INTRODUCTION
Since it is recognized that probably all living organisms are concerned with parasitism, either as hosts or as parasites (May, Reference May1992; Thompson, Reference Thompson1994; Windsor, Reference Windsor1998; Poulin and Morand, Reference Poulin and Morand2000), researchers attempt to integrate parasitology and ecology to provide a better understanding of ecosystem functioning. Recent findings have shed light on how parasites modulate food-web length and community structure (for study cases see: Thompson et al. Reference Thompson, Mouritsen and Poulin2005; Hernandez and Sukhdeo, Reference Hernandez and Sukhdeo2008a; Amundsen et al. Reference Amundsen, Lafferty, Knudsen, Primicerio, Klemetsen and Kuris2009; for reviews see: Lafferty et al. Reference Lafferty, Dobson and Kuris2006, Reference Lafferty, Allesina, Arim, Briggs, De Leo, Dobson, Dunne, Johnson, Kuris, Marcogliese, Martinez, Memmott, Marquet, McLaughlin, Mordecai, Pascual, Poulin and Thieltges2008), and stress that these effects increase with the complexity of their life-cycle (simply because the more complex the cycle, the greater the number of organisms concerned with infection). The ecological significance of parasites is even more obvious for those whose transmission relies on the predation of an intermediate host by a definitive host (trophically transmitted parasites, Marcogliese, Reference Marcogliese2004). The reason for this is that most of the infected intermediate hosts display physiological, morphological, and/or behavioural alterations, and the manipulation hypothesis predicts that some of these parasite-induced changes are parasite adaptations to increase predation risk by definitive hosts (Holmes and Bethel, Reference Holmes, Bethel, Canning and Wright1972; see Combes, Reference Combes1991 and Moore, Reference Moore2002 for reviews).
In their recent review on the ecological significance of manipulative parasites, Lefèvre et al. (Reference Lefèvre, Lebarbenchon, Gauthier-Clerc, Missé, Poulin and Thomas2008) described how they affect apparent competition phenomena (e.g. competitor A better exploits the resource than competitor B but the reverse is true when they share a parasite), energy flow along food chains, and habitat creation. In most of the studies devoted to ‘manipulated’ food webs, the authors have investigated the relationship between infected intermediate hosts and upper-trophic-level species to test the predictions of the manipulation hypothesis that infection should make the intermediate host more susceptible to predation, hence strengthening the trophic links involved in transmission (Moore, Reference Moore2002; Perrot-Minnot and Cézilly, Reference Perrot-Minnot, Cézilly, Thomas, Guégan and Renaud2009). As a consequence, infected intermediate hosts have been considered as potential predators, or at least, consumers, only in a few cases. The ‘top-down influences’ of infection (i.e. consequences downstream in the food chain) may nevertheless be of ecological importance when intermediate hosts are also key species in ecosystem functioning. For instance, in headwater streams, 20 to 73% of riparian leaf-litter inputs are estimated to be processed by benthic macroinvertebrates (Wallace and Webster, Reference Wallace and Webster1996; Covich et al. Reference Covich, Palmer and Crowl1999). Infection with Acanthocephalus tahlequahensis (Acanthocephala: Echinorhynchidae) has been shown to significantly decrease the detritus consumption of the isopod Caecidotea communis, which thereby affects both the recycling of dead organic material and the dynamic of invertebrates using the material processed by isopods as food or habitat (Hernandez and Sukhdeo, Reference Hernandez and Sukhdeo2008a,Reference Hernandez and Sukhdeob).
Amphipods, which are also common hosts of various manipulative parasites, are considered as key species in this functional process (Dangles and Malmqvist, Reference Dangles and Malmqvist2004; Piscart et al. Reference Piscart, Genoel, Dolédec, Chauvet and Marmonier2009b). For instance, Piscart et al. (Reference Piscart, Genoel, Dolédec, Chauvet and Marmonier2009b) have shown that their leaf-litter breakdown activity represents about 75% of the overall leaf-litter recycling in the streams of Western France. However, the functional role of amphipods is not limited to their shredding activity, as several studies pointed out the predatory behaviour of Gammarus species (Dick, Reference Dick and Platvoet1996; MacNeil et al. Reference MacNeil, Dick and Elwood1997; Kelly et al. Reference Kelly, Dick and Montgomery2002a,Reference Kelly, Dick and Montgomeryb, Reference Kelly, Dick, Montgomery and MacNeil2003, Reference Kelly, Bailey, MacNeil, Dick and McDonald2006; Piscart et al. Reference Piscart, Dick, McCrisken and MacNeil2009a). They can thus influence the size, location, growth and reproduction of prey populations, and such effects on lower trophic level organisms may be even greater since amphipods have been found to predominate the macroinvertebrate fauna in terms of biomass in many riverine communities (MacNeil et al. Reference MacNeil, Dick and Elwood1997; Dangles and Malmqvist, Reference Dangles and Malmqvist2004; Piscart et al. Reference Piscart, Genoel, Dolédec, Chauvet and Marmonier2009b).
