Published online by Cambridge University Press: 06 August 2004
This paper examines the efficiency of acquired resistance in protecting the fish host, rainbow trout (Oncorhynchus mykiss), against the trematode parasite Diplostomum spathaceum, and the hypothesis that fish recognize areas where infective stages are aggregated and show avoidance behaviour. We found that when fish with a low level of infection were held in restricted cages in natural conditions they became infected and developed cataracts as a result of this infection. This suggests that acquired resistance is insufficient in protecting fish against the parasite or the deleterious effects of infection in conditions where fish could not avoid the parasite. Behavioural experiments in the laboratory showed that fish reacted to the parasite cercariae by avoiding the infection source, which decreased the rate of parasite establishment. We conclude that by using a combination of behavioural avoidance and physiological resistance, fish could defend against the parasite more effectively.
Free-living organisms are continuously exposed to a variety of pathogens and parasites, and the process of infection has provided a selective pressure that should have made hosts behave in a manner that prevents or limits infection. A major physiological barrier in controlling infections is the immune system, which in vertebrates generally includes non-specific immunity replenished by more specific responses after the first encounter with a pathogen (e.g. Manning, 1994; Turner, 1994; Wakelin, 1996). Another mechanism, which tends to decrease the exposure to parasites, is the active avoidance behaviour of the hosts when they actively avoid sources of infection. Such behaviour has been described in several parasite–host systems (see reviews by Hart (1994) and Moore (2002)). The present paper explores the roles of these two processes, acquired resistance and parasite avoidance behaviour, in preventing the infection of fish hosts by the trematode Diplostomum spathaceum.
The life-cycle of D. spathaceum includes three hosts, fish eating bird, snail and fish. The parasite is transmitted from snail to fish as free-living cercariae that penetrate the skin of the fish and then migrate to the lens of the eye, where they form long-lived metacercarial stages. Extensive metacercarial infection in fish may cause cataract (Shariff, Richards & Sommerville, 1980), which can impair the vision of fish and cause reduced feeding ability and growth of the host (Crowden & Broom, 1980; Owen, Barber & Hart, 1993; Buchmann & Uldal, 1997). The parasite also increases the vulnerability of the fish to predation (Seppälä, Karvonen & Valtonen, 2004). Therefore it is likely that the parasite imposes a strong selective pressure on fish to develop means of identifying and avoiding sources of infection.
During the first infection and migration through fish tissues, D. spathaceum cercariae elicit an immune response (Bortz et al. 1984; Stables & Chappell, 1986; Whyte et al. 1987; Whyte, Chappell & Secombes, 1989, 1990; Höglund & Thuvander, 1990), which decreases the number of parasites that establish in subsequent infections (Höglund & Thuvander, 1990; Whyte et al. 1990; Karvonen et al. 2004a). Whyte et al. (1990) found that 70–80% of cercariae failed to establish when presented to immunized fish, indicating that the majority of the cercariae are rejected by immunity, but some are still able to establish to the lens. Thus, the actual ability of the physiological resistance to protect the fish is determined by the amount of damage caused by the parasites that succeed in avoiding the immune system. However, detailed quantitative studies on these aspects in D. spathaceum are lacking. As such, the first aim of this study was to determine how effectively acquired resistance could protect fish against the parasite in natural infection conditions with natural temporal changes in exposure. We exposed fish with low-level infection, and presumably acquired resistance, to natural infections in restricted cages in a lake system, and examined the intensity of infection and parasite-induced cataract under circumstances where the possibility to avoid the infection was excluded.
Workers have generally assumed that maintaining immunity against pathogens is costly in terms of trade-offs with other life-history traits (see Sheldon & Verhulst, 1996; Zuk & Stoehr, 2002). Therefore natural selection should favour the development of alternative mechanisms to resist or prevent infections as long as these are energetically less expensive than the costs of infection. This would include behavioural responses so hosts would take avoidance behaviour of circumstances where the infection is most likely to take place (Hart, 1994; Moore, 2002). Prevalence of D. spathaceum infection in snails is usually low (Väyrynen et al. 2000). Moreover, the parasite cercariae are not actively host seeking (Karvonen et al. 2003), but maintain themselves in the water column using short swimming bursts. Therefore, cercariae are likely to be found patchily distributed in water with major concentrations in close proximity to the infected snail hosts. Thus it would be advantageous for a fish to be able to recognize such patches and take avoidance behaviour before heavy infection occurs. Increased ventilation rate, heart rate and swimming activity in fish have been observed associated with the infection by the parasite (Laitinen, Siddall & Valtonen, 1996), suggesting that fish might become aware of the infection, but detailed experimental evidence on parasite avoidance behaviour in this system is lacking. The second aim of this study was to experimentally investigate if fish could respond to the parasite cercariae by escaping the infection source and in such way decrease the number of establishing parasites.
