INTRODUCTION
Cystic hydatidosis is a zoonotic infection caused by the taeniid cestode parasite Echinococcus granulosus. A number of domestic animal species (sheep, goats, cattle, pigs and other herbivores) act as the main intermediate hosts for the parasite while canids are the definitive hosts. Infection in humans causes substantial morbidity and mortality in endemic countries (Jenkins et al. Reference Jenkins, Romig and Thompson2005; Moro and Schantz, Reference Moro and Schantz2009).
Many countries have had active control programmes for hydatid disease that have been based on public education about the parasite and control of infection in dogs. However, few control programmes have had a major impact on disease transmission (reviewed by Craig and Larrieu, Reference Craig and Larrieu2006) and alternative strategies are required to improve the effectiveness of disease control measures. One potential new strategy is vaccination of the intermediate hosts. For this purpose, a vaccine has been developed utilizing a defined recombinant antigen (EG95) (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Health1996). The vaccine has been shown to induce more than 95% protection against hydatid infection in vaccinated sheep (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Health1996, Reference Lightowlers, Jensen, Fernandez, Iriarte, Woollard, Gauci, Jenkins and Heath1999). Woollard et al. (Reference Woollard, Gauci, Heath and Lightowlers2001) used truncated regions of EG95 (EG95-1, EG95-2 and EG95-3) antigen and found that the host-protective epitopes of EG95 antigens were conformational. A substantial amount of research has been undertaken on various aspects of the EG95 vaccine (Woollard et al. Reference Woollard, Gauci, Heath and Lightowlers1998, Reference Woollard, Gauci and Lightowlers2000a,Reference Woollard, Heath and Lightowlersb, Reference Woollard, Gauci, Heath and Lightowlers2001; Chow et al. Reference Chow, Gauci, Cowman and Lightowlers2001, Reference Chow, Gauci, Cowman and Lightowlers2004, Reference Chow, Gauci, Vural, Jenkins, Heath, Rosenzvit, Harandi and Lightowlers2008). However, little information is available about the cellular source of the EG95 antigen in E. granulosus oncospheres.
Recent studies have localized the host-protective antigens of Taenia ovis (Jabbar et al. Reference Jabbar, Kyngdon, Gauci, Walduck, McCowan, Jones, Beveridge and Lightowlers2010a,Reference Jabbar, Świderski, Mlocicki, Beveridge and Lightowlersb), T. saginata and T. solium (Jabbar et al. Reference Jabbar, Verástegui, Lackenby, Walduck, Gauci, Gilman and Lightowlers2010c) to one or both of two types of oncospheral penetration gland cell. The ultrastructure of these cells in T. ovis oncospheres has been defined from ultrathin serial sections examined in transmission electron microscopy (Jabbar et al. Reference Jabbar, Crawford, Młocicki, Świderski, Conn, Jones, Beveridge and Lightowlers2010d). Previously, a brief report of a conference presentation has suggested that EG95 may also localize to penetration gland cells in E. granulosus (Holcman et al., unpublished observations ), but no further studies have been published. Here we use immunohistochemical and immunogold-labelling methods to identify the presence of the EG95 specifically and unequivocally in penetration gland cells of E. granulosus oncospheres.
MATERIALS AND METHODS
Collection of the parasite
Mature gravid E. granulosus were collected from the intestine of the naturally-infected wild dogs (dingo/domestic dog hybrids) obtained with the assistance of Vertebrate Pest Control Officers, Tumbarumba, NSW, Australia. The intestine was removed from the dog and kept on ice during transport to the School of Animal and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW. Eggs were recovered from gravid worms as previously described (Jenkins and Thompson, Reference Jenkins and Thomson1995).
Collection, hatching, activation and culture of oncospheres
Mature eggs were re-suspended in water, counted using a Modified Fuchs-Rosenthal counting chamber and diluted or concentrated according to the required number of eggs. Hatching and activation of oncospheres was undertaken as described by Woollard et al. (Reference Woollard, Heath and Lightowlers2000b). Briefly, following centrifugation (200 g, 10 min) eggs (10 000–40 000) were resuspended in 12 ml of artificial gastric fluid (AGF; 1% HCl, 1% pepsin (Sigma-Aldrich, NSW, Australia)) in water and incubated at 37°C in a water bath for 1 h with re-suspension carried out by gentle inversion of the tube every 10 min. The suspension was then centrifuged (200 g, 10 min) and the supernatant removed, leaving approximately 500 μl of AGF above the eggs. This was subsequently re-suspended in 12 ml of artificial intestinal fluid (AIF) solution (1% pancreatin (Sigma-Aldrich), 1% NaHCO3 and 5% sheep bile in water) and incubated as above. The hatched and activated oncospheres were washed (centrifugation 200 g, 10 min) at least 5 times with pre-warmed (37°C) culture medium RPMI-1640 (RPMI; Invitrogen, Vic, Australia). After washing, the oncospheres were resuspended in 1 ml of RPMI and an aliquot was examined microscopically in a Modified Fuchs-Rosenthal counting-chamber to determine the proportions of activated and non-activated oncospheres. The oncospheres were defined as non-activated if they were immobile and had centrally located hooks or were designated as activated if they showed motility. Then 5 ml of pre-warmed (37°C) Percoll (Sigma-Aldrich) were mixed with the oncospheral suspension, followed by centrifugation at 1200 g for 20 min at 37°C. Oncospheres were removed from the surface with a Pasteur pipette, added to excess RPMI plus 1:100 antibiotic/antimycotic solution (AAS, Sigma-Aldrich) and washed 5 times by centrifugation at 200 g for 10 min, in RPMI-AAS at 37°C.
