail’ vaccine, but this has not been possible.
As part of an immunization programme, using the ‘infection and treatment’ live vaccination method to control ECF in Uganda, we have evaluated the efficacy of live vaccination of calves in the field in Uganda in relation to the carrier state. This involved monitoring the long-term carrier status of the component stocks of the vaccine using PCR-based techniques that we have developed. Both anti-PIM ELISA (at 48 days post-vaccination) and RLB analysis (at 17 days post-vaccination) showed that 13 out of 15 of the calves (87%) appeared to have been successfully vaccinated. Two of the calves failed to sero-convert following vaccination and also remained RLB negative throughout the course of the study. If these animals were successfully immunized this suggests that neither the generation of an antibody response against PIM, nor the induction of a carrier state of the parasite, is required for induction of the cellular immunity, that is believed to underpin the infection and treatment live vaccination method (McKeever et al. 1994). However, it is likely, given the lack of sero-conversion, that vaccination was unsuccessful in these calves, although they appeared resistant to local challenge. Although the cattle on the farm were dipped weekly with acaricide, there was strong evidence that a significant T. parva challenge remained on the farm. Before the vaccination programme was introduced on the farm, under a similar weekly acaricide dipping regime, calves frequently showed symptoms of acute ECF. Also unvaccinated cattle on the farm were infected with many genotypes of T. parva.
It was not possible to differentiate between the Muguga and the Serengeti-transformed stocks, using primers designed from the PIM locus in vaccinated cattle as the PIM sequences of these two stocks were identical. It was, however, possible to design stock-specific primers that differentiated between the Muguga/Serengeti stocks and the Kiambu 5 stock, based on PIM. Using these primers we found that both the Muguga and Serengeti-transformed stocks induce a short-term carrier state and are not detectable in any of the vaccinated cattle after day 87 post-vaccination. The Kiambu 5 stock, however, induces a long-term carrier state and all the vaccinated cattle that were PCR positive for the Kiambu 5 stock at 48 days post-vaccination were still infected with this stock, according to PCR data, at 303 days post-vaccination. This suggests that the persistence of the carrier state is markedly different between the different components of the ‘Muguga Cocktail’ vaccine. The induction of a carrier state in cattle immunized by the infection and treatment method has been shown previously using the T. parva Marikebuni stock and animals shown to be carriers at 3 and 7 months post-immunization by xenodiagnosis, based on both examination of the salivary glands of ticks, and also transmission to uninfected cattle (Kariuki et al. 1995). A more recent study on cattle experimentally infected with the Muguga and the Marikebuni stocks showed that a long-term carrier state was induced by infection with the Marikebuni but not with the Muguga stock (Skilton et al. 2002). In summary, the ‘Muguga Cocktail’ probably consists of 2 major distinct components, one of which (Kiambu 5) results in a long-term carrier state in cattle and the other (Muguga) results in a short-term carrier status. The consequences of this for the induction of immunity by infection and treatment by live vaccination constitutes an important area for future research.
There was considerable individual variation in the induction of a carrier state in animals by the different T. parva vaccine stocks. Two of the vaccinated calves, although infected with the Muguga stock at 17 days post-vaccination, did not appear to carry the Kiambu 5 stock at any of the time-points throughout the trial. All the successfully vaccinated calves (with the exception of calf number 10) were infected with Muguga/Serengeti stock at 17 days post-vaccination although calf number 10 was a carrier for the Kiambu 5 stock at this time-point, indicating that it was successfully vaccinated. Interestingly, the Muguga stock was detectable at day 241 post-vaccination in this calf. Possible