INTRODUCTION
Leishmania parasites cause a broad spectrum of diseases in man and other animals, ranging from self-curing cutaneous leishmaniasis (CL) to fatal visceral leishmaniasis (VL). The latter, caused by Leishmania donovani or L. chagasi (Syn: L. infantum) that infects macrophages of the liver, spleen and bone marrow, is responsible for one fourth of the 2 million new cases of human leishmaniasis that occur each year, and an estimated 60 000 deaths worldwide (Kaye and Aebischer, 2011).
So far, there is no vaccine approved for any type of human leishmaniasis, but acquisition of immunity following subclinical or resolved infection supports the notion that protective vaccination is feasible (Carvalho et al. Reference Carvalho, Teixeira and Johnson1981; Badaro et al. Reference Badaro, Jones, Carvalho, Sampaio, Reed and Barral1986). LACK (Leishmania homologue of receptors for activated C kinase) is one of the most promising antigens for the development of a vaccine not only because it is highly conserved among all Leishmania species and developmental parasite stages, but also because of its immunopathogenic role in murine L. major infection (Gonzalez-Aseguinolaza et al. Reference Gonzalez-Aseguinolaza, Taladriz, Marquet and Larraga1999; Julia and Glaichenhaus et al. Reference Julia and Glaichenhaus1999). However, the type of infection, antigen form, and the route of administration have been proved to be critical for vaccine efficacy. Intravenous (i.v.), subcutaneous (s.c.) and intranasal (i.n.) immunizations with recombinant LACK protein failed to prevent the onset and progression of CL in BALB/c mice (McSorley et al. 1998; Okuno et al. Reference Okuno, Takeuchi, Matsumoto, Otsuka and Matsumoto2002; Coelho et al. Reference Coelho, Tavares, Carvalho, Chaves, Teixeira, Rodrigues, Charest, Matlashewski, Gazzinelli and Fernandes2003; Pinto et al. Reference Pinto, Pinheiro, Rayol, Larraga and Rossi-Bergmann2004; Benhnini et al. Reference Benhnini, Chenik, Laouini, Louzir, Cazenave and Dellagi2009), whereas plasmid vectors carrying the LACK gene given by the s.c. or intramuscular (i.m.) routes were effective (Mendez et al. Reference Mendez, Belkaid, Seder and Sacks2002). As to VL caused by L. donovani or L. chagasi/infantum, no protection was achieved in mice with s.c. or i.m. pCI-neo-LACK (Melby et al. Reference Melby, Yang, Zhao, Perez and Cheng2001; Marques-Da-Silva et al. Reference Marques-Da-Silva, Coelho, Gomes, Vilela, Masioli, Tavares, Fernandes, Afonso and Rezende2005), unless live viral vectors are used in the booster dose (Ramos et al. Reference Ramos, Alonso, Marcen, Peris, Castillo, Colmenares and Larraga2008; Gonzalo et al. Reference Gonzalo, del Real, Rodriguez, Rodriguez, Heljasvaara, Lucas, Larraga and Esteban2002).
The nasal mucosa presents an attractive, non-invasive route for the delivery of DNA vaccines. Aiming at developing a non-viral and needle-free vaccine against CL and VL, we have successfully used this route to administer pCI-neo-LACK (Pinto et al. Reference Pinto, Pinheiro, Rayol, Larraga and Rossi-Bergmann2004; Gomes et al. Reference Gomes, Pinto, de Melo, Lima, Larraga, Lopes and Rossi-Bergmann2007). BALB/c mice challenged with L. amazonensis or L. chagasi as early as 1 week after the booster dose developed significantly milder infections that were accompanied by the expansion of mixed Th1 and Th2-type cells in the lesion-draining lymph nodes and spleens, respectively, indicating that active immunity rather than tolerance to LACK was involved. Compared to parenteral routes, little is known or has been done in order to understand the long-term immunity development and maintenance induced by Ag (soluble or particulate) and DNA given via the mucosal route, critically important for effective vaccine development and vaccination strategies against leishmaniasis or other diseases (Guan et al. Reference Guan, Wen, Deng, Zhang, Chen, Wu, Ruan and Tan2011; Matsuo et al. Reference Matsuo, Koizumi, Akashi, Nakagawa, Fujita, Yamamoto and Okada2011).
