Introduction
From a parasite's perspective, their hosts represent nutrient- and energy-rich ‘islands’ within an energy-low, and often hazardous, environment. Getting from one host to another constitutes a challenging transmission event for the parasite, especially for parasites with complex life cycles that include a wide spectrum of different hosts. As a result, parasites have evolved several adaptations to improve their chances of transmission success (Poulin, Reference Poulin1998). One strategy is to multiply asexually in an intermediate host and produce many genetically identical dispersal stages in order to increase the odds that at least a few individuals will reach the next intermediate or definitive host (Poulin, Reference Poulin2011; Poulin and Lagrue, Reference Poulin and Lagrue2015). This strategy seems to be especially suitable for direct transmission in aquatic habitats where asexually produced free-living stages can disperse and seek out potential target hosts. Consequently, this ability has evolved in digenetic trematodes of which most life cycles are at least partially aquatic (Cribb et al. Reference Cribb, Bray, Olson and Littlewood2003). In the first intermediate mollusc host, numerous free-living dispersal stages, or cercariae, are produced asexually. After leaving their first intermediate host, the cercariae disperse and seek out their next host, which, depending on the trematode species, can be an invertebrate or vertebrate, ranging from crustaceans, insects, fish, amphibians or reptiles to mammals. The transmission success of the short-lived cercarial stage is a key determinant of the parasite's fitness (Koehler et al. Reference Koehler, Brown, Poulin, Thieltges and Fredensborg2012), and therefore subject to strong selective pressure. Consequently, depending on the life strategies and the host the parasite has to reach, a huge diversity in size, shape and behaviour of trematode cercariae has evolved over time (Esch et al. Reference Esch, Barger and Fellis2002; Koehler et al. Reference Koehler, Brown, Poulin, Thieltges and Fredensborg2012).
Time is a crucial factor for the successful transmission of the short-lived cercariae that usually have a maximum survival of 24–72 h, depending on the species and environmental conditions (Morley, Reference Morley2012). Moreover, the functional life span during which the cercariae are infective is usually much shorter (Thieltges and Rick, Reference Thieltges and Rick2006). In order to enhance the chances of reaching a suitable host during this short transmission window, cercariae are released from their snail host according to emergence rhythms (Galaktionov and Dobrovolskij, Reference Galaktionov and Dobrovolskij2003) that are timed with the target host's activity pattern, placing the cercariae in the ‘host time’ (Combes et al. Reference Combes, Fournier, Moné and Théron1994). Upon their release, individual cercariae exhibit species-specific behaviours to locate and infect a suitable host. Depending on geo- and phototactic orientation, cercariae will migrate to places where they are most likely to encounter their respective hosts, the ‘host space’ (Combes et al. Reference Combes, Fournier, Moné and Théron1994; Morley, Reference Morley2012). In closer proximity to a potential host organism, tactile, visual and chemical cues allow the cercariae to seek out and penetrate a suitable host individual (Haas, Reference Haas2003).
Over the years, several experimental studies have looked at these species-specific dispersal patterns and elaborate host-searching behaviours of cercarial stages in their aquatic environments. More specifically, studies have investigated the swimming behaviour of cercariae in response to light and gravity (e.g. Feiler and Haas, Reference Feiler and Haas1988; Platt et al. Reference Platt, Burnside and Bush2008), their vertical and horizontal distribution in the water column (e.g. Mouritsen, Reference Mouritsen2001; McCarthy et al. Reference McCarthy, Fitzpatrick and Irwin2002; Haas et al. Reference Haas, Beran and Loy2008), and their reaction to thermal, chemical and tactile cues for host-finding and invasion (reviewed in Haas, Reference Haas2003), as well as swimming speed (Feiler and Haas, Reference Feiler and Haas1988; Fingerut et al. Reference Fingerut, Zimmer and Zimmer2003) and, more recently, swimming efficiency (Krishnamurthy et al. Reference Krishnamurthy, Katsikis, Bhargava and Prakash2016). However, these host-finding behaviours have only been analysed in detail for relatively few species (Haas, Reference Haas2003). Moreover, many study designs pool together cercariae to assess their microhabitat distribution at a certain point in time (e.g. horizontal and vertical distribution, McCarthy et al. Reference McCarthy, Fitzpatrick and Irwin2002), which does not allow to assess individual cercarial distributional patterns. Besides being time-consuming, observations of individual cercariae over a continuous period of time are difficult, and accurate data on parameters such as velocity, acceleration and the distance travelled per unit time are hard to obtain, and have only rarely been quantified (e.g. Feiler and Haas, Reference Feiler and Haas1988; Santos et al. Reference Santos, Karvonen, Pedrot, Faltýnková, Seppälä and Valtonen2007). Therefore, on a finer scale, we still have an extremely limited understanding of the interspecific variation in transmission strategies among many parasite species, which presumably reflects adaptations to particular conditions and selective pressures. Moreover, the intraspecific variation, i.e. the variation of individual-level traits of cercariae, has only rarely been included in assessments (e.g. Fingerut et al. Reference Fingerut, Zimmer and Zimmer2003; Santos et al. Reference Santos, Karvonen, Pedrot, Faltýnková, Seppälä and Valtonen2007). Altogether, this remains a serious shortcoming, since the question of how parasites move from one host to another remains a fundamental issue that can help shed some light on more basic biological questions (Thomas et al. Reference Thomas, Adamo and Moore2005).