explanations are either that the calf was infected with the Muguga stock at a level below the threshold of PCR detection for the 241 days post-vaccination and then it re-emerged at a detectable level, or the calf was infected by field ticks with a T. parva genotype that is genetically identical to the Muguga stock. The question of the detection threshold of the assay used to measure the carrier status of the cattle is important in this context and the sensitivity of the RLB assay for the detection of the T. parva has been measured and compared to a nested mini-satellite PCR assay (Oura et al. 2004). It is possible that the calves in this study that are RLB and PCR negative may still be infected with the parasite but at a level below the threshold of detection for the assay.
In order to evaluate possible evidence for local genotypes of T. parva breaking through the immunity induced by vaccination, PCR analysis was performed with primers flanking the mini-satellite MS 7 locus that can differentiate between the 3 component stocks of the ‘Muguga Cocktail’ vaccine (Muguga, Kiambu 5 and Serengeti-transformed) and also local genotypes. The 15 calves sampled prior to vaccination were all both T. parva antibody and PCR negative indicating that they had not been challenged with T. parva. However, on day 17 post-vaccination, MS 7 alleles of different sizes to any of the vaccine stocks were observed from the blood of 3 of the calves, indicating the possible presence of local T. parva genotypes. At this early stage post-vaccination the vaccine would not be protective so these genotypes cannot be classified to have ‘broken through’ the vaccine. Another possibility is that the unique genotypes seen on day 17 post-vaccination were present in the ‘Muguga Cocktail’ vaccine that contains more genotypes than previously known. This could be resolved by analysing the ‘Muguga Cocktail’ vaccine stabilate itself and looking for extra genotypes or experimental vaccination of animals, kept in tick-free conditions, and analysis at day 17 post-vaccination. In 4 of the vaccinated calves, genotypes of T. parva with different-sized alleles to the vaccine stocks, appeared at varying time-points between 48 and 303 days post-vaccination. These genotypes must have ‘broken through’ the immunity induced by vaccination and can be classified as ‘breakthrough’ genotypes. None of the calves that were infected with local genotypes showed signs of overt disease. These data indicate that the ‘Muguga Cocktail’ vaccine is not 100% protective against the challenge from local parasites in Uganda and suggest that the genetic heterogeneity we have detected among T. parva populations in this area is also reflected in diversity in the parasite antigens that are responsible for protective immunity.
We have used a panel of mini- and micro-satellite markers to characterize the component stocks of the ‘Muguga cocktail’ live vaccine. We conclude that 2 of the component stocks (Muguga and Serengeti-transformed) are genotypically and phenotypically very similar, the extent of these differences being at the level that might be expected between different passages of clones within a single stock. When the ‘Muguga cocktail’ was used in a vaccine trial 87% of the cattle sero-converted; however, the persistence of the carrier state was markedly different between the Muguga/Serengeti and the Kiambu 5 components. The Muguga and Serengeti-transformed stocks showed a short-term carrier status before being cleared by day 87 post-infection, whereas the Kiambu 5 stock was carried for the duration of the trial (303 days). We reveal evidence for limited breakthrough of local T. parva genotypes in the vaccinated cattle indicating that the ‘Muguga Cocktail’ vaccine is not 100% effective in protecting cattle in Uganda against the challenge from local genotypes of T. parva. The ‘breakthrough’ genotypes, however, did not appear to cause disease, indicating that the vaccinated cattle may be partially protected from infection with these genotypes.
C. A. L. Oura was funded on a Wellcome Trust Tropical Research Fellowship. We thank Mr J. B. Isabirye for kindly allowing us to bleed the cattle on his farm in the trial. We also thank the District Veterinary Officer Dr Xavier Magirigi for his help.