Despite the previous arguments, the feeding ecology of infected amphipods has received little interest. Crompton (Reference Crompton1970) reported an increased food intake in Gammarus pulex when infected with the bird acanthocephalan Polymorphus minutus. On the other hand, G. pulex infected with the fish acanthocephalan Pomphorhynchus laevis takes longer to consume leaf material (McCahon et al. Reference McCahon, Brown and Pascoe1988), or brine shrimp eggs (Pascoe et al. Reference Pascoe, Kedwards, Blockwell and Taylor1995), than uninfected conspecifics. Similarly, infection of G. pulex with Echinorhynchus truttae, another fish acanthocephalan, has been shown to decrease the tendency to kill live G. duebeni celticus (MacNeil et al. Reference MacNeil, Fielding, Dick, Briffa, Prenter, Hatcher and Dunn2003), and either to decrease (Fielding et al. Reference Fielding, MacNeil, Elwood, Riddell and Dunn2003, using a single prey density) or increase (Dick et al. Reference Dick, Armstrong, Clarke, Farnsworth, Hatcher, Ennis, Kelly and Dunn2010, using the functional response approach) the tendency to kill live Asellus aquaticus. Such results suggest that infection may affect the regulation of prey populations but it is premature to draw conclusions about the top-down influences of parasitism at the community level when our knowledge of the predatory behaviour of infected hosts is based on laboratory findings only.
Here, we combined laboratory and field data to assess how infection with the acanthocephalan parasite P. minutus affects the trophic ecology of Gammarus roeseli (Gervais, 1835), a freshwater amphipod of Balkan-European origin which is widespread throughout Western Europe (Karaman and Pinkster, Reference Karaman and Pinkster1977; Barnard and Barnard, Reference Barnard and Barnard1983). P. minutus is a trophically transmitted parasite which exploits amphipods as intermediate hosts and waterbirds as definitive hosts (Kennedy, Reference Kennedy2006). Infection with P. minutus is known to influence G. roeseli’s distribution (Médoc and Beisel, Reference Médoc and Beisel2009), reproduction (Dezfuli et al. Reference Dezfuli, Lui, Giovinazzo and Giari2008), physiology (Piscart et al. Reference Piscart, Webb and Beisel2007; Sures and Radszuweit, Reference Sures and Radszuweit2007), and behaviour (Marriott et al. Reference Marriott, Collins, Paris, Gudgin, Barnard, McGregor, Gilbert, Hartley and Behnke1989; Bauer et al. Reference Bauer, Haine, Perrot-Minnot and Rigaud2005; Médoc et al. Reference Médoc, Bollache and Beisel2006, Reference Médoc, Rigaud, Bollache and Beisel2009; Médoc and Beisel, Reference Médoc and Beisel2008), but nothing has been reported regarding its trophic ecology once infected.
We performed microcosm experiments under laboratory conditions to measure the consumption rates of uninfected and P. minutus-infected amphipods when feeding either leaf material, live or dead isopods (Asellus aquaticus). In the case where amphipods need to compensate for the cost of infection by increasing their food intake, we should observe a higher overall consumption activity in infected than in uninfected amphipods. However, the reverse could be observed if the parasite shifts the host's energy balance from costly feeding behaviours such as killing live prey to behaviours that are more conducive to transmission (e.g. swimming towards the water surface, Médoc et al. Reference Médoc, Bollache and Beisel2006). To get field information on the trophic ecology P. minutus-infected G. roeseli, we quantified the different neutral lipid classes of amphipods, namely triglycerides and free fatty acids, based on the assumption that feeding activities should be positively correlated to stocks of neutral lipids. We also determined the carbon (C) and nitrogen (N) isotopic signatures of amphipods and all the available food sources since they are known to indicate, respectively, the food sources and the trophic level of a consumer (DeNiro and Epstein, Reference De Niro and Epstein1978; Peterson and Fry, Reference Peterson and Fry1987). We expected the 15N/14N ratio to be consistent with our laboratory findings on the predatory behaviour of amphipods, with lower values for infected compared to uninfected amphipods if infection reduces predation, or vice versa. We also expected the 13C/12C ratio to be influenced by the habitat shift induced by infection with P. minutus (see Médoc and Beisel, Reference Médoc and Beisel2009), infected amphipods being mostly found in floating organic material.
