INTRODUCTION
In aquatic ecosystems, an increasing number of non-native species have become established in new locations. Studies of genetic diversity in invasive species contribute to an understanding of the potential for colonization and establishment, geographical patterns of invasions and range expansion. Reduced genetic diversity in newly established populations as a result of a colonization bottleneck due to the small number of initial colonists is generally expected and is often observed (e.g. Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O'Neil, Parker, Thompson and Weller2001; Hanfling, Reference Hanfling2007). Multiple introductions from different sources, or repeated introductions from the same source, however, may lead to invasive populations that are more genetically diverse than a single source population as different colonizing populations of the same species are likely to be genetically divergent and have different levels of genetic variation (e.g. Kirkpatrick and Barton, Reference Kirkpatrick and Barton1997).
Introduction processes may also be important in understanding host-parasite interactions in a novel area. Introduced species usually leave behind many of their co-occurring enemies (Torchin et al. Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003) and release from parasites and pathogens is a widely applied hypothesis to explain the proliferation of non-native species in their introduced regions (e.g. Keane and Crawley, Reference Keane and Crawley2002; but see MacLeod et al. Reference MacLeod, Paterson, Tompkins and Duncan2010). Introduction success of parasites with the host is associated with the presence of parasites on individuals in the host founder population and the ability of both host and parasite to persist in the new area (MacLeod et al. Reference MacLeod, Paterson, Tompkins and Duncan2010). Parasite loss during translocation or after arrival into a new area may result from a number of stochastic and selective pressures, e.g. absence of suitable intermediate hosts in the new range or a bottleneck during the translocation and colonization process (Dunn, Reference Dunn2009). Multiple introductions of aquatic organisms (e.g. in ballast water) may, however, increase the probability of introducing higher numbers of parasite species and, potentially, suitable intermediate hosts (Simberloff and Gibbons, Reference Simberloff and Gibbons2004). Moreover, introduced species tend to acquire generalist parasites from the local fauna (Poulin and Mouillot, Reference Poulin and Mouillot2003). The acquisition of native parasites by non-native species is relatively common and may have serious ecological impacts (Kelly et al. Reference Kelly, Patterson, Townsend, Poulin and Tompkins2009). In the new range, introduced species may act as competent hosts for local parasites. This amplifies infection rates, which then ‘spill back’ to the native host and thereby increase parasite numbers in the ecosystem. On the other hand, if introduced species are not suitable hosts for local parasites but still become infected, they may act as sinks for the parasites and thus dilute disease risk for native hosts (Poulin et al. Reference Poulin, Paterson, Townsend, Tompkins and Kelly2011).
In recent years, 4 Ponto-Caspian gobiid fishes (bighead goby Neogobius (Ponticola) kessleri, round goby N. (Apollonia) melanostomus, monkey goby N. (Babka) fluviatilis and racer goby N. gymnotrachelus (all species names follow Kottelat and Freyhof, Reference Kottelat and Freyhof2007) have spread beyond their native region due to human activities and/or natural expansion associated with increased water temperature. All 4 goby species have invaded the middle and upper stretches of the River Danube (Ahnelt et al. Reference Ahnelt, Bănărescu, Spolwind, Harka and Waidbacher1998). However, whereas high densities of N. kessleri and N. melanostomus have been recorded in the main channel, only scarce occurrence of N. gymnotrachelus and absence of N. fluviatilis has been recorded in the middle Danube (Jurajda et al. Reference Jurajda, Černý, Polačik, Valová, Janáč, Blažek and Ondračková2005). On the other hand, the latter 2 species have successfully invaded the middle stretch of the River Vistula (Baltic Sea watershed) via the central corridor, which follows the rivers Dnieper and Pripyat (Black Sea watershed) through the Pripyat-Bug canal to the rivers Bug (also known as the Western Bug) and Vistula (Gulugin and Kunitsky, Reference Gulugin and Kunitsky1999; Grabowska et al. Reference Grabowska, Pietraszewski and Ondračková2008). Freshwater populations of N. melanostomus and N. kessleri do not occur in the middle Vistula (Kakareko et al. Reference Kakareko, Plachocki and Kobak2009). Of the 4 goby species presently expanding their ranges, N. melanostomus appears to be the most invasive and is categorized as of ‘high invasiveness risk’ (Gozlan et al. Reference Gozlan, Britton, Cowx and Copp2010). Since the late 1980s, this species has been introduced (or has expanded) into the Great Lakes of the USA (Jude et al. Reference Jude, Reider and Smith1992); the middle and upper Danube (Wiesner, Reference Wiesner2005) and, subsequently, the River Rhine via the Rhine-Main-Danube canal (Borcherding et al. Reference Borcherding, Staas, Krueger, Ondračková, Šlapanský and Jurajda2011); and the Baltic (Kakareko et al. Reference Kakareko, Plachocki and Kobak2009) and North Seas (van Beek, Reference van Beek2006). In most of these new areas, the species has reached high population densities over a relatively short period (e.g. see Polačik et al. Reference Polačik, Janáč, Jurajda, Adámek, Ondračková, Trichkova and Vassilev2009; Borcherding et al. Reference Borcherding, Staas, Krueger, Ondračková, Šlapanský and Jurajda2011).
