Published online by Cambridge University Press: 07 August 2001
The burrowing mite Sarcoptes scabiei is the causative agent of the highly contagious disease sarcoptic mange or scabies. So far, there is no in vitro propagation system for S. scabiei available, and mites used for various purposes must be isolated from infected hosts. Lack of parasite-derived material has limited the possibilities to study several aspects of scabies, including pathogenesis and immunity. It has also hampered the development of high performance serological assays. We have now constructed an S. scabiei cDNA expression library with mRNA purified from mites isolated from red foxes. Immunoscreening of the library enabled us to clone a full-length cDNA coding for a 102.5 kDa protein. Sequence similarity searches identified the protein as a paramyosin. Recombinant S. scabiei paramyosin expressed in Escherichia coli was recognized by sera from dogs and swine infected with S. scabiei. We also designed a small paramyosin construct of about 17 kDa that included the N-terminal part, an evolutionary variable part of the helical core, and the C-terminal part of the molecule. The miniaturized protein was efficiently expressed in E. coli and was recognized by sera from immunized rabbits. These data demonstrate that the cDNA library can assist in the isolation of important S. scabiei antigens and that recombinant proteins can be useful for the study of scabies.
Scabies or sarcoptic mange, caused by infection with the parasitic mite Sarcoptes scabiei, is a widespread, highly contagious disease (Burgess, 1994). The parasite has been found in more than 40 different mammals, including man. During the infection, mites burrow in the skin to feed and reproduce, and their activities cause an intense irritation leading to itching and scratching. Sensitization of the host to the mites and their products probably plays an important role in the pathogenesis of the disease (Burgess, 1994). In its extreme form scabies can develop into a severe hyperkeratotic form where several thousands of mites can be found in the lesions. This form of crusted scabies is frequently observed among immunocompromised individuals, e.g. HIV-patients (Schlesinger, Oelrich & Tyring, 1994; Chosidow, 2000).
The classical method for the diagnosis of scabies is by microscopical demonstration of the mites and their eggs in skin scrapings. This method is, however, time-consuming and insensitive due to the low numbers of parasites often present in the samples. Recently the use of enzyme-linked immunosorbent assays (ELISA) for the detection of antibodies to S. scabiei has been successfully employed for several host species including man (Arlian et al. 1994; Bornstein, Thebo & Zakrisson, 1996; Normaznah et al. 1996; Bornstein & Wallgren, 1997; Hollanders et al. 1997).
Currently, there is no in vitro propagation system for S. scabiei, and mites used for antigen preparation must, therefore, be isolated from infected hosts. The red fox (Vulpes vulpes) is particularly susceptible to mange and naturally infected animals normally carry high numbers of mites (Mörner, 1992). Dead foxes also constitute one of the sources from which large quantities of mites have been isolated and successfully used for the production of antigen in different ELISA-systems (Bornstein & Wallgren, 1997, Bornstein et al. 1996). Nevertheless, the difficulties involved in using live animals, as sources for antigen, are evident. An in vivo propagation system was utilized after establishing S. scabiei var. canis on rabbits (Arlian, 1989). However, the low parasite intensity in this species makes the method unsuitable for growth of mites intended for the preparation of larger batches of antigen.
Lack of parasite material has not only limited the establishment of control programmes based on serological systems, but has also limited the possibilities to study other aspects of scabies, such as pathogenesis and immunity (Burgess, 1994). To overcome these and other problems, recombinant DNA methodology might be one of our best options to study this disease. For this purpose we have constructed an S. scabiei cDNA library in Escherichia coli and screened it for the expression of immunodominant antigens. Several clones coded for paramyosin and we could isolate the complete open reading frame from the cDNA library. After overexpression of S. scabiei paramyosin we could also show that the recombinant protein is recognized by sera from infected animals.