In the current study, we apply a novel approach, using the motion tracking software EthoVision XT (Noldus Information Technologies), to provide a detailed analysis of the movement and behavioural traits of individual cercariae of four different species. Although other studies have used specialized video tracking approaches, such as laser illumination and high-speed cameras (Fingerut et al. Reference Fingerut, Zimmer and Zimmer2003; Krishnamurthy et al. Reference Krishnamurthy, Katsikis, Bhargava and Prakash2016), to the best of our knowledge, this is the first approach using a widely available off-the-shelf software package that allows easily reproducible and comparable studies. We selected four common trematode species of the mud-snail Potamopyrgus antipodarum (Gray 1843) that serves as an important first intermediate host to a diverse fauna of trematodes in New Zealand freshwaters (Hechinger, Reference Hechinger2012; Lagrue and Poulin, Reference Lagrue and Poulin2015): Coitocaecum parvum (Opecoelidae), Maritrema poulini (Microphallidae), Apatemon sp. (Strigeidae) and Aporocotylid sp. I (Aporocotylidae). These four species belong to different families and represent different trematode life histories with two target host groups, either fish or crustaceans (Table 1). We predict that the cercariae of these four species exhibit different movement and behavioural patterns, reflecting their individual host-searching strategies, based on their respective target hosts’ ecology and distribution. Specifically, our aims are (i) to assess, quantify and illustrate the differences in movement, velocity and acceleration, as well as the active/resting time, of the individual cercariae of the different species, and (ii) to demonstrate the broad applicability of the motion tracking software to the study of cercarial movement.
Table 1. Overview of the trematode species used in the swimming assessment, their life cycle and approximate cercarial size and morphotype

Materials and methods
Snails, P. antipodarum, were collected from macrophytes, sediment and stones along the shoreline of Lake Waihola, New Zealand (46°01′14S, 170°05′05E) using dip nets. Snails were brought back to the laboratory and separated into individual wells of 24-well plates with small amounts of filtered lake water and incubated for 24 h at 20 °C under a light source to induce cercarial shedding and identify patent trematode infections. Cercariae were identified based on morphological features, using the keys of Winterbourn (Reference Winterbourn1973), Hechinger (Reference Hechinger2012) and Presswell et al. (Reference Presswell, Blasco-Costa and Kostadinova2014). We isolated snails infected with the following trematode species: C. parvum (Opecoelidae), M. poulini (Microphallidae), Apatemon sp. (Strigeidae) and Aporocotylid sp. I (Aporocotylidae) (Table 1, Fig. 1). Infected snails were grouped according to their trematode species and maintained in aquaria with aerated lake water and aquatic plants for food at a constant temperature (16 °C) and under a 12/12 h light/dark photoperiod until the beginning of the experiments.