Production of antigens
Recombinant antigens (EG95, EG95-1, EG95-2 and EG95-3) were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins and purified as previously described (Lightowlers et al. Reference Lightowlers, Lawrence, Gauci, Young, Ralston, Maas and Health1996; Woollard et al. Reference Woollard, Gauci, Heath and Lightowlers2001). The cDNA encoding EG95 protein was also cloned into the vector pMAL-C2 (Maina et al. Reference Maina, Riggs, Grandea, Slatko, Moran, Tagliamonte, McReynolds and Guan1988) and expressed and purified as a maltose binding protein (MBP) fusion as described by Woollard et al. (Reference Woollard, Gauci, Heath and Lightowlers1998). GST and MBP (control) proteins were also prepared.
Production of polyclonal antisera
All the procedures were performed in accordance with the requirements of the University of Melbourne Animal Ethics Committee. Four 1-year-old Dorset-Merino sheep were used to raise antisera against EG95-1, EG95-2 and EG95-3. Three animals were injected with 50 μg of each of the antigens combined plus 1 mg of Quil-A (Superfos Biosector a/s, DK-Vedbaek, Denmark) while a fourth animal received GST together with 1 mg of Quil-A subcutaneously on days 0, 31 and 61. Sera were obtained from blood samples collected prior to the first immunization and on day 70 after the first immunization.
Antisera against EG95-MBP and MBP were raised in mice using 2 groups of 6 mice (6 to 8-week-old Balb/c mice). On day 0, the animals were bled to collect pre-immunization sera and subsequently injected subcutaneously with 100 μg of either EG95-MBP or MBP plus 100 μl of Freund's Incomplete Adjuvant (Sigma-Aldrich) on days 0, 15, 30, 45 and 75. The mice were bled from the tail to check the titre and on day 85 all the animals were bled via cardiac puncture under anaesthetic.
Two 6 to 8-month-old Dorset-Merino sheep were used to raise antisera against EG95-GST. Each animal was injected with 50 μg of EG95-GST protein together with 1 mg of Quil-A subcutaneously on day 0 and subsequently on day 31. Sera were obtained from blood samples collected prior to the first immunization and on day 40 after the first immunization.
Western blotting
Western blot analysis was used to determine the specificity of antibodies raised against each host-protective antigen. Electrophoresis of recombinant proteins was carried out in 13% SDS PAGE and electroblotted using a Trans-Blot® SD Semi-Dry Electrophoretic Cell (Bio-Rad Labs, CA, USA) to a nitrocellulose membrane (Hybond ECL™ – Amersham Biosciences, UK). The membrane was blocked with 5% skim-milk powder in phosphate-buffered saline (PBS, 145 mm NaCl, 2·7 mm KCl, 12 mm Na2HPO4, 1·2 mm KH2PO4, pH 7·4) plus 0·05% Tween 20 (PBST; Sigma-Aldrich) overnight at 4°C. After washing, the membrane was incubated with immune serum diluted 1:1000 in PBST at ambient temperature for 1 h. Pre-immune serum at equivalent dilution was used as a negative control. The membrane was washed and incubated with secondary antibodies labelled with horseradish peroxidase (IgG-HRP) (rabbit anti-sheep, Zymax™ Zymed, USA; sheep anti-mouse, Sigma-Aldrich) diluted 1:4000 in 5% skim-milk powder in PBST at ambient temperature for 1 h. After washing, enhanced chemiluminescent substrate for detection of HRP (Pierce ECL Western Blotting Substrate, Quantum Scientific, Australia) was added to the membrane and exposed to X-ray film. The developed film was scanned using a Personal Densitometer SI - 375 (Molecular Dynamics).
Enzyme-linked immunosorbent assay (ELISA)
Animals immunized with EG95-GST, EG95-MBP, truncated EG95-1, -2 and -3-GST, or control proteins GST and MBP, were assessed for specific antibody titres against the respective immunizing antigens using ELISA, essentially as described by Kyngdon et al. (Reference Kyngdon, Gauci, Rolfe, Velasquez Guzman, Farfan Salazar, Verastegui Pimentel, Gonzalez, Garcia, Gilman, Strugnell and Lightowlers2006). The secondary antibodies used for ELISA were the same as those used for Western blotting. The optical density (O.D. 450 nm) for each serum was plotted and the titre read as the dilution at which the O.D. equaled 1·0.
Immunohistochemistry
The labelled streptavidin-biotin method was used for immunohistochemical localization of antigen as described by Jabbar et al. (Reference Jabbar, Kyngdon, Gauci, Walduck, McCowan, Jones, Beveridge and Lightowlers2010a). Briefly, gravid segments and hatched-activated oncospheres were fixed in absolute methanol. Following fixation, the oncospheres were embedded in low melting point agarose (LMP Preparative Grade for Large Fragments, Promega, WI, USA) and then in paraffin followed by routine histological procedures.