Living mites of both sexes and different developmental stages of S. scabiei were isolated from the skin of wild red foxes as described previously (Bornstein & Zakrisson, 1993). Briefly, pieces of skin with the fur trimmed to about 10-mm were left in Petri dishes at room temperature under an electric light. Mites that migrated onto the underside of the lid were collected and stored at −70 °C until further use.
A total of 160 mg of mites were washed with PBS and then homogenized in a glass grinder at 4 °C. Total RNA was extracted with an RNAgents kit from Promega (Madison, WI). Subsequently, mRNA was isolated by oligo(dT) cellulose chromatography (Amersham Pharmacia Biotech, Uppsala, Sweden) and double-stranded cDNA was synthesized from about 5 μg of S. scabiei mRNA with the ZAP-cDNA system (Stratagene, La Jolla, CA). The cDNA was size-fractionated on a spin-column packed with Sephacryl S-400 and ligated into the EcoRI–XhoI sites of the UNI-ZAP XR vector (Stratagene). After in vitro packaging into λ-phage (Gigapack Gold II, Stratagene), the primary library was analysed by X-gal staining according to the manufacturer's description and then amplified on E. coli XL-1 Blue MRF′.
A protein extract from S. scabiei mites was prepared as previously described (Bornstein & Zakrisson, 1993). From the extract, proteins with a size ranging from 90 to 200 kDa were selected on 10% gels by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and electroeluted (Jacobs & Clad, 1986). Two New Zealand White rabbits were injected i.m. with 200 μl of the eluted proteins (11 μg)+200 μl of Freund's complete adjuvant. The rabbits were then boosted with the same amount of S. scabiei protein and Freund's incomplete adjuvant during weeks 4, 6 and 19. Blood was drawn every second week starting during week 8 and ending during week 20. The amplified library was screened with 1:1000 dilutions of rabbit immune sera. Plaques reading positively were re-screened at a lower density and the process was repeated until plaque-pure populations were obtained. The library was also screened with 1:100 dilutions of pooled sera from S. scabiei-infected dogs.
High titre phage clones were excised as pBluescript SK phagemids with the ExAssist helper phage (Stratagene) according to the manufacturer's instructions, and sequenced with the T3 promoter primer. One of the clones, pPU1, was identified to code for paramyosin. Nested deletion clones of pPU1 and cDNA sequence-specific primers were used to determine the entire sequence of the pPU1 insert. To obtain a cDNA clone containing sequences upstream of the 5′-end of the pPU1 insert, a primer was synthesized (5′-GTT CGC TGT CTC TAA TTG TGC-3′) complementary to nucleotides 46–66 of the sense strand of the cDNA insert. The gene-specific primer and the T3 promoter primer were used in a PCR with template DNA from the cDNA library. The resulting PCR product was treated with Klenow DNA polymerase and T4 DNA polymerase to obtain blunt ends. Following purification using the Wizard PCR prep system (Promega), the product was cloned into the SmaI site of pUC19. After sequencing the inserts of 2 clones, a contigous paramyosin cDNA sequence was assembled and analysed with the GCG program package (Devereux, Haeberli & Smithies, 1984).