Fig. 1. (A) Spreadex gels showing PCR products generated using a selection of micro- and mini-satellite primers to amplify DNA from the vaccine stock used in Kenya, Theileria parva Marikebuni (Lane 1), and the 3 component stocks in the ‘Muguga cocktail’ live vaccine, T. parva Muguga (Lane 2), T. parva Serengeti-transformed (lane 3) and T. parva Kiambu (lane 4). (B) Phenogram showing genetic relationships of 4 vaccine stocks. Alleles were sized on Spreadex gels and the number of loci that differed between each pair was entered into a lower triangular distance matrix format of the clustering calculator programme and viewed as a phenogram using Treeview.

Table 1. Summary of sero-conversion of calves (at 48 days post-vaccination), carrier status of Theileria parva by a reverse line blot (RLB) assay and carrier status of the Muguga and Kiambu 5 ‘Muguga cocktail’ vaccine stock components pre-vaccination and at 17, 48, 87, 164 and 303 days post-vaccination

Fig. 2. A reverse line blot (RLB) assay to measure the carrier status of 15 cross-bred calves both pre- and post-vaccination with the ‘Muguga Cocktail’ live vaccine against Theileria parva. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). All the PCR products from DNA corresponding to the oligonucleotides, when added in the control panel on the left, gave positive reactions indicating that the oligonucleotides were correctly hybridized to the blot. The PCR products from the 15 calves were added to lanes 1–15. Time-points pre-vaccination and on day 17, day 48, day 122, day 164 and day 303 post-vaccination are shown. On day 17 post-vaccination calf number 9 was not sampled and on day 303 post-vaccination calf numbers 8 and 15 were not sampled.

Fig. 3. Agarose gels (1%) showing PCR products amplified from DNA extracted from the blood of 15 calves (lanes 1–15) both pre-vaccination and on day 17, day 48, day 122 and day 303 post-vaccination. On day 17 post-vaccination calf number 9 was not sampled and on day 303 post-vaccination calf numbers 8 and 15 were not sampled. PCR products amplified with Muguga-derived primers (left panel) and Kiambu 5-derived primers (right panel) are shown. Negative controls (−) using no DNA and positive controls (+) using either Kiambu 5 or Muguga DNA are run on the right-hand lanes of the gels.

Fig. 4. Carrier status of 10 cattle vaccinated in previous years. (A) Reverse line blot (RLB) assay. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). The PCR products from the 10 calves were added to lanes 1–10. (B) Agarose gel (1%) showing PCR products amplified from DNA extracted from the blood of the 10 cows (lanes 1–10) with Muguga/Serengeti-derived primers (left panel) and Kiambu 5-derived primers (right panel). Positive controls using either Kiambu 5 or Muguga DNA are run on the righthand lanes of the gels (labelled +), M, 50 bp marker. (C) Spreadex gels showing PCR products amplified using 5 mini- and micro-satellite primers to amplify DNA extracted from the blood of the 5 vaccinated cattle that were RLB positive (not including sample 8) for T. parva (Cows 2, 4, 6, 7, and 10). PCR amplifications with Kiambu 5 (K), and Muguga (M) DNA are run on the right-hand lanes.

Fig. 5. Carrier status of 10 unvaccinated cattle. (A) Reverse line blot (RLB) assay. Species-specific oligonucleotide probes were applied to the horizontal rows of the RLB and are shown to the left of the blot (T/B catch-all – Theileria/Babesia catch-all, T – Theileria). The PCR products from the 10 cows were added to lanes 1–10. (B) Agarose gel (1%) showing PCR products amplified from DNA extracted from the blood of the 10 cows (lanes 1–10) with Kiambu 5-derived primers (left panel) and Muguga/Serengeti-derived primers (right panel). Positive controls using either Kiambu 5 or Muguga DNA are run on the right-hand lanes of the gels (labelled +), M, 50 bp marker. (C) Spreadex gels showing PCR products amplified using 4 mini-satellite primers to amplify DNA extracted from the blood of the 7 unvaccinated cattle that were RLB positive for Theileria parva (Cows 3, 4, 6, 7, 8, 9 and 10). PCR amplifications with Kiambu 5 (K), and Muguga (M) and Serengeti-transformed (S) DNA are run on the right-hand lanes (Serengeti-transformed stock is not run with MS 16 but amplifies an identically sized PCR product to the Muguga stock).

Fig. 6. Spreadex gels showing PCR products amplified using MS 7 mini-satellite primers to amplify DNA extracted from the blood of 15 calves (lanes 1–15) both pre-vaccination and on day 17, day 48, day 122, day 241 and day 303 post-vaccination. PCR amplifications with Kiambu 5 (K), Muguga (M) and Serengeti-transformed (S) DNA are run on the right of the gel.
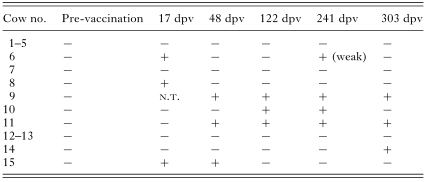
Table 2. ‘Breakthrough’ of local genotypes of Theileria parva