The experiment was conducted in 2002 in 3 cages, measuring 120×80×100 cm, anchored in the littoral zone of the lake Konnevesi in Central Finland. Based on the infection trials on the same location in previous years, the area had a population of infected snails providing natural level of infection in fish. The water depth at the location was 1·5 metres and the distance between the cages was 10 metres. A group of 1-year-old rainbow trout (Oncorhynchus mykiss) was obtained from a fish farm located close to the study site. The farm used surface water supply and the majority of these fish had a low-level infection with D. spathaceum (infection prevalence=72·7%, mean parasite load=1·32±0·20 (all figures indicate mean±S.E. unless mentioned otherwise)).
On 13 May, 20 fish were introduced into each of the cages. After 14 days, fish were brought to the laboratory and studied for parasite-induced cataract under anaesthesia (MS-222 as anaesthetic) using the slit-lamp microscope (Kowa portable SL-14). Cataract intensity was assessed from both eyes using a subjective scale: 0=no cataract, 1=cataract covering horizontally less than 50% of the lens area, 2=cataract covering less than 100%, 3=cataract covering 100%, 4=intensive cataract covering 100% and lens appearing totally opaque and white. After examination, the fish were returned to cages i.e. the same fish groups were followed throughout the experiment. To obtain a thorough insight into the dynamics associated with cataract formation, the examination procedure was then repeated every 14 days (i.e. on days 28, 42, 56, 70, 84, 98, 112, 126, 140) until day 154 of the experiment when all fish were euthanized and examined for infection, and parasite intensity was assessed. To determine the degree of parasite exposure during each experimental period, each cage was also presented with 15 fin-marked fish, which were dissected for parasites after each 14 day-period and replaced with a new group. All fish were fed with commercial fish pellets every second day during the experiment. Fish length in the groups of 20 fish increased from 194·2±1·4 mm to 231·4±1·9 mm and weight from 68·5±1·6 g to 147·7±5·4 g during the experiment.
The experiment was conducted in 2003 in 6 tanks, where individual fish were introduced with D. spathaceum cercariae, and the time required for avoidance response was measured. Each tank measured 215×18 cm (water depth 12 cm, total volume 46 l) and provided with 5 l/min flow-through of water. Tanks were illuminated from 45 cm above using 2 standard 60 W lamps providing a light intensity of 450 lux just above the tanks. One end of each tank was covered with black plastic creating a dark, 25×18 cm shelter for the fish and fixed their position to a desired location. This allowed the introduction of cercariae to the proximity of fish. A small tube was used to introduce cercariae and led through the plastic cover into the water in the centre of the shelter. The cercarial suspension poured through the tube was dispersed to the water underneath the shelter within a few seconds as verified by pre-trials with a coloured suspension. Fish used in the experiment had a low-level infection in some individuals (infection prevalence=28·1%, mean parasite load=0·33±0·08, length=150·1±1·2 mm, weight=31·1±0·7 g). One fish was introduced into each tank and the use of tanks was randomized. Fish were allowed to settle to the experimental conditions for 48 h. Parasite cercariae were obtained from 10 naturally infected Lymnaea stagnalis snails and the cercarial density in the combined suspension from the snails was estimated from ten 1 ml subsamples. Suspension of 6-h-old cercariae, known to be highly infective (Karvonen et al. 2003), was used to infect the fish (n=20). Infection dose was 2500 cercariae per fish (1·5 dl of suspension), which corresponded to a density of 460 cercariae/l in the shelter area. Comparable cercarial densities are likely to be found in proximity of shedding snails in nature (Karvonen et al. 2004b). Control fish (n=10) received 1·5 dl of water.
Just before the introduction of cercariae, the incoming water was turned off and the light intensity was decreased to 15 lux (3·3% from initial intensity) to encourage the fish to leave the shelter. Immediately after this, cercariae suspension was introduced to the fish and the time of the avoidance response from the shelter was recorded. Control fish received water with no cercariae. A maximum response time of 10 min was set, after which the fish was removed from the tank if no response had occurred. All fish were maintained in larger holding tanks for 48 h after the experiment, providing sufficient time for parasites to reach the eye. Afterwards, all fish were euthanized and parasites counted by dissecting the eye lenses.