Unlike sterile cure, the persistence of low parasite numbers at the infection primary site, ensured by regulatory T cells (Mendez et al. Reference Mendez, Reckling, Piccirillo, Sacks and Belkaid2004) was associated with the protective and durable immunity driven by IFN-α-producing CD4 T cells in human or mice experimental models (Muller, 1992; Aebischer et al. Reference Aebischer, Moody and Handman1993; Sassi et al. Reference Sassi, Louzir, Ben Salah, Mokni, Ben Osman and Dellagi1999; Sacks and Noben-Trauth, 2002); however, the molecular or immunological mecanisms associated with lasting immunity development are still unknown.
In the present study, we aimed to investigate and showed for the first time the systemic biodistribution of intranasally administered pCI-neo-LACK by detection of specific mRNA, and its association with memory duration/protection against L. chagasi infection, key to understanding the factors related to vaccine-induced immunity and needed for vaccine to be considered effective.
MATERIALS AND METHODS
Animals
BALB/c mice were originally purchased from Jackson Laboratory (Bar Harbor, Maine, USA). They were bred and maintained at our own facilities, using sterilized bedding, filtered water and pelleted food. Female animals were used at 4–6 weeks of age. The experimental protocols were approved by the Ethical Committee for Experimental Animal Use established in the Federal University of Rio de Janeiro.
Parasites and antigens
Leishmania chagasi strain MHOM/BR/1974/M2682 amastigotes were routinely isolated from the spleens of infected mice and cultured at 25 °C as promastigotes in DMEM medium pH 6·8 supplemented with 20% heat-inactivated fetal bovine serum, 2 mm l-glutamine, 25 mm HEPES, and 20 μg/ml of gentamicin (herein named DMEM 20% HIFCS). For L. chagasi antigen (LcAg), late-log-phase culture promastigotes were centrifuged, washed 3 times in phosphate-buffered saline (PBS) and disrupted by 3 rounds of freezing and thawing. Protein content was determined by the Lowry method. The recombinant LACK antigen was a kind gift of Dr Vicente Larraga (Centro de Investigaciones Biologicas, Madrid, Spain). For pCI-neo-LACK, the gene encoding the p36 Leishmania infantum LACK protein was inserted downstream of the cytomegalovirus promoter in the EcoRI/XbaI site of the pCI-neo expression vector (Promega), as described (Gonzalez-Aseguinolaza et al. Reference Gonzalez-Aseguinolaza, Taladriz, Marquet and Larraga1999). Endotoxin-free control and LACK-encoding plasmids were isolated using the EndoFreePlasmid Mega kit (Qiagen) according to the manufacturer's instruction.
Vaccination
Mice were i.n. vaccinated with pCI-neo-LACK as previously described in (Gomes et al. Reference Gomes, Pinto, de Melo, Lima, Larraga, Lopes and Rossi-Bergmann2007). Briefly, animals held upright received 10 μl of PBS containing 15 μg of pCI-neo-LACK in each nostril. Controls received PBS alone or empty pCI-neo-plasmid. A booster dose was given 7 days later.
Infection and parasite burden
One week, 3 months or 6 months after the booster vaccine dose, the animals were infected by the i.v. route with 107L. chagasi promastigotes at the stationary phase of growth. On day 30 of infection the parasite burden in each liver and spleen was determined by Limiting Dilution Assay, as described by Marques-Da-Silva et al. (Reference Marques-Da-Silva, Coelho, Gomes, Vilela, Masioli, Tavares, Fernandes, Afonso and Rezende2005). Briefly, each organ was weighed and homogenized in DMEM 20% HIFCS. Serial dilutions of single-cell suspensions were cultured for 12 days at 25 °C. The original number of parasites in each organ was calculated from the reciprocal of the highest dilution containing promastigotes.
Cutaneous hypersensitivity reaction
On day 1 of i.v. infection vaccinated and non-vaccinated mice were injected in the hind footpad with 20 μg of LcAg in 20 μl of PBS. Footpad swelling was measured with a dial caliper, and the results were expressed as the difference between the thickness of the injected and pre-injected footpads.