MATERIALS AND METHODS
Feeding experiments
Amphipod sampling
Amphipods were collected with a 500 μm mesh pond net in the river Nied (Laquenexy, north-eastern France, 49°05′ N and 6°19′ E), where the prevalence of P. minutus exceeds 10% (Médoc et al. Reference Médoc, Bollache and Beisel2006). Concerning infected G. roeseli, we focused our sampling effort on floating materials since they are described as their main habitat in the study site (Médoc and Beisel, Reference Médoc and Beisel2009). On the contrary, uninfected amphipods are widespread throughout the natural habitats of the study site (Médoc and Beisel, Reference Médoc and Beisel2009), so we sampled all these habitats and caught the amphipods we needed from this subsample to avoid any habitat effect. All the amphipods used in this study (i.e. feeding experiments, neutral lipid and stable isotope analyses) were sampled in the same way. The yellow-orange cystacanth (the infective stage of P. minutus inside its intermediate host), visible through the invertebrate's translucent cuticle, distinguished infected G. roeseli from their uninfected counterparts. To avoid size or parasitic-load effects, only medium-sized amphipods (9±1 mm in total length, from the tip of the rostrum to the base of the telson) harbouring 1 cystacanth were selected. We did not make distinction between the sexes.
In the laboratory, infected and uninfected individuals for feeding experiments were maintained separately in plastic tanks (33 cm long×25 cm wide×13 cm high) at a density of 20 ind./L. Each tank was filled with filtered site-water and equipped with glass pebbles and artificial plants to reduce cannibalism. The oxygen demand was supplied by a filter pump that also generated a flow inside the tanks. Alder-leaf discs (Ø: 20 mm) were provided to satiation as the sole food source. Amphipods were deprived of food for 24 h before experiments to standardize consumption rates. Housing took place in a thermo-regulated room to ensure a stable water temperature (14±1°C) and the alternating periods of light and dark were each 12 h.
Isopod consumption
The isopod Asellus aquaticus was collected by hand sorting in the river Moselle (Metz, North-eastern France, 49°07′ N and 6°10′ E). We deliberately chose a prey that is absent from the location where G. roeseli was collected in order to use naïve prey during predation tests (i.e. no common history between the predator and its prey). Furthermore, isopods are common prey of amphipods of the Gammarus genus (MacNeil et al. Reference MacNeil, Dick and Elwood1997). Medium-sized isopods were selected regardless of the sex (6±1 mm in total length), but gravid females were excluded to avoid the confounding influence of eggs on prey consumption. In the laboratory, they were maintained for 15 days under the regime described above for amphipods to acclimatize them to the water of the river Nied.
The experimental design consisted of 10 aquaria (33 cm long×25 cm wide×13 cm high), each with an air pump, enclosed between 2 hermetically fixed, porous partitions. Each aquarium was thus equally divided into 2 Experimental Units (EU). This design was expected to protect individuals from air-pump perturbations while allowing for proper oxygenation and water circulation. Each EU (13 cm long×14·5 cm wide×25 cm high) contained 2·3 L of filtered water drawn from the river Nied and 20 translucent glass pebbles (Ø: ~15 mm) were placed on the bottom, convex size down, to provide a standard substrate. The aquaria were placed inside a wide vat (140 cm long×110 cm wide×40 cm high) filled with tap water and equipped with a cooler (Huber TC40E) and a pump to ensure water circulation. This device acted as a ‘water bath’ system allowing a high degree of thermal stability among the various EUs (14±1 °C), water temperature being checked regularly. The experimental design was illuminated by 4 light tubes (Philips, 36 W) mounted 60 cm above the water's surface, with alternating periods of light and dark each being 12 h.
Twelve isopods were placed inside each of the 16 EUs. After a 30-min acclimatization period, 8 uninfected amphipods were added to half of the EUs while the other half received 8 P. minutus infected amphipods (i.e. N=8 per infection status). One control group of 4 EUs with isopods alone allowed to monitor for deaths due to experimental conditions. To test whether P. minutus infection affects G. roeseli’s tendency to kill live prey or its affinity for this prey type, the same feeding experiment was repeated by replacing live isopods with dead individuals previously sacrificed by thermal shock (immersion in water at 45°C for 30 s) (N replicates=12 per infection status). The EUs were checked regularly in search of dead amphipods and the number of totally consumed isopods was recorded after a 48-h exposure to G. roeseli. At the end of the experiments, amphipods were dissected to confirm parasitic load and infection status.
Leaf consumption
Freshly fallen alder leaves (Alnus glutinosa) were collected from the natural habitat and 220 disks were cut using a cork borer (Ø: 18 mm). All the disks were dried in an oven at 60°C for 48 h and pooled in groups of 5, corresponding to the number of disks amphipods were allowed to feed on during the experiment. Each group was weighed to the nearest mg to determine the amount of leaf material provided to amphipods at the beginning of the experiment, and placed in stream water using 250 μm-mesh net bags for 2 weeks prior to the experiment to establish fungal and bacterial biofilms on the disks. A net of 250 μm-mesh prevented invertebrate colonization. When conditioned, the disks were distributed in 44 top-opened cylindrical EUs (Ø: 11 cm, 1 group of 5 disks per EU) each equipped with 4 glass pebbles serving as a refuge for amphipods. The EUs were placed into a large vat (the same as for predation tests) filled with aerated stream water. The bottom of each EU was made of a 1 mm-mesh net and all the EUs were placed on a rigid grid 15 cm above the bottom of the vat to allow the supply of the aerated stream water to each EU (≈0·76 L of water per EU). This design was expected to minimize the sources of disturbance while allowing standard conditions between EUs.