In this study, we compared the parasite community and genetic structure of both native and introduced freshwater populations of 4 goby species that are presently expanding their ranges. Native populations originated from the lower Danube. In accordance with recent distribution and density patterns, introduced populations of N. kessleri and N. melanostomus were sampled in the middle Danube and N. fluviatilis and N. gymnotrachelus in the middle Vistula. Our aims were addressed to compare (1) parasite diversity and similarity in parasite component communities and infracommunities, and (2) genetic variability between native and non-native goby populations introduced within the same river system (River Danube; N. kessleri and N. melanostomus) and in fish hosts introduced from a different watershed (River Vistula; N. fluviatilis and N. gymnotrachelus). We expect that introduced populations with reduced genetic diversity due to a small number of colonists will show reduced parasite diversity as a small founding population may be one factor contributing to insufficient transmission of native parasites along with the host (Colautti et al. Reference Colautti, Ricciardi, Grigorovich and MadIsaac2004). In contrast, introduced populations may also have a higher probability of introducing greater numbers of parasite species into the new area. Consequently, we expect that introduced populations with high genetic diversity will show higher parasite diversity and similarity to native populations than introduced populations with reduced genetic diversity.
MATERIALS AND METHODS
Host and parasite collection
Native populations of N. kessleri, N. melanostomus, N. fluviatilis and N. gymnotrachelus were sampled in the Bulgarian section of the Danube; N. kessleri, N. melanostomus and N. gymnotrachelus near the town of Vidin (N 43°57′35″, E 22°53′16″), N. fluviatilis and N. gymnotrachelus near the villages of Gomotartsi and Koshava (N 44°05′33″, E 22°58′09″), 16–20 km upstream of Vidin. Introduced populations of N. kessleri and N. melanostomus were sampled in the Austrian section of the Danube, near the town of Orth an der Donau (N 48°07′23″, E 16°42′46″). The introduced population of N. fluviatilis was sampled from the Vistula, near the town of Torun (N 53°00′21″, E 18°36′23″). The introduced population of N. gymnotrachelus was sampled in the Wloclawski Reservoir (on the River Vistula) near the village of Soczewka (N 52o32′58″, E 19o34′29″). Geographical distribution of the 4 goby species including native range and range of introduction with indicated sampling sites is shown in Fig. 1. The fish were collected during autumn 2006 from the shoreline zone of each river, either by electrofishing or by using a beach seine depending on habitat conditions. Fish of the most frequent size class were selected for parasite dissection in the lower Danube based on an analysis of length-frequency distribution. Subsequently, fish of the same size class were selectively collected in the introduction area to minimize the effect of fish length on parasite community structure in comparative studies. Collected fish were transported alive in river water to the laboratory, where they were individually sacrificed prior to measurement for standard length (SL, to the nearest 1 mm; Table 1) and dissection. The caudal fin of each fish was preserved in 96% ethanol for DNA extraction. Fish were examined under a binocular microscope for the presence of metazoan parasites according to standard methods (Ergens and Lom, Reference Ergens and Lom1970). Collected parasites were preserved in 4% formaldehyde (Acanthocephala, Digenea, Cestoda, Bivalvia), in a mixture of ammonium picrate and glycerine (Monogenea), or in a mixture of glycerine and alcohol (Nematoda). Preserved digeneans and cestodes were stained in ferric acetocarmine, dehydrated in a gradual alcohol series, and mounted into Canada balsam (Ergens and Lom, Reference Ergens and Lom1970). Parasites were identified using a light microscope equipped with phase-contrast, differential interference contrast and the Lucia 5.0 Image Analysis System.

Fig. 1. Geographical distribution of 4 invasive Ponto-Caspian gobies in 2006 (native area of distribution = dark, non-native = cross-hatched) with indication of sampling sites (black circles).