Cytokines
Thirty days after infection, single-cell suspensions were prepared from spleens and incubated at 37 °C in 24-well flat-bottomed plates at 5 × 106 cells/ml in DMEM 10% HIFCS supplemented with 50 μ m 2-mercaptoetanol in the presence or absence of LcAg (50 μg/ml), rLACK (5 μg/ml) or medium alone for 72 h. The cytokine production in the cell-culture supernatants was determined by ELISA following the manufacturer's instructions (R & D Systems, Minneapolis, USA).
mRNA-LACK expression
At 1 week, 1 month, 2 months, 3 months, or 4 months after vaccination, mRNA-LACK was measured in the spleen, cervical lymph nodes (cLN), popliteal lymph nodes (pLN) and brain by RT-PCR. The total RNA was extracted from each organ using a Trizol® reagent and the cDNA prepared using a First-Strand cDNA synthesis kit. The PCR amplification was performed using specific primers for LACK (Forward (ATGAACTACGAGGGTCACCT) and Reverse (ACGTCCTTGGTGTGCTTCA) followed by electrophoresis on 1·5% agarose. Actin was used as endogenous control (Forward (GTTGCTATCCAGGCTGTGC) and Reverse (GCATCCTGTCGGCAATGC) for indirect quantitation of LACK-mRNA expression using gel band densitometry.
Statistics
Data were statistically analysed using the Prisma software. Means of normally distributed variables were compared by ANOVA analysis simple factorial test and by one-way ANOVA-Tukey's honestly significant difference (Tukey's HSD) post-hoc method and were considered significantly different when P < 0·05
RESULTS
Systemic expression of mRNA following intranasal administration of pCI-neo-LACK
The biodistribution of plasmids was evaluated at 1 week, 1, 2, 3 or 4 months post-vaccination in nasal-associated and peripheral organs. LACK-mRNA was detected by RT-PCR not only in the cervical lymph nodes but also in the spleen, popliteal lymph nodes and brain from 7 days up to 3 months (Fig. 1A). LACK-mRNA expression reached a maximum at 1 month in all organs, particularly cervical lymph nodes, being still detectable after 3 months, mainly in the popliteal lymph nodes. At 4 months, LACK-mRNA was undetectable in all the organs examined (Fig. 1B). Actin was used as an endogenous reference and showed normal expression in all organs at all times (data not shown).

Fig. 1. Expression of mRNA transcripts in peripheral organs after different times of vaccination. Mice received 2 i.n. doses of 30 μg of pCI-neo-LACK with 1 week interval. After the indicated times of the last dose, the total RNA was extracted from the spleen, cervical lymph nodes (cLN), popliteal lymph nodes (pLN) and brain. After synthesis from RNA, cDNA was amplified by RT-PCR. (A) 50 μl of cDNA samples from individual mice (3/group) were applied onto a 1·5% agarose gel yielding the 349 bp amplicons. As reference, 1 kb size marker was applied. The intensity of the bands in (A) and their respective actin bands (not shown) were quantitated by densitometry, and the results expressed as the ratio between LACK and actin (B). Means±s.d. (n = 3), *P <0·05 as compared with spleen. The data are representative of 3 independent experiments.
Intranasal vaccination with pCI-neo-LACK primes animals for cutaneous hypersensitivity
The cutaneous reaction to locally injected L. chagasi antigen was assessed as an index of cell-mediated immune response. Mice pre-immunized with pCI-neo-LACK and challenged in the footpad with antigen 1 week or 3 months, but not 6 months, later exhibited a significant (P < 0·05) swelling as a compared to PBS and empty pCI-neo controls (Fig. 2). The swelling measured at 18 h in all groups referred to the injected volume, whereas the reaction seen after 24 h is typical of a delayed-type hypersensitivity response. These results show that the intranasal vaccination with pCI-neo-LACK leads to a Th1-type immunity in the footpad that is maximum at 3 months and coincides with mRNA expression in the popliteal lymph nodes seen in Fig. 1.