Deparaffinized sections (3–5 μm) were permeabilized in PBS (NaCl 124 mm, Na2HPO4 10 mm, KH2PO4 6 mm, pH 7·4) containing 0·1% Triton X-100 (BDH Chemicals, VIC, Australia) for 30 min at ambient temperature on a rocker platform. After washing 3 times in PBS plus 0·025% Triton X-100 (PBSTr), the sections were blocked for non-specific binding (1% bovine serum albumin (BSA), Sigma-Aldrich; 20% rabbit or goat serum, Sigma-Aldrich in PBS) for 2 h at ambient temperature after which they were rinsed briefly in PBSTr and incubated with an optimal dilution of sheep (EG95-GST, 1:25 000; fragments of EG95-1, EG95-2 and EG95-3-GST, 1:1000) or mouse (EG95-MBP; 1:1000) primary antibodies in 1% BSA plus 1% either rabbit or goat serum in PBS at 4°C overnight. Controls included sections without any antibody, with secondary antibodies and pre-immune, anti-GST or MBP sera at the same dilution as that of the specific antisera. After washing, the sections were incubated for 2 h at ambient temperature with diluted (1:1000) biotinylated secondary antibody F(ab)2 fragment of biotin-conjugated secondary antibodies (rabbit anti-sheep and goat anti-mouse; Jackson ImmunoResearch Labs, PA, USA). The sections were washed 3 times in PBSTr and endogenous peroxidase activity eliminated with 3% aqueous H2O2 for 5 min at ambient temperature. Following a quick rinse in PBSTr, the sections were incubated with labelled streptavidin-peroxidase conjugate (Dako LSAB®2 Streptavidin-HRP, NSW, Australia) for 30 min at ambient temperature. After washing, the sections were incubated with the chromagen (Vector®NovaRed™ substrate Kit for Peroxidase, Vector Labs, CA, USA) for 2 min at ambient temperature and, following washing, counterstained with Gill's haematoxylin and mounted in DPX (DPX Neutral Mounting Medium, Ajax Finechem Pty. Ltd, NSW, Australia). Photographs were taken at 1000× magnification using an Olympus (BX41) microscope fitted with an Olympus DP71 camera and DP Controller software (Olympus, Japan).
Immunogold labelling
Hatched and activated oncospheres were gently pelleted (centrifugation 200 g, 10 min) and the supernatant discarded. About 2–3 μl droplets of the suspension were sandwiched between type A brass freezer hats (ProSciTech, Thuringowa, Qld, Australia). The enclosed oncosphere suspensions were then frozen using a Leica EM High Pressure Freezer (Vienna, Austria). The freezer hats enclosing the frozen oncosphere suspensions were split apart and stored in liquid N2 in cryo-vials prior to freeze-substitution in a Leica EM automated freeze-substitution unit.
Frozen oncosphere pellets were freeze-substituted in 0·1% uranyl acetate in acetone at –90°C for 48 h and the temperature raised to –50°C at 6°C/h. The pellets were scraped out of the freezer hats, rinsed in 3×30 min changes of acetone. The samples were then infiltrated with a graded series of Lowicryl HM20 low temperature resin (Polysciences, Warrington, PA, USA) in acetone consisting of 25% (8 h), 50% (overnight), 75% (8 h) and 100% resin (overnight). The infiltrated samples were placed in a fresh change of 100% resin in gelatin capsules, polymerized under UV light for 48 h at −50°C, and brought to room temperature at 6°C/h. The soft sample blocks were then hardened under UV light for a further 24 h at room temperature.
The oncospheres embedded in blocks were sectioned with a diamond knife on a Leica Ultracut R microtome (Vienna, Austria) and ultra-thin sections (90 nm) collected onto pioloform-coated 100 mesh hexagonal gold grids (ProSciTech, Thuringowa, Qld, Australia). The grids were blot dried with the section side up on filter paper overnight prior to immunolabelling. The sections were blocked for non-specific antibody binding sites by incubating the grids on 20 μl droplets of blocking buffer (1% BSA in PBS containing 0·01% of each of Tween 20 and Triton X-100) for 30 min. Grids were then incubated on 20 μl droplets of optimally diluted primary antibodies (1:10 000) against EG95-MBP in blocking buffer overnight at 4°C. Controls were reacted with anti-MBP antisera at the same dilution as that of the specific antisera. Grids were washed 3 times on 20 μl droplets of blocking buffer for 5 min each and then incubated on 20 μl droplets of diluted (1:100 with blocking agent) colloidal gold conjugated goat anti-mouse secondary antibodies (12 nm Colloidal Gold-AffiniPure Goat Anti-Mice IgG, Jackson ImmunoResearch Labs) overnight at 4°C. Labelled grids were rinsed 3 times on the drops of blocking buffer, then 3 times on drops of PBS, followed by 3×30 s immersions in distilled water before air drying. The immunolabelled sections on grids were sequentially stained with 2% uranyl acetate for 10 min and Triple Lead Stain for 5 min (Sato, Reference Sato1968) and viewed in a Phillips CM120 Biotwin transmission electron microscope at 120 kV. Images were captured with a Gatan Multiscan 600CW digital camera (Gatan Inc, CA, USA) using the appropriate software.