The full-length open reading frame of the S. scabiei paramyosin cDNA was amplified by PCR from the cDNA library using Pfu DNA polymerase (Stratagene), the forward primer (5′-ATG TCT GCT AGA TCA GCT AAA TTC-3′) and the reverse primer (5′-CGG GAT CCT TAA TTG GAT TGC TCT TC-3′). The underlined nucleotides form a linker containing a BamHI site. The resulting 2.6-kb PCR product was precipitated with ethanol, resuspended in TE-buffer and digested with BamHI. Following agarose gel purification using GeneClean (Bio101), the cDNA fragment was cloned into the XmnI–BamHI sites of the pMAL-c2 vector (New England Biolabs, Beverly, MA) to yield a maltose-binding protein (MBP) fusion. The resulting plasmid was designated pPU5. A 3′-end truncated version of the S. scabiei paramyosin cDNA was generated through digestion of pPU5 with SacI and XbaI, gel purification as above and ligation into SacI–XbaI sites of pPU16. The resulting plasmid was designated pPU58. The pPU16 vector is an MBP fusion expression vector derived from pMAL-c2 that allows for C-terminal fusion to a hexa histidine tag. A modified miniaturized form of paramyosin cDNA was constructed through the amplification and ligation of 3 different fragments into pPU16. A 105 bp fragment corresponding to the N-terminal part of paramyosin was cloned into the SacI–EcoRI sites of pPU16, followed by the addition of a 195 bp fragment corresponding to a central domain of paramyosin that was cloned into the EcoRI–XbaI sites. The last fragment corresponding to the 120 bp C-terminal end of paramyosin was cloned into XbaI–PstI sites. The resulting plasmid was designated pPU57. For amplification of the 5′ fragment, the forward primer (5′-GGT CGT CAG ACT GTC GAT GAA GCC-3′) and the reverse primer (5′-CCG GAA TTC GGT CAG AGC ACC GAG ATC AG-3′) were used. The central domain was amplified with the forward primer (5′-CCG GAA TTC AAG GCC ACC ACA CAC GCT CAA CA-3′) and the reverse primer (5′-TGC TCT AGA AGC CAA AGC ATC TCG TTG ATC T-3′). The 3′-end fragment was amplified with the forward primer (5′-TGC TCT AGA CAA GCT GAA TCA AAT CTA TCG-3′) and the reverse primer (5′-AAA CTG CAG ATA ATT GGA TTG CTC TTC TTG-3′). The underlined nucleotides are linkers introduced to facilitate the cloning. For the construction of the miniaturized paramyosin cDNA, pPU5 was used as a template in all the amplifications.
For the initial expression analysis, transformed E. coli (XL1-Blue MRF′) were grown in LB-medium with ampicillin (100 μg/ml) to an OD600 of about 0.5 at 37 °C. After sampling, the cultures were induced with 0.3 mM isopropyl-β-thiogalactopyranoside (IPTG). After 2 h of additional growth at 37 °C, samples were taken and analysed by SDS–PAGE and Western blot analysis.
For purification of full-length paramyosin, a culture of E. coli strain TOPP3 transformed with pPU5 was grown and harvested as described for the MBP system (Riggs, 1997). The recombinant fusion protein was affinity purified on an Amylose resin column (New England Biolabs) and the MBP was cleaved off with factor Xa (Riggs, 1997).
The miniaturized paramyosin was expressed in BL21-DE3 that was grown in a minimal medium with casamino acids and heavy metals (MM/CA) containing ampicillin (100 μg/ml) (Pryor & Leiting, 1997). At an OD600 of 0.8 the culture was cooled to 18 °C, induced with 0.5 mM IPTG and incubated at 18 °C for continued growth overnight. After harvest and cell lysis, the recombinant fusion protein was purified on a HiTrap Chelating column (Amersham Pharmacia Biotech). The recombinant protein was released from the MBP as above.
E. coli lysates were separated by SDS–PAGE in mini gels by standard procedures. Blotting of proteins to nitrocellulose filters was done by using an electrophoretic transfer cell. The quality of the protein transfer was checked by briefly incubating the membrane in a 0.2% Ponceau-S solution followed by rinsing in distilled water. The membranes were blocked with Tris-buffered saline with Tween 20 (T–TBS) containing 5% non-fat dry milk for 1 h. Membranes were briefly washed with T–TBS and then incubated for 1 h with different sera diluted in T–TBS containing 1% non-fat dry milk. Sera from immunized rabbits were diluted 1:1000 and sera from S. scabiei-infected dogs and S. scabiei-infected pigs were diluted 1:100. The membranes were rinsed and washed once for 15 min and twice for 5 min with T–TBS before they were incubated for 1 h with the appropriate anti-IgG peroxidase conjugate. The membranes were washed 1×15 min and 3×5 min with T–TBS before bound antibodies were visualized by chemiluminescence detection through exposure to film using the ECL-system (Amersham Pharmacia Biotech).