The experiment started with the addition of medium-sized amphipods: 3 uninfected in 20 randomly selected EUs and 3 infected in 20 others. Four supplementary EUs were free of G. roeseli to control for disk weight loss resulting from leaching. Four EUs per infection status (i.e. N replicates=4) were removed at 5 time-intervals: 4, 8, 12, 24 and 48 h. Amphipods and disks were washed and dried (48 h at 60°C) to determine individual feeding rates (i.e. the dry weight of leaf consumed divided by the dry weight of amphipods).
Neutral lipid analysis
The concentration of the different neutral lipid classes and especially triglycerides was used as a measure of nutritional condition (Fraser et al. Reference Fraser, Sargent, Gamble and MacLachlan1987; Napolitano and Ackman, Reference Napolitano and Ackman1989; Falk-Petersen et al. Reference Falk-Petersen, Sragent, Kwasniewski, Gulliksen and Millar2001). The use of neutral lipids was hence more powerful than the total lipid content which is an indicator of the physical condition of individuals (Plaistow et al. Reference Plaistow, Troussard and Cézilly2001). For each infection status, 30 medium-sized amphipods were sampled without distinction between the sexes from the river Nied within the same day. Since a single invertebrate provided insufficient lipid material, neutral lipids were analysed from the whole body of 3 pooled individuals (N replicates=10 per infection status). Before the extraction, cystacanths were removed from infected specimens. Lipids were extracted according to the method described by Folch et al. (Reference Folch, Lees and Stanley1957). Briefly, samples were homogenized in 20 vols of mixture of chloroform–methanol (2:1, v/v) and aliquots removed for protein determination (Bradford, Reference Bradford1976) (after evaporation of the solvent). Samples were filtered and washed twice with water containing 0·25% KCl (w/v). The chloroform lower phase was evaporated and total lipid contents were determined by weight. Neutral lipid classes, corresponding to triglycerides, free fatty acids, and cholesterol were resolved from each other on thin layer chromatography (TLC) plates using hexane-diethyl ether–acetic acid (80:20:1, v:v:v) as the developing solvent. The TLC plate was subsequently dried and neutral lipid contents were revealed after spraying with a solution of 10% cupric sulfate (w/v) in 8% phosphoric acid (v/v) and charred at 180°C for 15 min. Neutral-lipid spots were identified using authentic standards.
Because the stable isotope ratios of C (13C/12C, expressed as δ13C) and N (15N/14N, δ15N) of a consumer depends on its diet (DeNiro and Epstein, Reference De Niro and Epstein1978; Peterson and Fry, Reference Peterson and Fry1987), we determined the isotopic signatures of both infected and uninfected amphipods, and all the available food sources, to get field information on their trophic ecology. An enrichment in 15N is indeed observed between a consumer and its prey (Minigawa and Wada, Reference Minigawa and Wada1984), thus allowing estimation of the consumer's trophic position (Post, Reference Post2002; Vanderklift and Ponsard, Reference Vanderklif and Ponsard2003). Conversely, δ13C varies between the different sources of C, for instance benthic vs pelagic, freshwater vs marine, or C-3 vs CAM/C-4 plants (Post, Reference Post2002; Bearhop et al. Reference Bearhop, Adams, Waldron, Fuller and Macleod2004), with little or no 13C enrichment along the food chain.
For each infection status, 15 medium-sized amphipods were sampled without distinction between the sexes from the river Nied within the same day (N=15). We also picked up 3 samples (N replicates=3) of all the food sources available so as to have at least 0·5 and 1 mg of matter per sample (in dry weight) for animal and vegetal sources, respectively. This included 2 other macroinvertebrate consumers: Baetis rhodani (Ephemeroptera) and Calopteryx sp. (Odonata) larvae, 2 macrophytes: Myriophyllum spicatum and Potamogeton pectinatus, 2 terrestrial inputs: leaf litter and Salix babylonica roots, and the bacterial-fungal biofilm covering mineral substrates (perilithon, hereafter). Samples were frozen as soon as possible and stored at −20°C until processing. All animals were defrosted and digestive tracts were removed and discarded. Cystacanths were removed from infected amphipods. Samples were cleaned with distilled water and acidified by adding of 0·5 ml of 1 m hydrochloric acid to remove inorganic carbon from the cuticle. The resulting material was oven-dried (60°C for 48 h) and ground using a ball mill grinder (Mixer Mill MM 200, Retsch, Haan, Germany) to produce homogenous powder. Invertebrates were small (<10 mm) and/or lightweight (<7 mg dry weight) so whole bodies were analysed. Approximately 0·5 mg of each animal sample and 1 mg of each vegetation sample were weighed into tin cups. The stable-isotope ratios of C and N were measured using a continuous-flow stable isotope ratio mass spectrometer (CF-IRMS, Isoprime Ltd, Manchester, UK) interfaced with a Eurovector elemental analyser (EuroEA3028-HT) at the stable isotope geochemistry facility of the “PaléoEnvironnements et PaléobioSphère” laboratory (Lyon, France). Isotope ratios were reported as delta (δ) in part per thousand (‰) relative to international standard V-PDB (C) and atmospheric nitrogen (N). Repeated analyses of international standards (IAEA N1, USGS 25, IAEA CH6 and C3), along with 2 laboratory standards (tyrosine and triphenylamine), showed that the maximum standard deviations for δ 15N and δ 13C values were, respectively, 0·03‰ and 0·08‰. Standard deviations of samples analysed in duplicate (n=4) averaged 0·39‰ and 0·51‰ for C and N, respectively, and single measurements were carried out on all remaining samples.