Table 1. Number of sampled fish, mean standard length (SL), measures of parasite community structure and parasite diversity at the component and infracommunity level for four Neogobius species in their native (N – Bulgarian section of the River Danube) and introduced (I – Austrian stretch of the River Danube (AT) and the River Vistula in Poland (PL)) range

Microsatellite genotyping and genetic diversity
Samples from 259 individuals of 4 Neogobius species were genotyped for 16 polymorphic microsatellite loci (DDBJ/EMBL/GenBank database Accession numbers EF029924-EF029939) according to methods previously described and optimiZed by Vyskočilová et al. (Reference Vyskočilová, Ondračková, Šimková and Martin2007). DNA was extracted from ethanol-preserved tissue samples (fish fins) using the DNeasy Blood and Tissue Kit (Qiagen), and this was used as a template for PCR amplification following the described PCR mixture composition (including PCR primers) and PCR cycle conditions exactly. Samples were analysed using a standard fragment analysis procedure through capillary electrophoresis on an ABI PRISM 3130 Genetic Analyser (Applied Biosystems-Life Technologies).
Data analysis
Prevalence and mean parasite abundance were calculated for each fish species and locality sampled. Prevalence was expressed as the percentage of infected fish in a sample and mean abundance as mean number of parasites per all hosts in a sample. Metazoan parasite community structure was analysed at the infracommunity (IFC; including all parasites on a single host) and component community (all parasites in a host population) levels (Bush et al. Reference Bush, Lafferty, Lotz and Shostak1997).
Classification of core and satellite species followed the protocol of Hanski (Reference Hanski1982), with core species as locally abundant and regionally common species (prevalence > 50%, mean abundance > 10) and satellite species as locally and regionally rare species (prevalence < 10%, mean abundance < 1). Parasite diversity was characterized by total species richness, Shannon diversity index and Berger-Parker dominance index at the component community level, and mean IFC richness and Brillouin diversity index at the IFC level (Magurran, Reference Magurran2004). Similarity in parasite communities among populations was evaluated using the Jaccard index based on presence-absence data (qualitative similarity) and the Bray-Curtis index based on abundance data (quantitative similarity). Diversity indices and similarity between parasite communities were calculated using PAST software (PAlaeontologicalSTatistics v.1.77, http://folk.uio.no/ohammer/past/; Hammer et al. Reference Hammer, Harper and Ryan2001). The Mann-Whitney U test was used to compare differences in both quantitative and qualitative similarity at the IFC level between native and introduced populations for particular host species. A generalised linear model (GLZ) with Poisson error distribution was used to test for IFC species richness, and Kruskal-Wallis non-parametric ANOVA with multiple comparisons of mean ranks used for IFC diversity, when testing for differences among host species and for host population origin (i.e. native or introduced). Differences in parasite diversity between native and introduced populations at the component community level were tested by permutation test in PAST. Statistical analyses were performed using Statistica 9.1 (StatSoft, Inc) and R-statistics (Crawley, Reference Crawley2007).
The genetic data obtained were processed using GeneMapper Software version 4.0 (Applied Biosystems-Life Technologies) and statistically evaluated. Final data files contained in total 10 samples with missing data (only 1 unamplified locus from the microsatellite set per sample). Samples with a preponderance of missing data were excluded from analyses, assuming unsuitable DNA or sample quality. Data for individual species were evaluated separately in order to obtain accurate information about the species’ population structure. Despite 3 loci (NG52 in N. kessleri and N. melanostomus, NG28 and NG135 in N. melanostomus) being completely monomorphic, they were not excluded from further analyses. Despite these markers having zero heterozygosity and polymorphic information content in each species, we avoided the artificial introduction of He values in populations by intentionally excluding monomorphic loci from subsequent analyses.
The GenAlEx package v. 6.41 (Peakall and Smouse, Reference Peakall and Smouse2006) was used for adjusting data to Genepop format, determination of basic statistical parameters (e.g. allele frequencies), Principal Coordinate Analysis (PCoA) and Analysis of Molecular Variance (AMOVA) analyses. The genotypic linkage equilibrium between all pairs of loci and the Hardy-Weinberg equilibrium (HWE) were tested for by Hardy-Weinberg exact tests for each locus in each population separately, and across all loci by Fisher's method, using Genepop 4.0.10 (Raymond and Rousset, Reference Raymond and Rousset1995; Rousset, Reference Rousset2008). Probability values (P) were estimated by using the Markov chain method under the following parameters: dememorisation – 1000, batches – 100, iterations per batch – 1000. The same software was used to calculate expected and observed heterozygosities and F-statistics parameters (inbreeding coefficient of an individual relative to the population, inbreeding coefficient of an individual relative to the total sample; effect of populations compared to the total sample; and number of migrants for particular host species). The frequency of null alleles for each locus and population was calculated using the FreeNA software package (Chapuis and Estoup, Reference Chapuis and Estoup2007).