The quality of the primary cDNA library was assessed in 2 different tests. The first was a background test using an X-gal-staining protocol. Less than 1.5% of the plaques were blue, suggesting that more than 98.5% of them carried an insert. The second test was done to evaluate the sizes of inserts. A total of 20 plaques were randomly selected from the primary library and used in a PCR with vector specific T3 and T7 primers. All of the analysed plaques carried inserts, half of them had inserts between 0.5 kb and 1.0 kb, 6 had inserts between 1.1 and 1.8 kb and 4 had inserts above 1.8 kb. The largest insert was close to 3 kb. For the amplification of the library, a total of 106 plaque forming units (pfu) were used.
The initial screening of the amplified cDNA-library with hyperimmune rabbit sera identified a number of antibody-reactive plaques. After re-screening to single plaque clones and in vivo excision with the Exassist system, the 5′-ends of 12 clones were sequenced. The deduced amino acid sequences for 7 of these corresponded to myosin and for 3 of the clones, the sequences were homologous with paramyosin. The last 2 clones were both unique and no obvious match could be identified. The majority of the inserts were around 1.5 kb. However, 2 of the myosin clones were above 3 kb and 1 of the unique clones was around only 0.6 kb. The library was also screened with sera from dogs naturally infected with S. scabiei. From this screening, 3 clones corresponding to paramyosin were isolated.
The immunoscreening and sequence analysis had identified 6 clones as homologous with paramyosin, an invertebrate muscle protein. Since paramyosin has been recognized as an important antigen for various parasitic helminths, we decided to investigate the S. scabiei paramyosin in some detail. One of the clones, pPU1, homologous to paramyosin, contained an insert of about 2.1 kb. This sequence was used for designing an oligonucleotide primer complementary to a region close to the insert's 5′-end. The primer was used in combination with the T3 promoter primer to amplify and clone an 800 bp DNA product from the library. The DNA product corresponded to the upstream region of the cDNA and included the potential start codon. The nucleotide sequences of pPU1 and upstream extension clones ParaM5′ 14 and ParaM5′ 16 are shown as a continuous sequence in Fig. 1 together with the amino acid translation. The predicted initiation codon in surrounded by A at position −3 and T at position +4, a context which is identical to paramyosin cDNAs from Drosophila melanogaster (Becker et al. 1992) and Onchocerca volvulus (Dahmen et al. 1993). An additional ATG-codon starting at position +25 is not in a favourable translation context, since there is a T at the crucial −3 position. Upstream of the proposed start codon, several in-frame stop codons are present, including one tandem pair. The open reading frame (ORF) encodes a protein of 876 amino acids with a molecular weight of 102467 Dalton. With the exceptions of the amino-terminal 27 residues and 31 residues at the carboxy-terminus, the S. scabiei paramyosin amino acid sequence maintains both the 7 and the 28 repeat patterns characteristic for paramyosin (Limberger & McReynolds, 1990; Laclette et al. 1991; Becker et al. 1992; Landa et al. 1993). In Table 1 are sequence similarity data shown for S. scabiei paramyosin in comparison to other invertebrates, including several parasites. The complete S. scabiei paramyosin cDNA sequence has been deposited in GenBank under the accession number AF317670.

Fig. 1. Nucleotide sequence of Sarcoptes scabiei paramyosin cDNA and amino acid sequence of the predicted translation product. The recognition site for the XbaI site, used for the construction of MBP-paramyosin ΔXbaI, is indicated by a box. The N- and C-terminal parts and central domain of S. scabiei paramyosin that were used for the construction of the miniaturized paramyosin are indicated by solid lines.