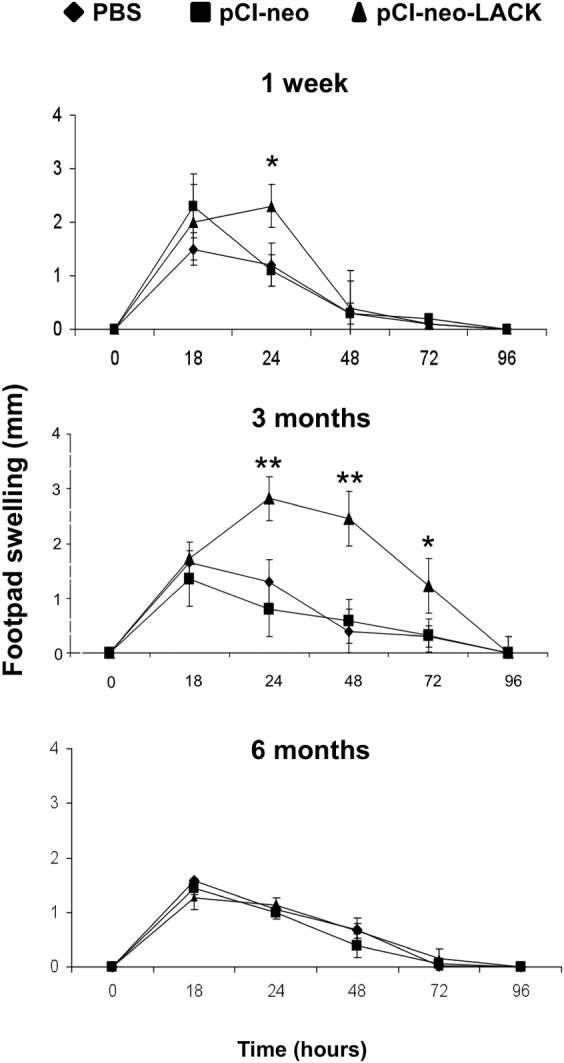
Fig. 2. Cutaneous hypersensitivity response after different times of vaccination. Mice received 2 i.n. doses of 30 μg of pCI-neo-LACK; 30 μg of pCI-neo plasmid; or 20 μl of PBS alone with 1 week interval. After 1 week, 3 months or 6 months of vaccination, the animals were i.v.-challenged with Leishmania chagasi, and on the following day they were s.c.- injected in the footpad with 20 μg of LcAg. The footpad swelling indicative of cutaneous hypersensitivity to parasite antigens was measured with a dial caliper at the indicated times. Arithmetic means±s.d. (n = 8). * P<0·05, ** P<0·01 as compared with pCI-neo controls.
Efficacy duration of vaccination with pCI-neo-LACK
At different times after vaccination, mice were infected with L. chagasi by the i.v. route. The differential parasite burdens seen in non-vaccinated control groups (PBS) at the different post-vaccination times is likely due to variations in parasite infectivity at the time of infection. Taking the PBS groups as a reference, animals given pCI-neo-LACK 1 week or 3 months earlier exhibited significantly (P < 0·05) lower parasite burden in both the liver and spleen, as compared with control groups (Fig. 3). The milder infection was compatible with their healthy appearance, contrary to the prostrate appearance of animals receiving empty plasmid or PBS. However, the protective immunity conferred by pCI-neo-LACK waned within the period of 3–6 months, as animals vaccinated 6 months earlier showed parasite burdens similar (P > 0·05) to non-vaccinated controls.

Fig. 3. Parasite burden in mice infected after different times of vaccination. Mice were vaccinated as described for Fig. 2. After 1 week, 3 months or 6 months of the booster dose, the animals were infected i.v. with 107 promastigotes of Leishmania chagasi. The parasite burden in individual organs was measured on day 30 of infection by limiting-dilution culture. Arithmetic means±s.d. (n = 6). *P <0·05 as compared with pCI-neo controls. Data is representative of 3 independent experiments.