The pair-wise population matrix of Nei genetic distance between native and introduced populations of particular species and the percentage of molecular variance (PhiPT) between and within these populations by AMOVA were also estimated using GenAlEx v. 6.41. PhiPT (θ PT) was calculated via AMOVA, without regional data structuring, as the proportion of variance among populations relative to total variance (variance among populations + variance within populations). The co-dominant genotype matrix of genetic distances generated was used as an input data set. In this case, the partition of variation within individuals is suppressed. Statistical tests of probability in AMOVA were based on random permutation (999 permutations) across the full data set. In the GenAlEX program, permutation tests are performed differently than in other packages (e.g. Arlequin) and the probability value P is calculated as the number of values ⩾ to the observed value (including observed value)/(number of permutations + 1).
RESULTS
Parasite diversity
The parasite fauna of N. kessleri, N. melanostomus, N. fluviatilis and N. gymnotrachelus comprised 21 parasite species within both the native and introduced range. Though none of the parasite species were found at all sampling sites, 6 taxa (Diplostomum spp., Apophalus sp., Nicolla skrjabini, Eustrongylides excisus, Pomphorhynchus laevis and Pseudoanodonta complanata) were common to all 4 goby hosts. Eight parasite species were recorded in both native and introduced goby populations, while 6 species were exclusive to native, and 7 to introduced, populations. Mean parasite abundance and prevalence varied among host species and between population origins (Table 2). In native populations, only P. laevis was denoted as a dominant species, reaching high prevalence and abundance in N. kessleri, N. melanostomus and N. gymnotrachelus. The same species was dominant in introduced populations of N. kessleri and N. melanostomus. In introduced N. gymnotrachelus, 2 dominant parasite taxa were found: metacercariae of Diplostomum spp. and Cyathocotylidae fam. spp. No one parasite species reached dominance in N. fluviatilis. The greatest number of satellite species was found in both native (80% of species) and introduced populations (50%) of N. melanostomus (Table 2).
Table 2. List of parasite species, prevalence (P,%) and mean abundance (A,± s.d.) of four Neogobius species in their native (N – Bulgarian section of the River Danube (BG)) and introduced (I – Austrian stretch of the River Danube (AT) and the River Vistula in Poland (PL)) range

mtc. – metacercariae; larv. – larval stage.
Parasite species reported from the region of the rivers Dnieper and Dniester (D) and the River Vistula (V) (Markevich, Reference Markevich1949; Koval, Reference Koval1959; Koval and Gerus, Reference Koval and Gerus1968; Koval et al. Reference Koval, Pashkevichute, Boshko, Kovalenko and Stavrobskyi1973, Reference Koval, Bagushchenko, Seregina and Pashkevichute1975; Niewiadomska, Reference Niewiadomska2003; Kvach, Reference Kvach2004; Pojmanska et al. Reference Pojmanska, Niewiadomska and Okulewicz2007).
At the component community level, parasite species richness did not differ between native and introduced populations of N. kessleri and N. melanostomus, both fish hosts that were introduced into the same river (permutation test, P > 0·05 for both species). Significantly lower species richness was found in introduced N. fluviatilis and N. gymnotrachelus compared to the native Danubean populations, i.e. in species originating from dissimilar drainages (Vistula; permutation test, P < 0·001 for both species; Table 1). Species richness did not differ among all 4 species in their native range or between the introduced sympatric species (permutation test, P > 0·05 for all comparisons). Introduced populations of N. kessleri, N. melanostomus and N. gymnotrachelus all had significantly higher Shannon diversity index values, while native populations had significantly higher Berger-Parker dominance index values. Conversely, introduced populations of N. fluviatilis had significantly lower diversity, and significantly higher dominance, than the native population (permutation test, p ⩽ 0·001 for all comparisons; Table 1). These results were associated with a relatively high abundance of Gyrodactylus proterorhini in the introduced population and a high abundance of P. laevis in the native populations of the other three goby species (Table 2).