To quantify the food source contributions to consumer's diet, linear mixing models based on isotopic mass balance are commonly used (e.g. Phillips, Reference Phillips2001). For instance, using 2 isotopic signatures (δ 15N and δ 13C), the contribution (f) of 3 food sources (A, B, C) to the diet of a consumer (M) is calculated with the following 3 end-member mixing model:
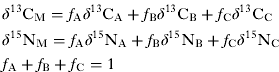
For a dual isotope system, this model is underdetermined and no unique solution exists for more than 3 food sources.
Here, the IsoSource mixing model (Phillips and Gregg, Reference Phillips and Gregg2003) was used to calculate the frequency distributions of feasible source contributions since the number of potential food sources exceeded 3 (leaf litter, S. babylonica roots, Potamogeton pectinatus, and perilithon). With this method, all possible combinations of each food source contribution (0–100%) are examined in small increments (1%) and those that sum to the observed mixture isotopic signatures within a small tolerance (0·1‰) are considered to be feasible solutions. Food sources were then combined using the aggregation method provided by Phillips et al. (Reference Phillips, Newsome and Gregg2005) to reduce the number of sources. We pooled leaf litter and S. babylonica roots into a single ‘terrestrial inputs’ source since the frequency distributions generated by IsoSource were similar. The contribution of terrestrial inputs, Potamogeton pectinatus, and perilithon to G. roeseli’s diet was then calculated using the previous equations (1) (Phillips, Reference Phillips2001). Uncertainties about source proportions that derive from variability in the stable-isotope composition of consumers and sources were determined using IsoError 1.04 developed by Phillips and Gregg (Reference Phillips and Gregg2001). C and N isotope ratios had to be corrected for trophic fractionation before the use of the model. A trophic enrichment of 3·4‰ was chosen for δ 15N based on commonly reported values (e.g. Post, Reference Post2002), and we assumed no trophic fractionation for δ 13C between food sources and primary consumers (France, Reference France1996).
Statistics
All data were examined for normality and homogeneity of variance using Shapiro-Wilk and Bartlett's tests, respectively. Because of the heterogeneity of variance, data concerning feeding experiments were tested non-parametrically. We used the Mann-Whitney U-test to assess differences in isopod or leaf consumption between infected and uninfected G. roeseli. Bonferroni's correction was used to account for the multiple comparisons. A Wilcoxon's signed rank test was used to compare the dry weight of control disks before and after the experiment. Data on lipids and isotopes met the assumptions underlying parametric tests. The total neutral-lipid concentration of uninfected amphipods was thus compared to that of infected ones with a Student's t-test. Differences among neutral-lipid classes were assessed using a multivariate analysis of variance (MANOVA), with infection status as independent variable and lipid classes as dependent variables. Concerning stable isotopes, we performed a one-way analysis of variance (ANOVA) with Tukey's HSD tests for multiple comparisons to compare the C and N ratios of both sources and consumers. Differences in the proportional contributions of organic matter sources to the diet of G. roeseli were assessed using Fisher's exact test. Statistical analyses were performed with Statistica software (v. 6.0, Statsoft Inc.). All tests were two-tailed and used a 5% type I error risk.
RESULTS
Isopod consumption
There were no deaths during the experiment, neither for isopods in controls, nor for amphipods feeding on A. aquaticus. This means that mortality in isopods was caused by predation only, while amphipods did not exhibit cannibalism. Regardless of the infection status of G. roeseli, there was a higher number of isopods consumed when dead than when alive (Mann-Whitney's U tests, uninfected amphipods: Z=3·43, P<0·001, infected: Z=3·70, P<0·001, Fig. 1). This is simply because, in a given time-interval, it takes longer to kill and consume a live prey than to consume a dead animal. There was no significant difference in the number of dead isopods consumed between the two infection statuses (Z=−1·38, P=0·18) whereas uninfected amphipods consumed a higher number of live isopods than did infected ones (Z=−3·26, P<0·001, Fig. 1).