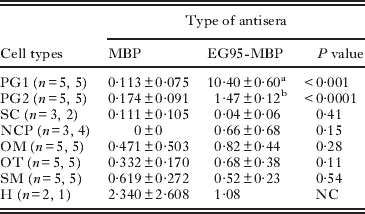
Quantification of immunogold labelling and statistical analysis
A quantitative analysis of the distribution of immunogold particles was performed to determine the immunolabelling density for EG95 in the cytoplasm of oncospheral penetration gland cells. Briefly, 5 oncospheres with intact ultrastructure and clear immunolabelling from the sections stained with EG95-MBP as well as MBP antisera were randomly chosen for counting the total immunogold particles in the penetration gland cells. Additionally, immunogold particles were also counted on other cell types, muscles, hooks, tegument as well as the oncospheral membrane which is a cytoplasmic layer (Holcman et al. Reference Holcman, Heath and Shaw1994). Total area for all cell types was measured for each oncosphere by the software Image J (National Institutes of Health, UAS) and the number of immunogold particles in each cell counted manually. The labelling density was calculated by dividing the immunogold particle (EG95) numbers by the area (i.e., EG95 number/μm2 cellular area). The distribution of immunogold particles was categorised according to different cell types to determine cell types associated with EG95. Statistical comparison of labelling density between treatment and control antisera for each cell type was performed using a t-test assuming unequal variances. The unit of analysis was the oncosphere and those oncospheres with 2 cells of a particular type used the sum of the gold particles divided by the sum of their areas as the response variable. A paired t-test compared the labelling density between the penetration gland cell type 1 (PG1) and 2 (PG2) within the oncospheres reacted with EG95-MBP antisera. Stata 11.0 for Windows (StataCorp, College Station TX) was used for analysis and a two-tailed P-value <0·01 was considered to be statistically significant.
RESULTS
Specificity and sensitivity of polyclonal antisera
Specific antibodies were found in all of the antisera raised against the various antigens (EG95, EG95-1, EG95-2, EG95-3-GST and EG95-MBP) of E. granulosus (Fig. 1). Antibody titres measured in ELISA for antisera used in the immunolocalization studies were as follows: EG95-GST (80 000), EG95-MBP (2000) and truncated EG95-GST fragments (5500).

Fig. 1. Western blot analysis of host-protective EG95 antigen of Echinococcus granulosus. (A) Coomassie-stained SDS–PAGE gel containing the antigens: Lane 1, glutathione S-transferase (GST); Lane 2, maltose-binding protein (MBP); and Lane 3, EG95-MBP; LMW, low molecular weight marker. The gel was transferred to a nitrocellulose membrane and probed with antisera against EG95-GST (B) or truncated fragments of EG95 (EG95-1, EG95-2 and EG95-3) (C) followed by incubation in horseradish peroxidase-conjugated rabbit anti-sheep antibodies and detected with chemiluminescent substrate. (D) Coomassie-stained SDS–PAGE gel containing the antigens: Lane 1, MBP; Lane 2, GST; and Lane 3, EG95-GST; LMW, low molecular weight marker. The gel was transferred to a nitrocellulose membrane and probed with sera against EG95-MBP (E) followed by incubation in horseradish peroxidase-conjugated sheep anti-mouse antibodies and reactively detected as above. All the antisera raised against EG95 were found to have specific reactivity with their respective antigens.
Immunohistochemistry
EG95 was detected in mature eggs, hatched oncospheres and activated oncospheres of E. granulosus. Antisera against EG95-GST and EG95-MBP exhibited similar patterns of staining (Fig. 2). No positive staining was seen with antisera against EG95-1, EG95-2 and EG95-3-GST even at high concentrations (1:100) of antisera (Fig. 2). Specific staining with EG95-GST and EG95-MBP showed a distinctive bilateral pattern of staining in mature eggs as well as in non-activated oncospheres, and appeared to occur within 2 pairs of cells situated bilaterally. The pattern of staining in non-activated oncospheres varied depending on the plane and orientation at which the individual oncospheres had been sectioned. However, a general pattern of staining became clear upon examination of a large number of sections (Fig. 2). The shape of cells containing the antigen varied from oval to irregular depending on the plane of sectioning. In activated oncospheres, the staining pattern was different from that seen with non-activated oncospheres. In activated oncospheres a more general staining pattern was evident throughout the oncospheral parenchyma in addition to the staining of 2 lateral pairs of cells (Fig. 2).
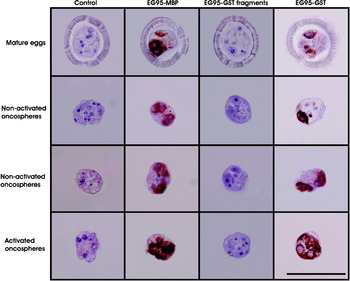
Fig. 2. Immunolocalization of EG95 antigen on sections of Echinococcus granulosus mature eggs, non-activated and activated oncospheres using specific antisera raised against either EG95-MBP, combined truncated EG95 fragments (EG95-1, EG95-2 and EG95-3), EG95-GST or control proteins (anti-GST and anti-MBP). Positive staining appears as a brown colour while the nuclei stain blue. Scale bar = 500 μm.