IFC richness was generally low, with a maximum of 7 parasite species found in 1 specimen of N. gymnotrachelus in its native range. IFC richness differed significantly between introduced and native populations (GLZ; d.f. = 1, P = 0·034) and among the host species (d.f. = 3, P < 0·001), though the effect of population origin differed among species (indicated by strong interaction: d.f. = 3, P < 0·001). Introduced populations of N. kessleri and N. melanostomus showed higher values of IFC richness compared to the lower mean IFC richness of introduced N. fluviatilis. No difference between populations was found for N. gymnotrachelus. Diversity at the IFC level, measured using the Brillouin diversity index, showed a similar trend to IFC richness (Kruskal-Wallis test, H 7,293 = 176·7, P < 0·001), i.e. the Brillouin diversity index was significantly higher in introduced populations of N. kessleri and N. melanostomus (multiple comparison test, P < 0·001 for both species) in contrast to significantly lower diversity in introduced N. fluviatilis (P = 0·007) and no difference between populations in N. gymnotrachelus (Table 2).
Microsatellite genetic diversity
Cross-species amplifications were performed on all species except N. kessleri, for which the analysed microsatellite panel was initially designed. Of the 16 loci tested, all 16 were amplified in N. kessleri, 9 in N. melanostomus, 9 in N. fluviatilis and 11 in N. gymnotrachelus. Only 6 microsatellite loci (NG215, NG71, NG111, NG92, NG135 and NG70) were amplified reliably in all 4 Neogobius species. Locus NG115 was amplified in all species’ populations except that of N. gymnotrachelus from Poland.
A summary of the amplified alleles of all loci and populations is given in Table 3. The total number of alleles in the population and average allelic richness were similar between native and introduced populations of N. kessleri and N. melanostomus; whereas a decrease in number of alleles and mean allelic richness was found for introduced populations of N. fluviatilis and N. gymnotrachelus. Calculation of average allelic richness using only data for the 6 universal microsatellite markers for each species confirmed these results (Table 3).
Table 3. Number and ranges (bp) of determined alleles of particular microsatellite loci in Neogobius populations

Frequency of null alleles (if identified) ranged from 0·001% (different loci in different populations) to 19·8% (NG28, N. gymnotrachelus, Bulgaria). Taking all Neogobius species into consideration, most null alleles were estimated for locus NG135 (N. kessleri 9·8%, N. gymnotrachelus 11·7%). The minimum number of null alleles was estimated at loci NG236 and NG195 in N. melanostomus, despite relatively low values of heterozygosity observed in this species (Table 4).
Table 4. Summary of population-genetic analysis in native (Bulgaria) and introduced (Austria, Poland) populations of four Neogobius species

The majority of loci (all loci in N. melanostomus) were in HWE (Table 5). Highly significant differences from expected HWE (P < 0·001) were observed, however, at locus NG236 in N. kessleri, NG184 in N. fluviatilis and NG28 and NG135 in N. gymnotrachelus populations. A slightly significant deviation from HWE (P < 0·05) was observed at locus NG132 in N. fluviatilis and NG52 in N. gymnotrachelus populations. In N. kessleri and N. fluviatilis populations, this may be a consequence of population structure as the number of null alleles at these loci was either low or null alleles were not found at all. In N. gymnotrachelus populations, significant deviation from HWE (Table 5) could have been caused by the presence of null alleles; their frequencies (only frequencies higher than 1% taken into consideration) ranging from 1·9% to 19·8% at the loci.
Table 5. Characterization of the Neogobius populations using parameters of F-statistics
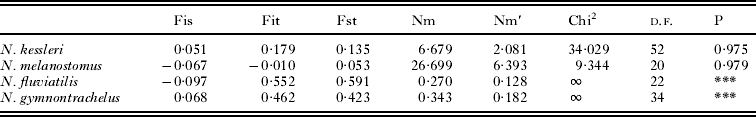
Interpopulation variability based on microsatellite data
In N. kessleri and N. melanostomus, 77% and 90% of molecular variance, respectively, was estimated within populations; whilst in N. fluviatilis and N. gymnotrachelus, the majority of variance (76 and 59%, respectively) was estimated between populations. θ PT values for N. kessleri, N. melanostomus, N. fluviatilis, N. gymnotrachelus were 0·226, 0·098, 0·759 and 0·592, respectively. Probabilities of a random value ⩾ the observed data value [P(rand ⩾ data)] for each dataset were 0·001.