Fig. 1. Isopod consumption by Gammarus roeseli infected with Polymorphus minutus. The number of dead or live isopods consumed (median and interquartile range) was obtained for uninfected (white bars) and infected (black bars) amphipods at the end of a 48-h experiment. Significant differences in isopod consumption between the two infection statuses are indicated with an asterisk above bars (Mann-Whitney U tests, * P⩽0·05, ns: non significant).
Leaf consumption
The dry weight of control disks did not significantly vary during the experiment (Wilcoxon's signed rank test, Z=1·46, P=0·14), and there were no deaths of amphipods suggesting that they did not exhibit cannibalism. Overall, leaf consumption increased with time following an exponential relationship for uninfected amphipods (R²=0·78, P<0·001, N=20), and a logarithmic relationship for infected ones (R²=0·50, P<0·001, N=20, Fig. 2). There was no significant difference in leaf consumption between the two infection statuses within the first 24 h (Mann-Whitney's U tests at 4, 8, 12 and 24 h: all P>0·05) but, after a 48-h exposure, leaf consumption was 2-fold higher for uninfected amphipods (≈1·4 g/g amphipod) than for infected ones (≈0·75 g/g amphipod, Z=2·31, P=0·028, Fig. 2). Because of the limited number of replicates (N=4 per infection status), this difference was not significant after Bonferroni's correction (0·05/5=0·01).

Fig. 2. Leaf consumption by Gammarus roeseli infected with Polymorphus minutus. The dry weight of leaf consumed per dry weight of amphipods (median and interquartile range) was obtained for uninfected (white dots) and infected (black dots) amphipods at different time-intervals. Adjustment curves (dotted for uninfected amphipods, solid for infected amphipods) were calculated with raw data. Significant differences in leaf consumption between the two infection statuses are indicated with an asterisk above dots (Mann-Whitney U tests without Bonferroni's correction, * P⩽0·05, ns: non significant).
Lipid contents
The total neutral-lipid concentration of infected amphipods (20·62±6·0 μg per 100 mg proteins) was not significantly different from that of uninfected amphipods (18·65±5·0 μg per 100 mg proteins, Student's t-test, t 18=1·4, P=0·621). However, when comparing the mean concentrations of triglycerides, free fatty acids, and cholesterol, the analysis of variance indicated a small but significant effect of infection status on the amphipod's lipid metabolism (MANOVA, F 3=5·16, P=0·011). This may be due to the triglyceride concentration that tends to be higher in infected compared to uninfected G. roeseli even if the difference was not significant (F-test, F18=2·20, P=0·155, Fig. 3).

Fig. 3. Concentrations of the neutral lipid classes (mean±s.d.) in uninfected (white bars) and Polymorphus minutus – infected Gammarus roeseli (black bars).
Stable isotope study
When depicted in a δ 13C-δ 15N bi-plot space, the stable-isotope ratios of both consumers and food sources suggested the existence of 2 distinct food webs (Fig. 4). The first (on the left of Fig. 4) showed the trophic links occurring between Myriophyllum spicatum, Baetis rhodani and Calopteryx sp. larvae. The stable-isotope ratios of this food web varied significantly between sources (ANOVA, 15N content: F 2,8=19·8, P<0·001; 13C content: F 2,8=90·2, P<0·001, Fig. 4). The increase in δ 15N (+0·7‰) and δ 13C (+0·9‰) between M. spicatum and B. rhodani indicated that B. rhodani mainly fed on M. spicatum. However, the 15N enrichment was lower than expected (+3·4‰±1 between two trophic levels; Post, Reference Post2002) and suggested that terrestrial inputs (leaf litter and/or S. balylonica roots) could be a significant part of B. rhodani's diet. The increase in δ 15N (+1·5‰) and δ 13C (+1‰) between B. rhodani and Calopteryx sp. were congruent with the hypothesis of a predator--prey interaction, B. rhodani representing a major food source for Calopteryx sp. larvae.

Fig. 4. Nitrogen and carbon stable isotope bi-plot (mean±s.d.) for uninfected and Polymorphus minutus-infected Gammarus roeseli, two other invertebrate consumers (Baetis rhodani and Calopteryx sp. larvae), and five potential food sources (leaf material; Myriophyllum spicatum; Potamogeton pectinatus; perilithon; Salix babylonica roots).
On the other hand, the δ 13C and δ 15N values of infected and uninfected G. roeseli indicated that their diet was mainly made of terrestrial inputs, with a potential contribution from P. pectinatus and perilithon, thus constituting a separate food web (on the right of Fig. 4). The stable isotope ratios along this food web varied significantly between sources (15N content: F 4,34=128·3, P<0·001; 13C content: F 4,34=12·1, P<0·001, Fig. 4). The δ 15N values of infected and uninfected G. roeseli were similar (Tukey's HSD post-hoc test, P=0·677), indicating the same trophic level, whereas δ 13C values were significantly different (Tukey's HSD post-hoc test, P<0·001), strongly suggesting differences in food sources.