Immunogold labelling
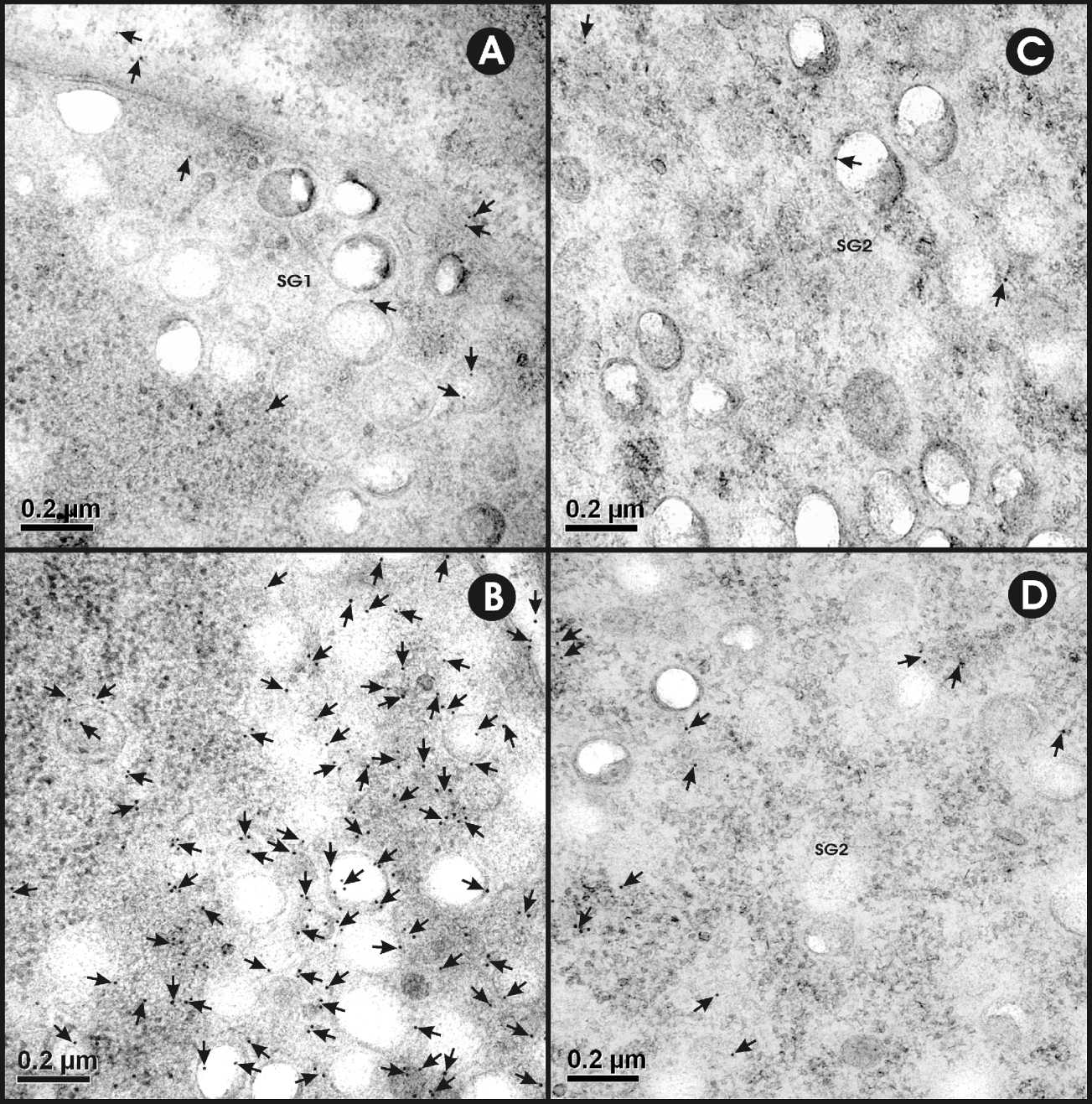
The bilaterally distributed cells recognized as containing EG95 at the light microscopic level were identified as penetration gland cells using transmission electron microscopy (Fig. 3). The cells were recognized as the penetration gland type 1 (PG1) cell which is a quadri-nucleated syncytium containing 2 lateral pairs of cell bodies interconnected by narrow cytoplasmic bridges (Jabbar et al. Reference Jabbar, Crawford, Młocicki, Świderski, Conn, Jones, Beveridge and Lightowlers2010d). The lateral pairs of PG1 cells were clearly separated from each other by plasma membranes (Fig. 3B). The PG1 cell was found to contain a spherical nucleus having heterochromatin islands and peri-nuclear material rich in free ribosomes, a well-developed granular endoplasmic reticulum and mitochondria. The granules of these cells are circular in shape with well-defined membranes (Fig. 3B). The E. granulosus oncospheres also contain the second type of penetration gland cell (PG2) which is syncytial occupying almost 50–60% of the oncospheral space. The granules of PG2 cells are larger, less electron dense and sparser than those of PG1.