Relationship between T cell response and protection induced by vaccination
To establish the critical immune responses associated with protection, splenocytes were obtained from infected mice and re-stimulated in vitro with LcAg or rLACK antigens. Their capacity to undergo blastogenesis and to produce key cytokines was used as a parameter of activation. We found that cells from animals vaccinated for 1 week or 3 months, but not 6 months prior to infection strongly proliferated in vitro after re-stimulation with either LcAg or rLACK (Fig. 4). No significant (P > 0·05) antigen-specific proliferative response was seen at any time-point in cells from control pCI-neo vector or PBS groups. When cytokines were measured in the cell supernatants, increased production of IFN-γ was observed at 1 week and 3 months, but not at 6 months post-vaccination with pCI-neo-LACK for (Fig. 5). Moreover, in mice challenged with parasites 3 months post-pCI-neo-LACK-vaccination, an increased production of IL-4 along with decreased IL-10 was found in the spleen (Fig. 5). After 6 months post-vaccination, animals were unable to suppress an IL-10 response upon infection challenge. The significant increase in IL-4 production by all groups infected at 6 months of vaccination, including the non-vaccinated PBS, may be due to their milder infection challenge relative to the groups at 1 week and 3 months after immunization rather than to vaccination itself.

Fig. 4. Parasite-specific lymphoproliferative response in mice infected after different times of vaccination. Mice (n = 6) were vaccinated and infected after the indicated times, as in Fig. 3. On day 30 of infection, their spleen cells were stimulated in vitro with LcAg (50 μg/ml); recombinant LACK protein (5 μg/ml); or medium alone, and the lymphoproliferative response was determined by 3H-thymidine incorporation. Arithmetic means±s.d. (n = 3). *P <0·05, **P <0·01 as compared with the respective PBS controls.

Fig. 5. Parasite-specific cytokine response in mice infected after different times of vaccination. Mice (n = 6) were vaccinated and infected after the indicated times, as in Fig. 3. On day 30 of infection their spleen cells were stimulated in vitro with LcAg (50 μg/ml); recombinant LACK protein (5 μg/ml); or medium alone, and IFN- γ, IL-4 and IL-10 were measured in the supernatants by ELISA. Arithmetic means±s.d. *P <0·05, **P <0·01 as compared with the respective PBS controls.
DISCUSSION
In this study, we demonstrated that naked pCI-neo-LACK plasmid is absorbed after intranasal vaccination and expressed not only in local but also in critical peripheral lymphoid organs to induce protective immunity against leishmaniasis. LACK transcripts were found in all tissues analysed, namely the cervical and popliteal lymph nodes, spleen and brain. Conventional RT-PCR was sensitive enough to detect expression for as long as 3 months after vaccination. LACK expression in the spleen and popliteal lymph nodes is of relevance for VL and CL, respectively, and may have accounted for protection achieved in our previous studies, where the mice were challenged i.v. with L. chagasi (Gomes et al. Reference Gomes, Pinto, de Melo, Lima, Larraga, Lopes and Rossi-Bergmann2007) and in the footpad with and L. amazonensis (Pinto et al. Reference Pinto, Pinheiro, Rayol, Larraga and Rossi-Bergmann2004) Studies using pCMVβ plasmid encoding the gene for β-galactosidase (Oh et al. 2003) or pVAX1 plasmid encoding an anti-caries DNA construct (Liu et al. Reference Liu, Fan, Xu and Li2008) showed mRNA expression in a number of organs including brain as early as 15 min after intranasal administration, decreasing after 1 month. Our demonstration that LACK-mRNA is also expressed in the brain is compatible with previous studies showing that plasmid DNAs up to 14·1 kb in size can be delivered to the brain by a direct transport to the olfactory bulb via the olfactory epithelium (Han et al. Reference Han, Kim, Byun, Hwang, Kim, Hwang, Park, Jung, Chun, Jeong and Oh2007), as the pCI-neo-LACK used here measures 6·4 kb. Since the brain is an immunologically privileged organ that normally tolerates the introduction of an antigen without eliciting an inflammatory response (Suter et al. Reference Suter, Biollaz, Gatto, Bernasconi, Herren, Reith and Fontana2003), a transient LACK expression in the brain may not be of relevance.
The way intranasal pCI-neo-LACK primes the immune system for anti-leishmanial immunity is not clear. Since LACK and its mammalian homologue RACK are cytosolic proteins (Gonzalez-Aseguinolaza et al. Reference Gonzalez-Aseguinolaza, Taladriz, Marquet and Larraga1999; Mochly-Rosen et al. Reference Mochly-Rosen, Khaner and Lopez1991), it is feasible that transfection of antigen presenting cells lead to endogenous protein synthesis, processing in the proteasomes and presentation of MHC class I-restricted epitopes to effector CD8+ T cells.