The contribution of each food source to the diet of infected and uninfected G. roeseli was calculated using the equations previously described (1) (Phillips and Gregg, Reference Phillips and Gregg2001). Based on a 15N enrichment of 3·4‰ (Post, Reference Post2002) and assuming no trophic fractionation for 13C, terrestrial inputs represented a major proportion of the diet for infected (70±8%) as well as for non-infected amphipods (58±7%). The contribution of P. pectinatus was about 27±9% regardless of infection status. Although perilithon was a consistent part of the assimilated diet of uninfected amphipods (15±4%), its contribution to that of infected ones was negligible (3±3%, Table 1).
Table 1. Contribution of organic matter sources (terrestrial inputs, Potamogeton pectinatus, perilithon) to the diet assimilated by uninfected and Polymorphus minutus-infected Gammarus roeseli

DISCUSSION
The present study combined laboratory and field data with the aim of revealing changes in the trophic ecology of acanthocephalan-infected amphipods relative to their uninfected conspecifics. Stable isotopes and neutral lipids were successfully used together, to complement the results of feeding experiments whose ecological relevance, when alone, may be questionable.
Contrary to Crompton's assumption (Crompton, Reference Crompton1970) that infected animals need to feed more to compensate for the cost associated with infection, we found no difference in the number of dead isopods consumed between infected and uninfected G. roeseli. Similarly, infection with the fish acanthocephalan Echinorhynchus truttae has no influence on the consumption of dead chironomids by G. pulex (Fielding et al. Reference Fielding, MacNeil, Elwood, Riddell and Dunn2003). Although our experiment was not designed to quantify the energy diverted to P. minutus, this result means that under laboratory conditions and in the presence of an ‘easy-to-eat’ food source (i.e. dead prey), G. roeseli did not feed more to compensate for the cost associated with infection. This is consistent with the fact that we did not find a marked difference in the total amount of neutral lipid contents between infected and uninfected amphipods. Thus, even under natural conditions, infected amphipods, which have a more important content of triglycerides than uninfected ones, seem to eat as much as uninfected ones and did not show apparent neutral lipid depletion resulting from a decrease in the feeding activity.
The results of our feeding experiments differed greatly when isopods were alive, the number of isopods consumed being significantly lower for infected than for uninfected amphipods. Similar differences were found in G. pulex infected with E. truttae and feeding on live isopods (Fielding et al. Reference Fielding, MacNeil, Elwood, Riddell and Dunn2003), or live amphipods (MacNeil et al. Reference MacNeil, Fielding, Dick, Briffa, Prenter, Hatcher and Dunn2003). Conversely, Dick et al. (Reference Dick, Armstrong, Clarke, Farnsworth, Hatcher, Ennis, Kelly and Dunn2010) showed that G. pulex preying on isopods displays a Type-II functional response rising more steeply and with a higher asymptote when infected with E. truttae, which suggests that infection increases predation. How infection modifies the diet of infected hosts may thus depend on the host-parasite system while laboratory results may vary with the experimental approach used. Concerning our results, one may argue that the parasite larva encysted in the abdomen of G. roeseli was a physical constraint to its motion, hence decreasing its efficiency in capturing live prey. This is, however, improbable because a previous study investigating the same host/parasite system demonstrated that infected amphipods swim faster than their uninfected counterparts (Médoc and Beisel, Reference Médoc and Beisel2008). Because infected amphipods ate as many dead isopods as uninfected ones, the assumption that infection reduced the affinity of the host for this particular prey types can be rejected. The low number of live isopods consumed by infected amphipods thus argues for a reduced tendency to kill living prey. In other words, P. minutus infection significantly reduced the predatory behaviour of G. roeseli.
Data on leaf consumption are more surprising. Indeed, as shown in the first feeding experiments, infection had no apparent influence on the consumption of dead isopods, and thus we expected the same with leaf disks. This was not the case and after 48 h the amount of leaf material consumed by infected amphipods was half of that consumed by uninfected ones. Amphipods shred the leaves to subsequently feed on bacteria and fungi from their attached biofilms (Dangles and Malmqvist, Reference Dangles and Malmqvist2004; Piscart et al. Reference Piscart, Genoel, Dolédec, Chauvet and Marmonier2009b). We expect this shredding activity to be to some extent costly, thus the difference we observed does not necessarily mean that infected compared to uninfected amphipods ate less leaf material, but rather that they invested less energy in shredding. Similarly, G. pulex takes longer to consume leaf material when infected with the fish acanthocephalan Pomphorhynchus laevis (McCahon et al. Reference McCahon, Brown and Pascoe1988) but, conversely, it shows unchanged daily feeding rates when infected with E. truttae (Fielding et al. Reference Fielding, MacNeil, Elwood, Riddell and Dunn2003). The way a host's biology is influenced by infection may thus differ among host/parasite systems.