Fig. 3. Ultrastructure of penetration gland type 1 (PG1) in a non-activated oncosphere of Echinococcus granulosus. (A) Oncosphere showing a lateral pair of PG1 cells. (B) High magnification image of the lateral pair of PG1 cells and surrounding structures. Abbreviations: GER, granular endoplasmic reticulum; M, muscles; Mt, mitochondria; N, nucleus; NC, nerve cell; OM, oncospheral membrane; OT, oncospheral tegument; PG1 and PG2, penetration gland type 1 and type 2 cells; PM, plasma membrane; SC, somatic cell.
The specificity of the immunolabelling was evident from the lack of reactivity seen using control antisera (anti-MBP and anti-GST antisera; Fig. 4A, C; Table 1). Maximum specific immunogold labelling density (P<0·001) for EG95 was found in the cytoplasm and the secretory granules of the PG1 cell (Fig. 4B; Table 1). The cytoplasm and the secretory granules of the PG2 cell also contained immunogold particles in samples reacted with anti-EG95 in comparison to control antisera but these were in significantly lower abundance (P<0·001) compared to those in the PG1 cell (Fig. 4B, D). Cellular processes were found emanating from the PG1 cell in different directions and these also contained numerous immunogold particles. Other cell types including the nerve cell and its neurosecretory granules contained low distribution of immunogold particles (Fig. 5; Table 1). The distribution of immunogold particles in different cell types per unit area is summarized in Table 1. Quantitative evaluation of the density of the immunogold particles associated with the various oncospheral structures and cells revealed no difference between oncospheres reacted with test and control antisera other than PG1 and PG2 cells (Table 1).
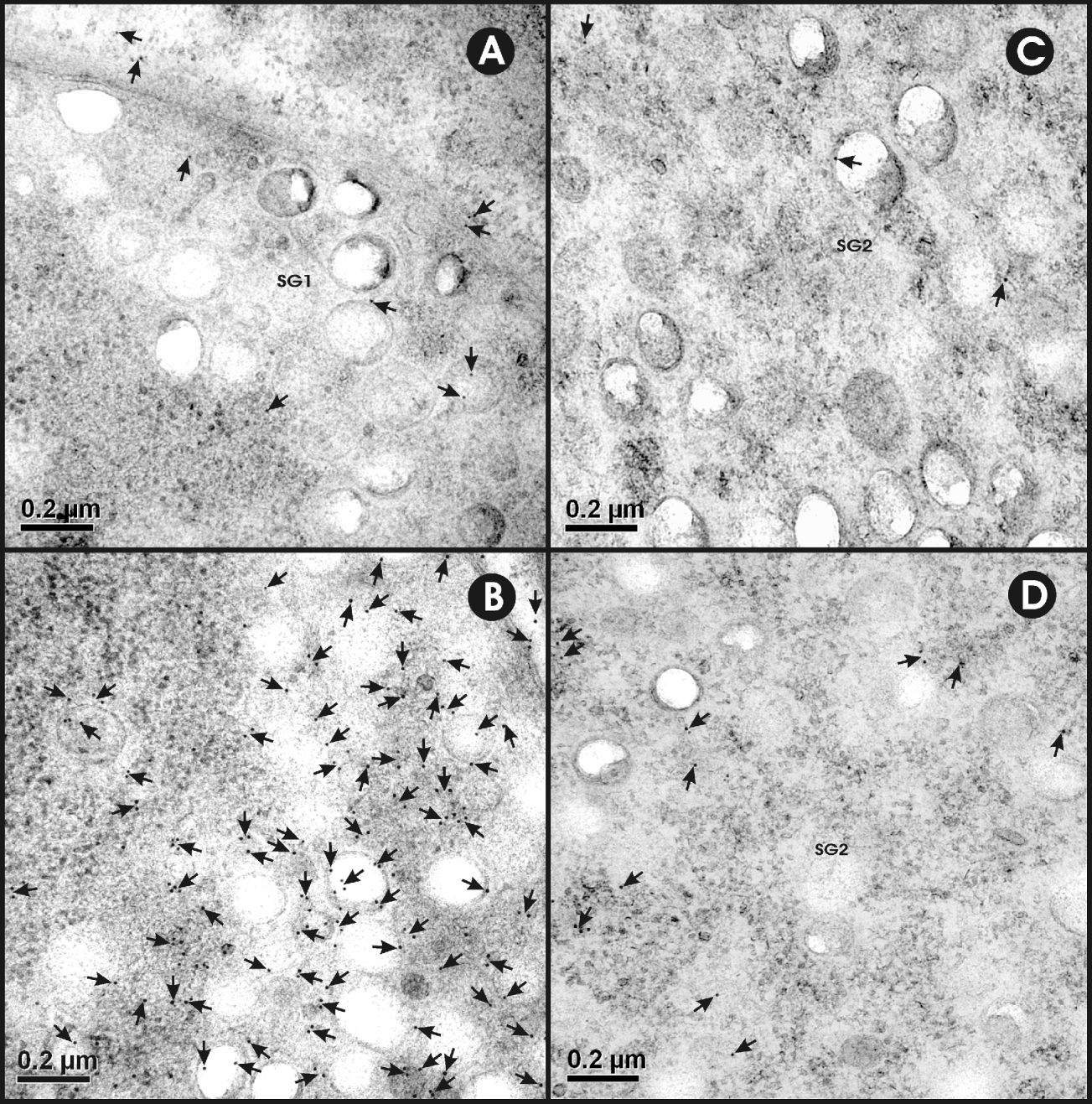
Fig. 4. Ultrastructural immunolocalization of EG95 in penetration gland type 1 (PG1) cell and type 2 (PG2) cells of Echinococcus granulosus non-activated oncospheres. Antisera used were raised against EG95-MBP and MBP. (A) The cytoplasm and secretory granules (SG1) of the PG1 cell stained with control antisera against MBP. (B) Immunogold labelling in the cytoplasm and SG1 of PG1 cell (black dots near the tip of arrows). (C) The cytoplasm and secretory granules (SG2) of PG2 cell stained with control antisera raised against MBP. (D) Immunogold labelling in the cytoplasm and SG2 of PG2 cell (arrows).