In leishmaniasis, it is widely accepted that IL-12 leading to IFN-γ production is critical for protection, whereas IL-13/IL-4 and IL-10 are key cytokines in disease progression (Cummings et al. Reference Cummings, Tuladhar and Satoskar2010). In the present work the inability of i.n. pCI-neo-LACK vaccination to sustain protection for up to 6 months was likely due to loss of the suppressive control over IL-10 production, despite the elevated IFN-γ production at this stage. These results indicate that the effective immunity conferred by pCI-neo-LACK vaccination is more a function of reduced IL-10 than increased IFN-γ. We also found a positive correlation between increased IL-4 and protection in animals infected 3 months after vaccination. Contrary to CL caused by L. major where IL-4 is known as a disease exacerbating cytokine (Locksley et al. Reference Locksley, Heinzel, Holaday, Mutha, Reiner and Sadick1991), in the VL model IL-4 is necessary to optimize a Th1 response by IFN- γ production during chemotherapy (Alexander et al. Reference Alexander, Carter, Al-Fasi, Satoskar and Brombacher2000) and vaccination (Basu et al. Reference Basu, Bhaumik, Basu, Naskar, De and Roy2005).
Although peripherally expressed antigens may be critical for immune sensitization against leishmaniasis, the contribution of mucosal priming should also be considered, as stronger expression of LACK transcripts were found in the cervical lymph nodes. The observation that non-genic i.n. vaccination with Leishmania amazonensis antigens (LaAg) comprising LACK (Pinto et al. Reference Pinto, Cortezia and Rossi-Bergmann2003) or lipophosphoglycan (LPG) (Pinheiro et al. Reference Pinheiro, Pinto, Guedes, Agrellos, de Mattos, Saraiva, De Mendonca and Rossi-Bergmann2007) can also generate peripheral immunity against L. amazonensis infection further supports the notion that mucosal sensitization is important for successful vaccination.
In general, mucosal vaccination presents many advantages over s.c. and i.m. routes as it is needle-free, painless, and requires less-stringent sterile conditions. Besides, i.n. delivery of plasmids has been shown to be superior to i.m. delivery in terms of biodistribution, intensity of antigen expression and memory duration (Asanuma et al. 2007). Those characteristics may be critical for the differential outcome of L. chagasi infection when pCI-neo-LACK is used for protective i.n. as compared with non-protective i.m. vaccination (Melby et al. Reference Melby, Yang, Zhao, Perez and Cheng2001; Marques-Da-Silva et al. Reference Marques-Da-Silva, Coelho, Gomes, Vilela, Masioli, Tavares, Fernandes, Afonso and Rezende2005) but the possibility that sensitization of the nasal-associated immune system drives protective responses in the periphery, e.g. suppression of parasite-specific IL-10 production, should also be considered.
Other studies using non-mucosal vaccines, either protein (Koutsonanos et al. Reference Koutsonanos, Vassilieva, Stavropoulou, Zarnitsyn, Esser, Taherbhai, Prausnitz, Compans and Skountzou2012) or DNA (Tang et al. Reference Tang, Yin, Tian, Huang, Shi, Fu, Wang, Wu, Hao and Ni2011) on their own have also shown short-term protection in mice. We found that as long as antigenic stimulation is present, there seems to be protection against the disease, probably due to constantly stimulated effector cells. Mucosal vaccination has emerged as a successful alternative for parenteral vaccination against leishmaniasis (Pinto et al. Reference Pinto, Cortezia and Rossi-Bergmann2003, Reference Pinto, Pinheiro, Rayol, Larraga and Rossi-Bergmann2004; Gomes et al. Reference Gomes, Pinto, de Melo, Lima, Larraga, Lopes and Rossi-Bergmann2007). Due to the easiness of application and transient expression, the present i.n. pCI-neo-LACK vaccine may be of value to people visiting an endemic area. Strategies involving appropriate adjuvants or slow-release delivery systems, such as biodegradable nanoparticles that improve targeting and DNA transfection of antigen-presenting cells will likely promote extended protection, and render the vaccine also suitable for people living in an endemic area.