The open reading frame for S. scabiei paramyosin was ligated into the expression vector pMAL-c2 in fusion with the open reading frame of the maltose binding protein (MBP) resulting in the plasmid pPU5. An E. coli culture harbouring pPU5 was induced for the expression of the MBP-paramyosin fusion protein and was analysed by SDS–PAGE. The pPU5 expressed a comparatively low level of a 145 kDa protein, corresponding to the predicted size of the full-length fusion protein (Fig. 2A). Confirmation of this 145 kDa protein band as a S. scabiei-derived paramyosin was obtained by Western blotting, using a rabbit hyperimmune serum raised against native S. scabiei antigens (Fig. 2B). As a contrast, none of the pre-immune sera reacted with the recombinant antigen (data not shown). The Western blot data indicated that only a fraction of the induced protein was of full-length. This could be due to either E. coli-induced degradation of the recombinant protein or the result of translation associated problems. Switching to other strains of E. coli did not have any significant effect on the ratio of the full-length protein versus shorter fragments. However, a higher expression of total recombinant protein could be obtained by selecting the TOPP3 strain (data not shown). In contrast, a construct with the N-terminal part of the S. scabiei paramyosin in frame with the MBP (MBP-paramyosin ΔXbaI) was expressed to very high levels (Fig. 2A). This fusion protein contained about 40% or the first 344 amino acid residues of the S. scabiei paramyosin and the resulting product was readily recognized by rabbit antisera (Fig. 2B). The MBP-paramyosin ΔXbaI fusion protein appeared to be well tolerated by the host cells, but a fraction of the induced protein was of smaller size compared to the predicted molecular weight.

Fig. 2. Analysis of whole cell lysates of E. coli expressing Sarcoptes scabiei paramyosin and derivatives of that protein. Coomassie Blue stained SDS–polyacrylamide gel (10%, reducing condition) is shown in (A). The corresponding Western blot analysis using serum from a rabbit immunized with an S. scabiei protein extract is shown in (B). Lane 1, total E. coli lysate without any recombinant plasmid; lanes 2, 3 expression of MBP-paramyosin before and after induction with IPTG, respectively; lanes 4, 5 expression of MBP-paramyosin ΔXbaI before and after induction with IPTG, respectively; lanes 6, 7 expression of the miniaturized paramyosin in fusion with MBP before and after induction with IPTG, respectively. The sizes of the molecular weight markers are given in kDa (New England Biolabs pre-stained protein marker).
Sera from virtually all infected animals reacted extensively with E. coli protein, including some that reacted with the MBP. The full-length variant of the paramyosin fusion protein was therefore affinity purified from an E. coli extract, using an amylose resin column. The MBP was cleaved from paramyosin using the protease factor Xa and the purified protein was analysed by Western blot. Sera from experimentally infected dogs as well as experimentally infected pigs reacted with the recombinant paramyosin (Fig. 3A and B). However, some of the tested sera did not react with the recombinant paramyosin (Fig. 3B).

Fig. 3. Western blot analysis of Sarcoptes scabiei paramyosin with sera from infected animals. Full-length paramyosin was expressed in fusion with the maltose binding protein (MBP) in the E. coli TOPP3 strain. The recombinant protein was purified on an amylose resin column and the MBP was cleaved from paramyosin with the specific protease factor Xa. (A) Immunoblot of recombinant paramyosin after incubating with sera from naturally infected dogs. (B) Immunoblot with sera from 2 experimentally infected pigs (lanes 1 and 2) and serum from a control pig (lane 3). The bars indicate the size of full-length paramyosin.
Paramyosin is evolutionary rather conserved among various species. We therefore decided to construct a miniaturized version of paramyosin. The cDNA sequences corresponding to the N- and C-terminal parts of paramyosin, unique to S. scabiei, were amplified and cloned in fusion with a fragment coding for a relatively divergent, central domain of S. scabiei paramyosin. The resulting miniaturized form of paramyosin had dual affinity-tags attached to it: MBP at its N-terminus and a His-tag at its C-terminus. The recombinant protein could be produced at high levels and was recognized by rabbit serum (Fig. 2A and B). There were almost no small-sized fusion proteins present in the induced samples, suggesting that this short fusion protein was well tolerated by E. coli. The His-tag was efficient for a rapid affinity purification and after removal of the MPB tag by factor Xa cleavage, the miniaturized version of paramyosin was about 17 kDa and was recognized by sera from rabbits immunized with S. scabiei proteins. The results from Western blot analysis with sera from infected dogs and swine, however, were rather weak and inconclusive.