Fig. 5. Ultrastructural immunolocalization of EG95 in the cytoplasm of penetration gland type 1 (PG1) cell in comparison with penetration gland type 2 (PG2) and nerve cells in E. granulosus oncospheres. (A) Micrograph showing the secretory granules (SG1) from PG1 cell and neurosecretory granules (NSG) of the nerve cell with no immunolabelling after reacting with antiserum against MBP. (B) Micrograph showing high immunolabelling density in the cytoplasm and SG1 whereas NSG and secretory granules of PG2 cell (SG2) are relatively devoid of immunolabelling except for few gold particles.
Table 1. Localization of EG95 antigen in non-activated oncospheres of Echinococcus granulosus
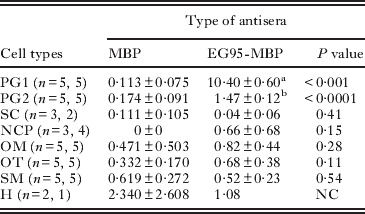
PG1 and PG2, penetration gland cell types 1 and 2; SC, somatic cell; NCP, nerve cell process; OM, oncospheral membrane plus underlying amorphous substance; OT, oncospheral tegument; SM, somatic muscles; and H, hook. n = n1, n2 indicates the number of oncospheres examined; n1 indicating the number of oncospheres examined with the anti-MBP antisera and n2 the number examined with anti-EG95-MBP. The number of cells was the same in control and treatment except for SC, NCP and H. NC = not conducted due to limited number of observations. Superscripts (a,b) indicate P<0·001 by paired test.
DISCUSSION
Immunohistochemical localization of EG95 in E. granulosus using light microscopy demonstrated that the antigen is distributed in the cytoplasm of 4 cells located bilaterally in the oncosphere. Ultrastructural localization of the antigen using immunogold labelling in transmission electron microscopy revealed this cell type to be one of those associated with the oncospheral penetration gland.
In his observations on the structure of cestode oncospheres, Reid (Reference Reid1948) introduced the term penetration gland and hypothesized that the secretion of these glands may help the parasite to penetrate the tissues of the intermediate host. Subsequently, several authors have speculated about the potential antigenic nature of penetration gland secretions (Silverman, Reference Silverman1955; Silverman and Maneely, Reference Silverman and Maneely1955; Heath, Reference Heath1973; Rajasekariah et al. Reference Rajasekariah, Mitchell and Rickard1980a,Reference Rajasekariah, Rickard and Mitchellb) but there has been no direct evidence to support this hypothesis. A potential role for these structures in host invasion is supported indirectly by evidence that during the first few hours of host invasion, the glands constitute a large volume as compared with the total size of the oncosphere and they secrete macroscopic material (Silverman and Maneely, Reference Silverman and Maneely1955). Indirect experimental evidence supports the hypothesis that oncosphere secretions are antigenic. Rickard and Bell (Reference Rickard and Bell1971) achieved a high level of immunity against T. ovis in sheep in which they had previously implanted activated oncospheres within diffusion chambers having a pore size too small to allow intact oncospheres to escape, but large enough to allow the release of excretory/secretory (ES) products. However, these authors reported that only 0·5% of the total implanted T. ovis oncospheres developed normally (Rickard and Bell, Reference Rickard and Bell1971) and it is possible that immunity stimulated by the implants may have been due to the disintegration of oncospheres releasing cellular proteins. Rajasekariah et al. (Reference Rajasekariah, Mitchell and Rickard1980a) achieved a high level of immunity against Taenia taeniaeformis infection in mice using antigens collected from the sonicated non-activated oncospheres, indicating that host-protective antigens were present in non-activated oncospheres of T. taeniaeformis. A similar situation was found to occur for E. granulosus. Osborn and Heath (Reference Osborn and Heath1982) showed that the secretory products of E. granulosus oncospheres contained host-protective antigens and Heath and Lawrence (Reference Heath and Lawrence1996) demonstrated that protective antigens were also present in freshly activated oncospheres of E. granulosus. In both Taenia species and E. granulosus, host-protective antigens are present in the oncospheres as well as being secreted during post-oncospheral development. The results of the studies presented here demonstrate that the EG95 antigen is localized predominantly in the PG1 cell. This finding is consistent with recent observations made in relation to the localization of host-protective antigens in T. ovis oncospheres (Jabbar et al. Reference Jabbar, Kyngdon, Gauci, Walduck, McCowan, Jones, Beveridge and Lightowlers2010a,Reference Jabbar, Świderski, Mlocicki, Beveridge and Lightowlersb). In T. ovis 3 different antigens were localized predominantly in the PG1 cell.