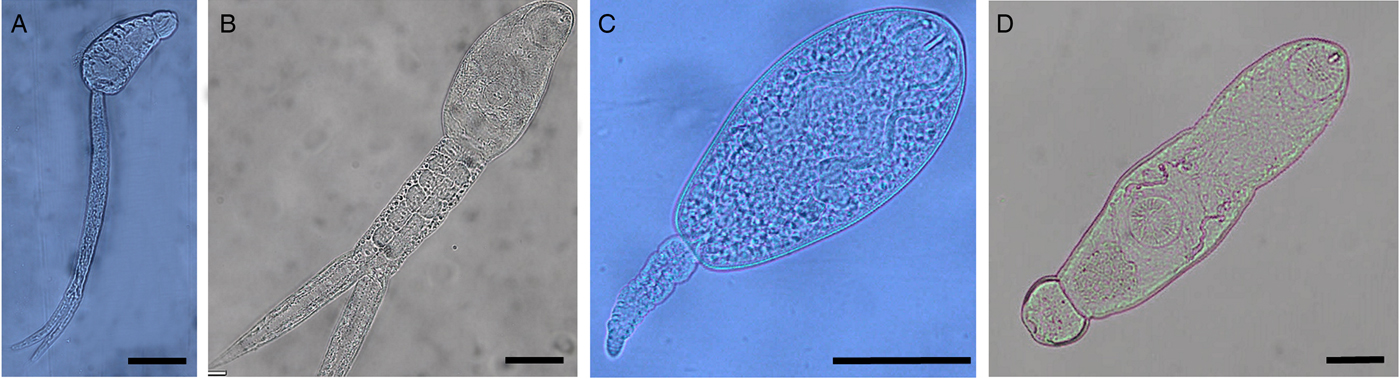
Fig. 1. Cercariae of trematode species used in the behavioural assessment. (A) Aporocotylid sp. I; (B) Apatemon sp.; (C) Maritrema poulini; (D) Coitocaecum parvum. Scale bars: 50 µm.
In order to obtain cercariae for the swimming assessment, infected snails were placed in a small Petri dish containing 2 mL of filtered lake water and incubated in a temperature chamber at 16 °C under constant illumination for 2 h. Since cercarial activity and infectivity decreases with age, all cercariae used in the experiments were of the same ‘age’ (<2 h). For the experiment, active cercariae were haphazardly selected and individually transferred to a microscope well slide containing a measured volume of filtered lake water at 16 °C. The water volume had to be adjusted according to the size of the cercariae of the different species (Table 1) and the required magnification rate, so that the cercaria could move freely while being recorded. The larger cercariae of C. parvum and Apatemon sp. were placed in 100 µL of water (magnification 0.8×), whereas the smaller cercariae of Aporocotylid sp. I and M. poulini were placed in 35 µL (1.2× magnification) and 25 µL (1.5× magnification), respectively. To ensure cercarial movement was not constrained and cercariae could move freely in the selected volumes of water, preliminary trials with different volumes of water were conducted and cercarial behaviour was observed. The volumes of water chosen for the experiments allowed the cercariae to move horizontally while the camera could record their movement; vertical movement was restricted in the selected water volumes, ensuring that cercariae remained in focus. Previous studies on cercarial mobility have found measurements in small containers or cuvettes to reflect the movement and distribution in the field (Santos et al. Reference Santos, Karvonen, Pedrot, Faltýnková, Seppälä and Valtonen2007; Haas et al. Reference Haas, Beran and Loy2008).
The well slide containing a single cercaria was transferred onto the dissecting microscope and left for a 2 min acclimatization period. Subsequently, each specimen was filmed and recorded for 5 min under a dissecting microscope (Olympus SZ61) fitted with a top-mounted Olympus DP25 camera. Lighting was provided by a SZX2-ILLT circular light unit to avoid unidirectional illumination and potential effects on cercarial phototaxis. For each trematode species, at least 17 individual cercariae were recorded for 5 min (300 s) this way, resulting in a dataset of at least 85 min of host-searching behaviour per parasite species. Based on the videos, the individual movement and behavioural patterns were automatically tracked using EthoVision XT 11.5 (Noldus Information Technologies). The programme distinguishes between the tracking subject and background based on grey values and contrast or colour values of the pixels in each video frame (see Noldus et al. Reference Noldus, Spink and Tegelenbosch2001). In detail, the following movement and behavioural patterns were analysed and quantified: total horizontal distance moved (mm), maximum velocity (mm s−1), maximum acceleration (mm s−2) and time spent moving vs time not moving (s). For the last variable, a subject was considered to be moving when it exceeded a start velocity of 0.04 mm s−1, and considered not moving when the velocity was below 0.02 mm s−1. Heatmaps and track plots of the cercarial movement patterns were created for each specimen to illustrate the behaviour of individual cercariae during the 5 min recording.