Interpopulation values in distance and identity between native and introduced populations calculated by pairwise population matrix of Nei Genetic Distance and Identity were 0·095 and 0·909, respectively, for N. kessleri, 0·017 and 0·983 for N. melanostomus, 1·018 and 0·361 for N. fluviatilis, and 0·770 and 0·463 for N. gymnotrachelus. PCoA analysis via covariance matrix also revealed strong genetic and geographical structuring in N. fluviatilis and N. gymnotrachelus, in contrast to relatively low or minimum structuring found in N. kessleri and N. melanostomus, respectively (Fig. 2).

Fig. 2. Two-dimensional Principal Coordinates Analysis (PCoA) plots using a covariance matrix with standardization of genetic data, showing distance between native and introduced populations of 4 Ponto-Caspian gobies.
Private alleles (137 in total) were observed in all Neogobius populations, with a lower number of private alleles found in introduced populations compared to native populations. The maximum number of private alleles was revealed in native Bulgarian N. fluviatilis and N. gymnotrachelus populations, indicating their isolation (Table 4). The highest number of migrants was observed in N. melanostomus (Table 5), corresponding to the lowest number of private alleles. The number of effective alleles, Shannon Information Index and heterozygosity were comparable between native and introduced populations of N. kessleri and N. melanostomus, but these parameters were lower in introduced populations of N. fluviatilis and N. gymnotrachelus compared to native populations. The latter 2 fish species were characterized by a higher F-index in introduced compared to native populations, possibly indicating more frequent occurrence of inbreeding in Polish populations (Table 4).
Similarity in parasite communities
At the component community level, the qualitative similarity between native populations of different host species was higher than inter- and intra-species similarity between (1) native and introduced populations in the same river system (Danube), and (2) populations from distant rivers (Vistula vs Danube). Qualitative similarity between introduced populations of N. fluviatilis and N. gymnotrachelus in the Vistula showed higher values in comparison to introduced Danubean populations of N. kessleri and N. melanostomus (Table 6). Quantitative similarity showed similar results, with the lowest values between native and introduced populations from distant rivers, and comparable similarity between the different species in their native range, and native and introduced populations in the Danube. Quantitative similarity was relatively low between the two sympatric host species in non-native populations of N. kessleri and N. melanostomus in the Danube and N. fluviatilis and N. gymnotrachelus in the Vistula (Table 6).
Table 6. Qualitative similarity based on the Jaccard index (above the diagonal) and quantitative similarity based on the Bray-Curtis index (below the diagonal) between particular populations of four Ponto-Caspian gobies (NF – N. fluviatilis, NG – N. gymnotrachelus, NK – N. kessleri, NM – N. melanostomus) in their native and introduced range. Sympatric populations are denoted in bold

At the IFC level, qualitative similarity was significantly lower in native host populations for all 4 goby species (M-W U test; Z = 8·5, P < 0·001; Z = 12·5, P < 0·001; Z = 25·5, P < 0·001; Z = 6·1, P < 0·001 for N. fluviatilis, N. gymnotrachelus, N. kessleri and N. melanostomus, respectively). A similar pattern was observed for quantitative similarity in N. fluviatilis, N. gymnotrachelus and N. kessleri (M-W U test; Z = 7·4, P < 0·001; Z = 7·3, P < 0·001; Z = 5·8, P < 0·001, respectively), though no interpopulation difference was found for N. melanostomus (M-W U test; Z = 0·1, P > 0·05). The lowest qualitative and quantitative similarity between IFCs was found for N. fluviatilis in both their native and introduced range.
DISCUSSION
This study compared genetic and parasite diversity between native goby populations from the rivers Danube and Vistula with those (1) introduced from within the same river system (Danube, Black Sea watershed; N. kessleri and N. melanostomus), and (2) introduced from sources outside the Danube (River Vistula, Baltic Sea watershed; N. fluviatilis and N. gymnotrachelus). Our results show that, whilst no differences were observed in either microsatellite diversity or parasite species richness between native and non-native populations (or even higher parasite diversity in non-native populations) in fish introduced from within the same river system, reduced genetic diversity and parasite species richness were observed in populations introduced from different river systems.