To determine the number of parasites acquired by these fish in conditions where avoidance was not possible, 30 fish were individually exposed by placing the fish in containers with 0·5 l of water and 230 cercariae. This corresponded to the cercarial density used in the behavioural trials. Infection time was 30 min during which the water was continuously aerated using aquaria pumps. After infection, fish were maintained in larger tanks for 48 h and parasites counted as described previously.
In the lake cages, 1 fish died during the experiment and was excluded from the data. All individuals in groups of 20 fish were infected at the end of the experiment. Mean parasite numbers in these fish increased significantly from 1·32±0·20 to 59·00±2·66 parasites per fish during the course of the experiment (ANOVA on log-transformed data; F5,113=868·52, P<0·001) and the pattern of infection did not differ between cages (F5,113=0·029, P>0·9). Dissection of the groups of 15 fin marked fish indicated that parasite exposure was low in the beginning of the experiment, reached a peak in mid July (days 56–70) during the highest water temperatures and then decreased slowly towards the end of the experiment (Fig. 1). This indicated that fish were exposed to a low number of cercariae in the beginning of the experiment. This also suggested that fish that possibly had not been exposed prior to the experiment (27·3% of the fish), received a low-level infection during the early days of the experiment, and developed resistance against the parasite before high exposure occurred (see Whyte et al. 1987). Cataract intensity in fish was low at the beginning of the experiment when 62·9% of the eyes were without cataract, but the intensity increased significantly with time, and on day 140 of the experiment, all eyes had cataracts with 39·0% of the eyes fully covered and 21·2% totally opaque (Fig. 2).

Fig. 1. Number of new Diplostomum spathaceum infections in the groups of 15 fish held in 3 cages in a lake system and dissected for parasites every 14 days. Bars indicate mean±S.D. The solid line indicates water temperature in the lake during the experimental period.

Fig. 2. Parasite-induced cataract intensity, measured using a subjective scale (see text), in fish held in 3 cages for a period of 154 days and studied every 14 days. Data from every second period are presented. Error bars indicate asymmetrical 95% confidence limits.
In the parasite avoidance experiment, 3 fish were excluded from the data because they did not settle in the shelter. All fish which were exposed to the parasite cercariae (n=18) escaped from the shelter with an average time of 3·07±0·54 min. The mean acquisition rate of parasites was 2·01±0·32 per minute and the average number of parasites acquired before the escape response was 7·06±2·06. Total number acquired also increased with time spent in the shelter (linear regression; F1=19·92, P<0·001; Fig. 3). Control fish (n=9) showed no response within the 10 min. The average number of parasites in fish infected individually in containers was 44·30±3·65, which indicates that parasites effectively infected these fish in the absence of escape possibility.

Fig. 3. Number of new Diplostomum spathaceum infections in fish (log scale) as a function of response time to the parasite cercariae (in sec, log scale) (linear regression, r2=0·71).
This paper investigates the role of physiological and behavioural defence in protecting fish hosts against the cercariae of an invasive trematode parasite, Diplostomum spathaceum. We found that initial infections that should have stimulated an acquired resistance did not protect fish against the parasite or subsequent cataract formation in a long-term experiment where fish were held in restricted cages under natural infection conditions. Behavioural experiments conducted in the laboratory showed that fish were able to respond to the presence of the parasite cercariae and perform avoidance behaviour, which decreased the number of establishing parasites. However, individual differences in response times were considerable, which resulted in different parasite numbers in fish and may have important implications for parasite distribution in fish populations.
Physiological defence systems in fish include non-specific resistance followed by acquired resistance after the first exposure to a pathogen (e.g. Manning, 1994). In a recent study, Karvonen et al. (2004a) showed that rainbow trout developed resistance against the parasite when held in cages in natural infection conditions similar to this study. However, those data could not be used to estimate the efficiency of resistance in protecting the fish against the harmful effects of infection. The present data suggest that acquired resistance is not adequate for protecting the fish as complete cataract coverage was observed in 39·0% of the eyes and 12·2% of the eyes were totally opaque by the end of the experiment. Although the direct effects of the infection and cataract formation on fish were not examined, we suggest that intensities of this magnitude may have serious effects on fish. For example, Seppälä et al. (2004) have shown that high cataract intensities comparable to this study (intensities 3–4, see Materials and Methods section) increase the predation vulnerability of fish. Furthermore, reduced feeding ability and stunted growth of fish have been reported in association with D. spathaceum infection (Crowden & Broom, 1980; Owen et al. 1993; Buchmann & Uldal, 1997). Therefore, it would seem necessary for the fish to decrease the exposure to the parasite through other mechanisms.