Research on the infection biology of S. scabiei and the immunology of scabies poses several interesting challenges. However, the difficulty in obtaining scabies mites regularly and in sufficient quantities has always been a critical limitation. Access to an S. scabiei cDNA expression library should, therefore, be useful for the study of several aspects of scabies, including molecular host–parasite interactions. Production of large quantities of recombinant S. scabiei proteins will not only be helpful for the characterization of various virulence factors but also for applied diagnostic and immunoprophylactic studies.
The cDNA library presented here was constructed from mRNA isolated from S. scabiei mites parasitizing red foxes. There is only 1 recognized species of Sarcoptes and, in general, there are only limited morphological differences among S. scabiei mites isolated from different host species (Burgess, 1994). Several studies have shown that S. scabiei mites from one species of mammal may be transmitted occasionally to other species although clinical observations and experimental studies suggest that those infections mostly are self-limiting (Arlian, 1989; Burgess, 1994). Consequently, S. scabiei isolates from different kinds of hosts are often classified as different varieties. In a recent molecular fingerprinting study, based on S. scabiei-specific hypervariable microsatellite markers, it was observed that genotypes of dog-derived S. scabiei mites and human-derived S. scabiei mites cluster by host species and not by geographical origin (Walton et al. 1999). Nevertheless, the one-species concept has found firm support in a recent phylogenetic study (Zahler et al. 1999). Through sequence analysis of the second internal transcribed spacer (ITS2) of the ribosomal RNA complex Zahler and coworkers demonstrated that Sarcoptes mites from various hosts is one and the same species (Zahler et al. 1999). Thus, the source of S. scabiei mites for construction of genetic libraries or for the purification of specific molecules will rarely be any problem. Still the unique interface between a particular host species and S. scabiei mites derived from that host, has to be taken into consideration in certain applications.
Immunoscreening of the cDNA library identified several independent clones and, with the aid of PCR, we have cloned the complete open reading frame for S. scabiei paramyosin including 5′UTR and 3′UTR. The role for paramyosin in helminth infections is well known and several studies have identified the protein as a target for protective immunity (for a review see Kalinna & McManus, 1997). The inherent immunogenic properties of paramyosin might explain why we isolated the cDNAs in this study, especially as we used hyperimmune sera from rabbits. However, we could also isolate clones coding for paramyosin when we used sera from S. scabiei-infected dogs. In order to verify the results we over-expressed paramyosin in E. coli and tested it by Western blot analysis. These results suggested that paramyosin is indeed recognized by infected animals. It might be argued that S. scabiei paramyosin is so similar to paramyosin from other species so that what we observe are cross-reacting antibodies originating from concomitant helminth infections. It has previously been recognized that both the N- and C-terminus of paramyosin are much less conserved (Dahmen et al. 1993; Limberger & McReynolds, 1990). We therefore designed and expressed a miniaturized version of paramyosin that included only the N-terminal part, a short stretch of the helical core that is evolutionary variable, and the C-terminal part of the molecule. The helical element included conformed to the structural description of paramyosin with periodic heptad repeats but it had less similarity with paramyosin from other species that were available for sequence comparison. The miniaturized paramyosin was readily expressed in E. coli and it was detected by anti-S. scabiei rabbit serum in Western blot experiments. However, the purified miniaturized protein was not easily detected by antibodies from dogs infected by S. scabiei, suggesting that important epitopes were removed during the subcloning. Overall, it appears that paramyosin is an important immunogen also in scabies, although the precise interaction between the host's immune system and S. scabiei paramyosin remains to be clarified. A recombinant paramyosin could potentially be used as an infection marker in immunoassays, e.g. ELISA. However, due to the large number of evolutionarily conserved epitopes, paramyosin could perhaps be applied as a general marker for helminth and ectoparasite infections.