A reduction in the number of parasites infecting a host species during the introduction process, known as ‘parasite loss’, has been identified as one important factor affecting invasion success (Torchin et al. Reference Torchin, Lafferty, Dobson, McKenzie and Kuris2003). Parasite loss may occur due to an absence of parasites in the host founder population, or through failure of parasites present to become established due, for example, to an absence of alternative or intermediate hosts (MacLeod et al. Reference MacLeod, Paterson, Tompkins and Duncan2010). Species richness based on measurements of parasite loss, however, requires knowledge of the parasite fauna in the actual source population, rather than simply using information limited to its native range (Colautti et al. Reference Colautti, Ricciardi, Grigorovich and MadIsaac2004). In this study, a comparison of known source and introduced populations was only performed for N. kessleri and N. melanostomus as we were unable to undertake genetic studies on gobies from the Dnieper. Genetic characterization using microsatellite loci confirmed the lower Danube as the source of populations in the middle Danube. Conversely, strong genetic differentiation between native (lower Danube) and non-native (Vistula) populations of N. gymnotrachelus and N. fluviatilis confirmed that the Danube was not the source population for non-native Vistula gobies. Introduced and native (source) populations of N. kessleri and of N. melanostomus showed no difference in parasite species richness at the component community level, with introduced populations even displaying higher infracommunity richness. On the other hand, populations of N. fluviatilis and N. gymnotrachelus introduced into the Vistula showed low parasite species richness. The native parasite fauna of the most likely source N. fluviatilis populations along the freshwater stretch of the River Dnieper includes at least 10 metazoan species (Markevich, Reference Markevich1949; Koval, Reference Koval1959; Koval and Gerus, Reference Koval and Gerus1968; Koval et al. Reference Koval, Pashkevichute, Boshko, Kovalenko and Stavrobskyi1973, 1975), a level comparable with that of native Danubian populations. Parasites of N. gymnotrachelus from the Dnieper have not yet been investigated (Y. Kvach, personal communication). We might expect a reduction in parasite species richness for at least N. fluviatilis, therefore, despite the unavailability of recent published data on parasite communities.
The difference in parasite loss observed between fish introduced to the middle Danube and the Vistula may be explained, in part, by river system connectivity. Introduction within the same river, where potentially suitable intermediate hosts are likely to occur, increases the chances of introduced parasites surviving and establishing viable populations. In contrast with the middle Danube, where gobies have moved upstream, the rivers Vistula and Dnieper are in historically separated drainage systems (Black Sea vs Baltic Sea) that have been artificially connected in the 18th century (Olenin, Reference Olenin and Briand2002). Thus, the reduced number of parasite species infecting gobies in the Vistula may be associated with insufficient adaptation of either the introduced host to local parasite fauna or of local parasites to new host species (Lively and Dybdahl, Reference Lively and Dybdahl2000).
Size of the founding population and number of founding events may also explain differences in parasite diversity between gobies introduced to the middle Danube and the Vistula. Reduced genetic diversity resulting from recent demographic bottlenecks (‘founder effect’) during colonization events (e.g. Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O'Neil, Parker, Thompson and Weller2001) is common in species introductions. As parasites are usually aggregated across host individuals, a small founder population will result in a lower number of parasite species transmitted (Poulin, Reference Poulin2007). Of the 4 species examined in this study, only N. fluviatilis and N. gymnotrachelus, both introduced hosts with reduced parasite species richness, appear to have passed through a genetic bottleneck. Microsatellite analysis of introduced populations of these two species showed a low number of migrants and a high F-index, indicating more frequent inbreeding. In addition, we also found low levels of heterozygosity and lower allelic richness compared to native populations. Mean microsatellite heterozygosity recorded by Neilson and Stepien (Reference Neilson and Stepien2011) for the Dnieper, the most likely source of N. fluviatilis in the Vistula (Gulugin and Kunitsky, Reference Gulugin and Kunitsky1999; Ohayon and Stepien, Reference Ohayon and Stepien2007), showed comparable values with the Danubean population in our study. A low number of colonists, therefore, may also have contributed to low parasite species richness and diversity in N. fluviatilis found in the Vistula. Unfortunately, comparable genetic data from Dnieper are not presently available for N. gymnotrachelus. A comparable number of effective alleles, Shannon's information index, and heterozygosity level between native and introduced populations of N. kessleri and N. melanostomus, and even a slight increase in allelic richness in introduced populations, all indicate that non-native middle Danube populations were founded by a large number of individuals and/or were exposed to continual supplementation of new genotypes. These results, therefore, support the hypothesis that multiple host introductions facilitate transmission of parasites, simply because the probability of parasite introduction increases with the number of introduced hosts and number of source populations.