Previous descriptions of the ultrastructure of E. granulosus oncospheres have referred to penetration gland cells (Świderski, Reference Świderski1983; Harris et al. Reference Harris, Heath, Lawrence and Shaw1989; Holcman et al. Reference Holcman, Heath and Shaw1994). Localization of the EG95 antigen corresponded with a cell type that Świderski (Reference Świderski1983) referred to as a PG1 cell, although a clear description of the ultrastructure of this cell type was not provided. The characteristics of the cell seen here to stain specifically for the presence of EG95 were similar to the PG1 cell type recently described in T. ovis oncospheres (Jabbar et al. manuscript submitted), and shown to contain host-protective antigens in this species. While the EG95 antigen was predominantly localized in the PG1 cell, a lesser but significant level of positive staining was also observed in the PG2 cell. This is similar to the observations made in relation to localization of the To45W, To16 and To18 antigens of T. ovis (Jabbar et al. Reference Jabbar, Kyngdon, Gauci, Walduck, McCowan, Jones, Beveridge and Lightowlers2010a). While these antigens were predominantly localized in the PG1 cell type, a lesser but unequivocally positive level of staining was also seen in the PG2 cell type. In both E. granulosus and T. ovis, the PG1 and PG2 cells have cytoplasmic granules which differ in their characteristics between the different cell types.
Holcman et al. (Reference Holcman, Heath and Shaw1994) described vesicles or granules in the tegument of E. granulosus after 2 days in in vitro culture. They indicated that the granules were of the same electron density as granules seen in the penetration gland. Several granule/vesicle types were described, including types with a disc-like core, dense granular contents or rod-like core. The characteristics of the granules seen in the oncospheres investigated here were different from those described by Holcman et al. (Reference Holcman, Heath and Shaw1994), possibly because of a difference in the fixation and staining methods used in the two studies. Also, the studies undertaken by Holcman et al. (Reference Holcman, Heath and Shaw1994) were with oncospheres after 2 days of in vitro culture whereas the oncospheres investigated here were non-activated.
Woollard et al. (Reference Woollard, Gauci, Heath and Lightowlers2001) found that the host-protective epitopes of EG95 were associated with conformational epitopes of the protein and that these epitopes were present in 3 overlapping protein fragments which spanned the full EG95 sequence. We showed that antisera raised against these fragments failed to induce antibodies that produced any detectable binding to the PG cells of E. granulosus oncospheres. This finding is consistent with the observations of Woollard et al. (Reference Woollard, Gauci, Heath and Lightowlers2001) that the immunodominant epitopes of EG95 are conformational.
Heath and Lawrence (Reference Heath and Lawrence1981) reported that the oncospheres could be killed in vitro when incubated with the antisera from infected sheep or from sheep immunized with E. granulosus oncospheral antigens. Lysis of E. granulosus oncospheres in the presence of immune serum in in vitro culture is dependent on antibody and complement-mediated attack (Heath et al. Reference Heath, Holcman and Shaw1994), suggesting that the host-protective antigens should be present on the surface of oncospheres. EG95 antigen was not observed on the oncospheral tegument and membrane of non-activated or recently activated oncospheres. In their studies on the killing of oncospheres in vitro by anti-oncospheral antibodies, Heath and Lawrence (Reference Heath and Lawrence1981) suggested that the E. granulosus oncospheres could be killed within a 24 h period after activation. Subsequently, Heath et al. (Reference Heath, Holcman and Shaw1994) reported that most or all of the metacestodes of E. granulosus developing in vitro were killed by day 6 when incubated with antisera collected from lambs injected with activated oncospheres. Woollard et al. (Reference Woollard, Heath and Lightowlers2000b) demonstrated that anti-EG95 antibodies could kill E. granulosus oncospheres in in vitro culture when they were assessed after 10 days of culture. Our inability to identify EG95 on the surface of freshly activated oncospheres suggests that the invading E. granulosus parasites may not be susceptible to attack by immune responses directed at the antigen and that susceptibility develops subsequently, during post-oncospheral reorganization. Further studies on post-activated oncospheres are required to know whether or when EG95 antigen appears on the surface of the parasite and becomes susceptible to antibody and complement-mediated attack.
ACKNOWLEDGEMENTS
We are grateful to Professor Ian Beveridge for his comments to improve the quality of this manuscript. The authors wish to acknowledge the expert technical assistance of Paul Benham and Faye Docherty. The statistical advice from Garry Anderson is also appreciated. Funding from National Health and Medical Research Council (grants 400109 and 628320) is acknowledged.