Statistical analyses were performed with PAST 3.15 (Hammer et al. Reference Hammer, Harper and Ryan2001) to test for differences between the movement patterns of the different species. In detail, we tested for statistically significant differences between species regarding their total horizontal distance moved, maximum acceleration and maximum velocity. Since data were normally distributed (Jarque–Bera test), analyses of variance (one-way ANOVA) and Tukey's pairwise post hoc tests were used to compare sample means. Boxplots were created to illustrate and compare intra- and interspecific variation of total distance moved, maximum acceleration and maximum velocity.
Results
The mean time of footage from which cercarial movement could be captured ranged from 295.1 to 298.9 s per 300 s video, allowing an accurate assessment of the individual cercarial movement patterns and a comparison between the different species (Table 2). The mean time cercariae spent moving ranged from 63.1% in Aporocotylid sp. I and 81% in C. parvum to 94.6 and 98.8% in M. poulini and Apatemon sp., respectively. The total distance moved, maximum velocity and maximum acceleration showed clear differences between the different cercarial morphotypes, with the furcocercariae being faster swimmers and moving greater distances (Table 2, Fig. 2). The ANOVA revealed significant differences between the different species regarding their distance moved (ANOVA: F 3, 66 = 422.3, P < 0.0001), velocity (F 3, 66 = 142.3, P < 0.0001) and acceleration (F 3, 66 = 153.5, P < 0.0001). Post hoc comparisons (Tukey's pairwise post hoc test) showed that distance moved differed among all groups (all P < 0.001), except between C. parvum and M. poulini (P = 0.893). Likewise, there were significant differences between all species regarding their maximum velocity and acceleration (all P < 0.001), except between Apatemon sp. and Aporocotylid sp. I (0.853 and 0.962, respectively). The heatmaps and tracks generated from the recorded videos illustrate the species-specific behavioural patterns of individual cercariae (Fig. 3). These images suggest that the proportion of the total area available that is actually explored by cercariae may also differ among the four species.
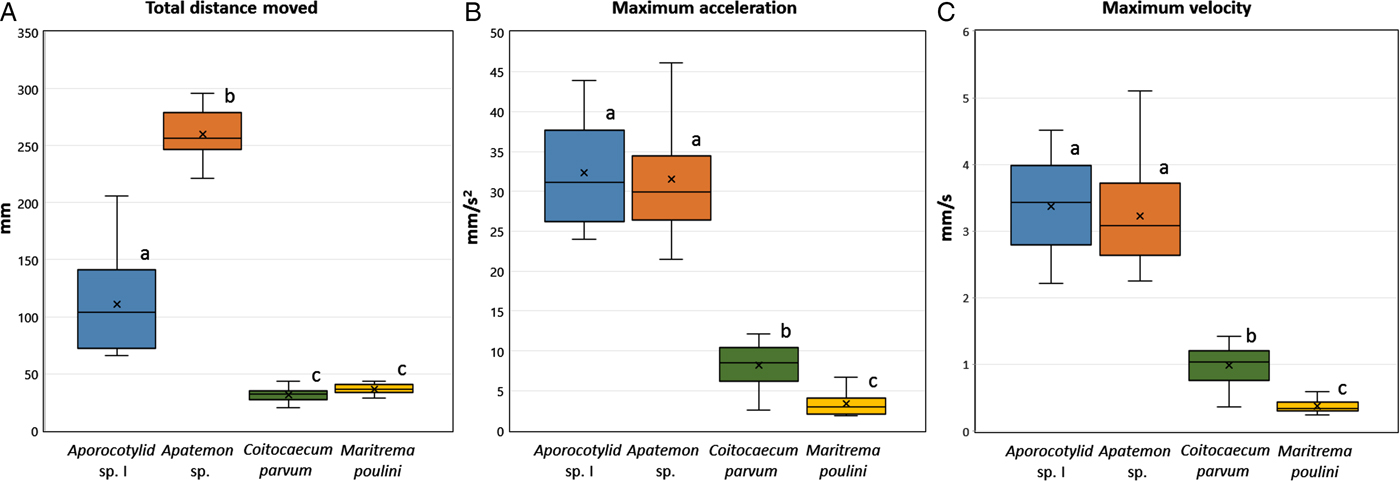
Fig. 2. Boxplots showing (A) the total distance moved during the 5 min recording, (B) the maximum acceleration and (C) the maximum velocity of the different trematode species. Each box comprises the interquartile range, whiskers indicate minimum and maximum values, the line across the box shows the median, and the × indicates the mean. Different letters above the individual boxplots (a–c) denote statistically significant differences between species (P < 0.05).