At the parasite component community level, similarity between different species in their native range was higher than that between conspecifics inhabiting the lower and middle Danube, though the majority of parasite species have been reported in gobies from both stretches of the Danube (Kakacheva-Avramova et al. Reference Kakacheva-Avramova, Margaritov and Grupcheva1978; Ondračková et al. Reference Ondračková, Dávidová, Pečínková, Blažek, Gelnar, Valová, Černý and Jurajda2005; Francová et al. Reference Francová, Ondračková, Polačik and Jurajda2011). Lower qualitative and quantitative similarity was observed between non-native populations of N. kessleri and N. melanostomus, despite comparable ecological requirements; both goby species occupying stony substrata and having amphipods, the intermediate hosts for the most common goby parasites, as the dominant prey item in their diet (Polačik et al. Reference Polačik, Janáč, Jurajda, Adámek, Ondračková, Trichkova and Vassilev2009). It is possible, however, that similarity in both parasite community and parasite diversity will increase for these two species over time as Ponto-Caspian gobies are susceptible to a relatively high number of non-specific parasites (Ondračková et al. Reference Ondračková, Dávidová, Blažek, Gelnar and Jurajda2009). Conversely, comparison of parasite communities in N. fluviatilis and N. gymnotrachelus from the Vistula showed high qualitative similarity in their non-native range, despite over 100 km between sampling sites and differing habitat preferences. Both species were infected by the same parasite species (i.e. Gyrodactylus proterorhini), imported with Neogobius hosts from the source population, and larval digeneans (i.e. metacercariae of Diplostomum spp. and Cyathocotylidae fam. sp.), parasites most probably acquired in the new area as these digeneans represent common parasites in Poland and have been reported for many fish host species (Niewiadomska, Reference Niewiadomska2003). At the IFC level, qualitative and quantitative similarities showed higher values in the range of introduction in all 4 goby species. IFCs are likely to represent a random sample of the parasite component or compound community (Holmes, Reference Holmes, Esch, Bush and Aho1996). As introduced species often lack adaptation to local parasite fauna in the new area (Lively and Dybdahl, Reference Lively and Dybdahl2000), we would expect them to be accidentally parasitized by local non-specific parasites widely distributed in different unrelated fish species (Poullin and Mouillot, Reference Poulin and Mouillot2003). This could lead to a relatively low parasite IFC similarity within the host population. Interestingly, our data showed the opposite result, i.e. that IFC similarity increased in introduced populations in both the middle Danube and Vistula. Timi et al. (Reference Timi, Lanfranchi and Luque2010) suggested that the influence of local environmental characteristics is higher for parasite IFCs than for component communities in one marine teleost fish species. One explanation for our findings, therefore, may be that habitat structure, which was similar in both regions of introduction (Kakareko et al. Reference Kakareko, Plachocki and Kobak2009; Polačik et al. Reference Polačik, Janáč, Jurajda, Adámek, Ondračková, Trichkova and Vassilev2009), could potentially lead to decreased diversity of potential intermediate hosts for many endoparasites.
In summary, our results showed no differences in parasite and genetic diversity in fish introduced to the middle Danube as a consequence of multiple host introductions and translocation within the same river system. On the other hand, significantly lower genetic and parasite diversity were found in fish introduced into the River Vistula from outside the drainage area. A decrease in microsatellite diversity compared to the source population (Nielson and Stepien, Reference Neilson and Stepien2011) was, however, only confirmed for N. fluviatilis. The reduced parasite diversity compared to that reported for this species in the literature (e.g. Markevich, Reference Markevich1949; Koval, Reference Koval1959; Koval and Gerus, Reference Koval and Gerus1968; Koval et al. Reference Koval, Pashkevichute, Boshko, Kovalenko and Stavrobskyi1973, Reference Koval, Bagushchenko, Seregina and Pashkevichute1975) is in line with the hypothesis of parasite loss during the introduction process connected to a low number of founders and insufficient adaptation of the host or parasite to the new, historically separated, region. Further studies that include source populations are necessary for comparative analysis of N. gymnotrachelus. Comparison of genetic structure and parasite composition in recently established populations with populations in their native range may then provide valuable information about the process of fish invasion.
ACKNOWLEDGEMENTS
We would like to thank the staff of the Donau-Auen National Park in Austria, the National Agency of Fishery and Aquaculture in Bulgaria and the Polish Angling Association (PZW) for sampling permissions. We are grateful to Teodora Trichkova, Milen Vassilev, Hubert Keckeis, Tomasz Kakareko, Joanna Grabowska and Miroslaw Przybylski for their help with fish sampling, and colleagues from the Institute of Vertebrate Biology and Faculty of Science for help with parasite dissections. We would also like to thank Kevin Roche for correction of our English.
FINANCIAL SUPPORT
This study was supported by the Grant Agency of the Czech Republic, Project No. 524/07/0188 and P505/11/1768.