S. scabiei mites live in the outer layers of the skin and do not feed on blood as many other parasitic arthropods. Despite this, many hosts can mount an efficient immune response in order to control the S. scabiei infection. This means that various components of the immune system are able to respond and act on the parasite. In protein lysates of Sarcoptes mites that have been isolated from infected animals as described above, we have detected host IgG antibodies (data not shown). This demonstrates that antibodies can be found associated with the mites. Thus, a potential approach for the control of S. scabiei could be vaccination. For this strategy, the identification of protective so called ‘concealed’ antigens could be pivotal (Willadsen, 1997). This class of antigens are often located in the internal organs of the parasite, are not normally exposed to the host and have a critical physiological role in the parasite. Concealed antigens have for instance been successfully employed for vaccination against the cattle-tick Boophilus microplus, and promising results to control the blow-fly Lucilia cuprina suggest that the use of concealed antigens can be extended to non-blood sucking ectoparasites (Tellam & Bowles, 1997; Willadsen, 1997). Thus, the expectation is that further exploration of the cDNA library will shed more light on the various S. scabiei antigens that are exposed to the host immune system during infection. The library will also be a valuable tool for the identification of other groups of proteins that could be used for immunological control of S. scabiei. Whether paramyosin will have a role as target for protective immunity in scabies remains to be proved.
This work was financially supported by grants from the National Veterinary Institute and from Pfizer Animal Health Inc., New York. We thank Professor A. Uggla, Dr S. Bornstein, Dr A. Tallsjö and Dr S. T. Tolling for helpful advice and support. This study is part of the EU research collaboration 833.

Fig. 1. Nucleotide sequence of Sarcoptes scabiei paramyosin cDNA and amino acid sequence of the predicted translation product. The recognition site for the XbaI site, used for the construction of MBP-paramyosin ΔXbaI, is indicated by a box. The N- and C-terminal parts and central domain of S. scabiei paramyosin that were used for the construction of the miniaturized paramyosin are indicated by solid lines.

Table 1. Sequence comparison between paramyosin from Sarcoptes scabiei and paramyosin from other invertebrates
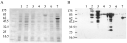
Fig. 2. Analysis of whole cell lysates of E. coli expressing Sarcoptes scabiei paramyosin and derivatives of that protein. Coomassie Blue stained SDS–polyacrylamide gel (10%, reducing condition) is shown in (A). The corresponding Western blot analysis using serum from a rabbit immunized with an S. scabiei protein extract is shown in (B). Lane 1, total E. coli lysate without any recombinant plasmid; lanes 2, 3 expression of MBP-paramyosin before and after induction with IPTG, respectively; lanes 4, 5 expression of MBP-paramyosin ΔXbaI before and after induction with IPTG, respectively; lanes 6, 7 expression of the miniaturized paramyosin in fusion with MBP before and after induction with IPTG, respectively. The sizes of the molecular weight markers are given in kDa (New England Biolabs pre-stained protein marker).

Fig. 3. Western blot analysis of Sarcoptes scabiei paramyosin with sera from infected animals. Full-length paramyosin was expressed in fusion with the maltose binding protein (MBP) in the E. coli TOPP3 strain. The recombinant protein was purified on an amylose resin column and the MBP was cleaved from paramyosin with the specific protease factor Xa. (A) Immunoblot of recombinant paramyosin after incubating with sera from naturally infected dogs. (B) Immunoblot with sera from 2 experimentally infected pigs (lanes 1 and 2) and serum from a control pig (lane 3). The bars indicate the size of full-length paramyosin.