Fig. 3. Heatmaps and tracks illustrating the typical species-specific behavioural patterns. Each example shows the movement of an individual cercaria during a 5 min recording chosen because its parameters (distance moved, velocity) matched the species mean values. Colours in the heatmap indicate the amount of time a subject spent at a specific position, from dark blue (very little time) to red (a lot of time). (A) Aporocotylid sp. I; (B) Apatemon sp.; (C) Maritrema poulini; (D) Coitocaecum parvum. Scale bars: 5 mm.
Table 2. Mean values (±s.d.) of the recording time per sample, and the time spent moving/not-moving as well as the mean distance moved, and the mean maximum velocity and acceleration of individual cercariae

Discussion
The main goal of the study was to quantify and illustrate the movement patterns of free-living trematode cercariae, while demonstrating the applicability of a novel approach based on automated video tracking. Specifically, we compared the swimming speed, distance travelled and active time of individual cercariae of four common New Zealand trematode species. Our results clearly show the different cercarial behavioural strategies used to search for the parasites’ respective target hosts.
Overall, both furcocercariae, Apatemon sp. and Aporocotylid sp. I, travelled far greater distances than the two other species. This most likely reflects the different target host densities of these groups, and thus the parasites’ chances of encountering a potential host. Both C. parvum and M. poulini require small crustaceans, such as the amphipod Paracalliope fluviatilis, that are abundant and often occur in high densities in the littoral zones among vegetation and rocks (Fenwick, Reference Fenwick2001; Friesen et al. Reference Friesen, Poulin and Lagrue2017) in total small-scale sympatry with the snail hosts. In contrast, Apatemon sp. and Aporocotylid sp. I require fish as second intermediate and definitive hosts, respectively, that are much sparser in the aquatic environment. Consequently, their fish-infecting cercariae need to cover more space to enhance their chances of encountering a suitable host during their limited lifetime. The differences in the total distance travelled between the two fish-infecting species are mainly due to the dissimilar activity patterns of the cercariae, with Aporocotylid sp. I showing the longest resting periods (37%), compared with the continuous swimming behaviour of Apatemon sp. (resting 1%). Since cercariae are non-feeding and their life span is limited by their restricted glycogen supply (Morley, Reference Morley2011; Studer and Poulin, Reference Studer and Poulin2014) that declines over the course of their life time (Lowenberger and Rau, Reference Lowenberger and Rau1994), these resting phases might be a crucial adaptation of the smaller bodied, and thus possibly less energy-rich, Aporocotylid sp. I. Since this is currently only hypothetical, future studies could test for a potential link between body size, glycogen content and activity levels of different cercariae. Both tracks and heatmaps of these two species illustrate the contrasting host-searching behaviours, i.e. continuous movement vs periodic resting periods (Fig. 3A and B). Likewise, the movement tracks of M. poulini and C. parvum larvae illustrate these species’ different small-scale search patterns for their high-density substrate-dwelling hosts (Fenwick, Reference Fenwick2001). While M. poulini cercariae cover the ground in small horizontal loops (Fig. 3C), C. parvum cercariae alternate between crawling and swinging their body in a repetitive and uniform pattern while attaching to the substrate with their short sucker-like tail (Fig. 3D). These general swimming patterns of cercariae correspond to previous descriptions of the various species’ larval movements (see Hechinger, Reference Hechinger2012 and references therein) but the automated tracking provides much more detailed quantitative data and a standardized illustration of the spatio-temporal distribution.