Overall, our findings suggest that P. minutus changes the life-history budgeting of its intermediate host G. roeseli. Natural selection among parasites favours those having the ability to manipulate the phenotype of their host in a way that increases their own transmission. Host behavioural manipulation by P. minutus is well known and includes a reverse geotaxis (Bauer et al. Reference Bauer, Haine, Perrot-Minnot and Rigaud2005; Médoc et al. Reference Médoc, Bollache and Beisel2006), a higher refuge use (Médoc et al. Reference Médoc, Rigaud, Bollache and Beisel2009), and a higher swimming speed (Médoc and Beisel, Reference Médoc and Beisel2008). Although their associated fitness gains in terms of increased trophic transmission are assumed and remain to be proven, we expect parasites that are able to shift the energy balance of their host in favour of manipulated traits to be favoured by natural selection. In turn, infected hosts should reduce their investment in behaviours that are less conducive to the parasite's transmission, for instance costly feeding activities (e.g. predation, shredding) or reproduction. This assumption is supported by the results of our feeding experiments and by the literature on the reproduction of P. minutus-infected G. pulex. Its pairing success is indeed significantly decreased while females are totally castrated (Bollache et al. Reference Bollache, Gambade and Cézilly2001, Reference Bollache, Rigaud and Cézilly2002). P. minutus should hence shift the energy balance of G. roeseli in such a way that its overall energy output is reduced. This could explain why infected compared to uninfected amphipods had a higher concentration of triglycerides without necessarily feeding more.
At the community level, 2 distinct food webs were identified based on stable-isotope tracers: a first one relying on the predator/prey interaction between Odonata and Ephemeroptera larvae, the latter being herbivorous, and a second one with G. roeseli feeding mostly on terrestrial inputs. Both Ephemeroptera and amphipods (whether infected or not) shared the same N-isotope signature, that of primary consumers, whereas the N-isotope signature of Odonata larvae argued for a higher trophic position, that of a predator. This may first appear inconsistent with laboratory findings stressing the potential predatory behaviour of (uninfected) amphipods (Dick and Platvoet, Reference Dick and Platvoet1996, Reference Dick and Platvoet2000; Kelly et al. Reference Kelly, Dick and Montgomery2002b; and present study). Actually, although amphipods are omnivorous, they are first of all opportunistic, feeding on what they find where they are (MacNeil et al. Reference MacNeil, Dick and Elwood1997; Maazouzi et al. Reference Maazouzi, Piscart, Pihan and Masson2009). For instance, the feeding strategy of the amphipod Dikerogammarus villosus was recently found to be much more plastic than previously expected (Maazouzi et al. Reference Maazouzi, Piscart, Pihan and Masson2009). Nicknamed ‘killer shrimp’, D. villosus was depicted as a strong predator by almost all the experimental studies (Dick and Platvoet, Reference Dick and Platvoet2000). Maazouzi et al. (Reference Maazouzi, Piscart, Pihan and Masson2009) determined the fatty acid composition of the D. villosus population from an artificial reservoir. The fatty acid composition, which is an efficient trophic marker, significantly differed between seasons and habitats. D. villosus is thus rather unspecialized; it adapts its diet and switches from carnivorous to herbivorous, or detritivorous, depending on spatially and temporally available food sources.
As shown by the N-isotope signatures and because of the previous arguments, the reduced predation due to P. minutus infection observed in the laboratory was not found under natural conditions. So, based on the predation experiment only, it would be premature to conclude that infection may affect the regulation of prey populations. This result emphasizes the need to complement laboratory results with field data before drawing conclusions at the community level.
The small but significant difference in δ 13C values between infected and uninfected G. roeseli was probably due to a lower perilithon consumption by infected (<9% of the diet) than by uninfected amphipods (7 to 24% of the diet). P. minutus is known to induce a reverse geotaxis in G. roeseli (Bauer et al. Reference Bauer, Haine, Perrot-Minnot and Rigaud2005; Médoc et al. Reference Médoc, Bollache and Beisel2006), which therefore is found inhabiting floating materials in its natural habitat (Médoc and Beisel, Reference Médoc and Beisel2009). Infected amphipods are thus located far from benthic food sources and, because they are opportunistic, they feed on what is available at the surface, for instance decomposing leaves found among floating materials. This could explain the apparent shift from perilithon to terrestrial inputs in the diet of infected amphipods.
To conclude, our findings emphasize that the knowledge of parasite life-history strategies is essential to fully understand the feeding behaviour of infected hosts. From an ecological perspective, predicting the top-down influences of infection may be confusing when the host is an opportunistic feeder and, as such, this study shows the benefits of combining field and laboratory data through a multiple approach.
ACKNOWLEDGEMENTS
We wish to thank Sophie Sroda for help during field work, François Fourel and François Martineau for stable isotope measurements and Bernard Kaufmann for his linguistic corrections.
FINANCIAL SUPPORT
This study was supported by the InBioProcess project (ANR-06-BDIV-007-InBioProcess (2007–2010) of the Biodiversity 2006 program of the National Research Agency (Agence Nationale de la Recherche, ANR), and funded by a grant from the Conseil Régional de Lorraine.