Parasite avoidance through behavioural modifications has been described in several parasite–host systems where infective stages are associated with faeces or hosts may become aware of the infection process and show avoidance behaviour (Hart, 1994, 1997; Folstad et al. 1991; Hutchings et al. 1998; Altizer, Oberhauser & Brower, 2000; Moore, 2002; Wilson et al. 2002). In this study, we demonstrated that fish recognized infection risk due to D. spathaceum cercariae and performed avoidance behaviour, which decreased the number of establishing parasites. Previous workers have reported, for instance, increased swimming activity in fish associated with the infection by Diplostomum spp. cercariae (Laitinen et al. 1996), which supports our findings as the increased swimming effort can be seen as an attempt to avoid the cercariae. However, to our knowledge, the present study is the first experimental demonstration of avoidance behaviour in the system. One pre-requisite for parasite avoidance is that hosts are able to detect the presence of parasites (Poulin, Marcogliese & McLaughlin, 1999). In this study, we did not determine the exact mechanism associated with parasite detection, but 3 mechanisms can be considered. First, fish may observe the cercariae visually, but we consider this unlikely since the cercariae are small and virtually transparent in water. Second, Poulin et al. (1999) showed that rainbow trout did not respond to odour of Diplostomum spp. cercariae. Third, the most probable mechanism of detection is the mechanical stimuli caused by the penetration of cercariae through gills and skin, although this allows some cercariae to successfully penetrate the fish before the actual avoidance takes place. However, it is possible that the acquired physiological resistance is sufficient to protect the fish during the recognition of infection and therefore the defence against the parasite would be completed through a combination of behavioural and physiological defence. Of course, individual differences in the ability of fish to respond to cercariae are likely as indicated by the present data, which may, together with individual differences in physiological resistance, be one of the reasons for the aggregated parasite distribution in natural fish populations (Pennycuick, 1971; Sweeting, 1974; Burrough, 1978; Karvonen et al. 2004a).
An intriguing additional aspect in parasite avoidance is the effect of conspecifics in shoals of fish. It is possible that avoidance behaviour in some individuals may trigger similar responses in others possibly without an actual contact with the parasite. Fish are also known to release alarm substances from epidermal club cells when being infected by Diplostomum spp. cercariae, which induces responses in conspecifics (Poulin et al. 1999). Furthermore, shoaling itself may serve as protection as it decreases the probability of an individual fish to encounter parasite cercariae. In fact, Sweeting (1974) reported that fish form tighter shoals when exposed to Diplostomum spp. cercariae. Shoaling and responses initiated by conspecifics may therefore alter the significance of avoidance behaviour for an individual fish. However, in the present study we focused on individual responses and interactions associated with avoidance behaviour, and studies on shoaling behaviour and parasites in fish require future work. We also emphasize that responses of different fish species to different cercarial numbers need to be explored.
To conclude, this study demonstrated that acquired resistance after initial infection by D. spathaceum failed to protect the fish against the deleterious effects of infection in natural conditions and in the absence of parasite avoidance. The level of physiological resistance is generally a compromise, determined first by pathogen pressure and on the other hand by factors such as risk of autoimmunity (see Zuk & Stoehr, 2002). Therefore maximal immune defence is not necessarily an optimal solution and we suggest that in this particular parasite--host system, parasite avoidance behaviour might serve as the first line of defence by decreasing the exposure to the parasite and may be replenished by the partial physiological resistance operating after the contact with the parasite has occurred. It is possible that the combination of these two mechanisms provides more efficient protection against the parasite with lower costs.
We thank the persons who assisted with this work and Konnevesi Research station for the facilities. Peter Hudson, Jukka Jokela and two anonymous referees gave valuable comments on the manuscript. The study was financed by the Biological Interactions Graduate School, the Finnish Cultural Foundation and the SUNARE project of the Academy of Finland.

Fig. 1. Number of new Diplostomum spathaceum infections in the groups of 15 fish held in 3 cages in a lake system and dissected for parasites every 14 days. Bars indicate mean±S.D. The solid line indicates water temperature in the lake during the experimental period.

Fig. 2. Parasite-induced cataract intensity, measured using a subjective scale (see text), in fish held in 3 cages for a period of 154 days and studied every 14 days. Data from every second period are presented. Error bars indicate asymmetrical 95% confidence limits.
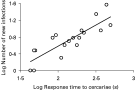
Fig. 3. Number of new Diplostomum spathaceum infections in fish (log scale) as a function of response time to the parasite cercariae (in sec, log scale) (linear regression, r2=0·71).