Despite their differences in body size, resting periods and overall distance travelled, the cercariae of Apatemon sp. and Aporocotylid sp. I show strikingly similar values for maximum acceleration and maximum velocity. While the cercariae need to be fast enough to successfully reach a fish that is encountered in the water column, they cannot afford to waste energy by being too fast, due to their limited glycogen reserves. Quantifications of swimming speed of other fish-infecting furcocercariae of European trematodes have produced similar values. Based on manual observations of video-recorded cercariae, Santos et al. (Reference Santos, Karvonen, Pedrot, Faltýnková, Seppälä and Valtonen2007) measured swimming speed ranges for Diplostomum pseudospathaceum and Ichthyocotylurus variegatus (both mean 3.9 mm s−1) similar to Apatemon sp. and Aporocotylid sp. I in this study (3.2 and 3.4 mm s−1, respectively), while morphologically similar cercariae with other target host groups (molluscs, leeches, anatids), such as Cotylurus brevis, Australapatemon spp. and Bilharziella polonica showed significantly slower velocities (0.9–2.4 mm s−1). Altogether, these results could suggest a critical/optimal speed of cercariae infecting fish to successfully reach and infect their fast moving and sparse targets. It would be promising to test this hypothesis by analysing the cercarial velocity and acceleration of further fish-infecting trematode species, such as Tylodelphys spp., under standardized conditions that are known to impact cercarial swimming speed, such as temperature (Feiler and Haas, Reference Feiler and Haas1988). Although swimming speed and cercarial behaviour have been suggested as a consistent measure to discriminate between trematode species (Santos et al. Reference Santos, Karvonen, Pedrot, Faltýnková, Seppälä and Valtonen2007), it seems that target host identity may be a better determinant of swimming patterns. Therefore, swimming speed might rather be used to distinguish between target hosts, which might be useful in cases where the second intermediate host of a trematode species is not yet identified.
Other studies have also recently applied new methods to investigate trematode dispersal patterns, e.g. by assessing the mobility and swimming efficiency of Schistosoma mansoni cercariae based on a combination of biological experiments, theoretical models and robotics (Krishnamurthy et al. Reference Krishnamurthy, Katsikis, Bhargava and Prakash2016), or by applying mixed models to statistically test temporal patterns in cercarial release (Hannon et al. Reference Hannon, Calhoun, Chadalawada and Johnson2017). Both examples show that there is an ongoing interest in studying the fundamental dispersal and host-searching patterns of trematode larvae. Evaluating these small-scale processes is essential for a better understanding of complex host–parasite interactions, ranging from parasite community structure to the transmission of potential disease agents. Further relevant questions that could benefit from detailed behavioural analyses of free-living parasite stages include the influence of biotic factors, pollutants and environmental changes on parasite transmission (Pietrock and Marcogliese, Reference Pietrock and Marcogliese2003; Morley et al. Reference Morley, Lewis and Hoole2006; Thieltges et al. Reference Thieltges, Jensen and Poulin2008). Taking cercarial longevity into account, future studies could investigate the energy expenditure of the different cercariae and provide estimates of the potential active dispersal capabilities of these larval trematodes in their aquatic environment. Furthermore, since these free-living parasite stages are ubiquitous components of zooplankton communities (Kuris et al. Reference Kuris, Hechinger, Shaw, Whitney, Aguirre-Macedo, Boch, Dobson, Dunham, Fredensborg, Huspeni, Lorda, Mababa, Mancini, Mora, Pickering, Talhouk, Torchin and Lafferty2008; Morley, Reference Morley2012; Preston et al. Reference Preston, Orlofske, Lambden and Johnson2013; Soldánová et al. Reference Soldánová, Selbach and Sures2016) and constitute important food items to a variety of predators (Thieltges et al. Reference Thieltges, Amundsen, Hechinger, Johnson, Lafferty, Mouritsen, Preston, Reise, Zander and Poulin2013), it would be revealing to investigate whether cercariae with certain movement patterns are better at evading potential predators. Moreover, while cercariae have been the focus of various studies on trematode transmission, the free-swimming miracidial stages and their host-searching behaviours have received far less attention (e.g. Kalbe et al. Reference Kalbe, Haberl and Haas1997), but are equally crucial for the parasites’ successful completion of their life cycle. Experimental investigations of these questions using video tracking software can provide detailed insights into the behavioural patterns of the free-living parasite stages at a resolution we could not achieve with the naked eye. EthoVisionXT has already proven valuable to study a wide variety of organism, ranging from insects and fish to large-bodied mammals (reviewed in Noldus et al. Reference Noldus, Spink and Tegelenbosch2001). Our results demonstrate that the programme can also be successfully applied to much smaller organisms, such as the free-swimming larvae of trematodes. Altogether, automated video tracking provides a powerful tool for such detailed analyses of parasites’ host-searching strategies and will allow future studies to quantify and illustrate detailed parasite movement in host space and time.
Acknowledgements
We are grateful to Olwyn Friesen and Clement Lagrue for help in the field and laboratory, and to Sheri Johnson and Anne Besson for providing access to EthoVision. We also thank two anonymous referees for their comments and suggestions that improved the manuscript.
Financial support
This work was supported by the German Research Foundation (DFG, SE 2728/1-1).








