Published online by Cambridge University Press: 17 October 2003
The liver fluke, Fasciola hepatica relies on a well-developed muscular system, not only for attachment, but for many aspects of its biology. Despite this, little is known about the system beyond the gross organization of the main somatic muscle layers. In the present study, a range of techniques have been applied to F. hepatica in order to understand more about various aspects of muscle organization, biochemistry (in terms of muscle proteins) and identity of isolated muscle fibres. Scanning electron microscopy has provided a direct visualization in situ of the somatic muscle layers and the organization of the muscle fibres within the ventral sucker. The muscle bundles contributing to the main somatic muscle layers are made up of up to 10 individual muscle fibres. Phalloidin staining for actin, in conjunction with confocal microscopy, confirmed the presence of 2 main somatic muscle layers (outer circular, inner longitudinal), beneath which lies a third layer of oblique muscle fibres. The use of propidium iodide in combination with phalloidin staining for actin demonstrated that the cell bodies associated with the 2 main somatic muscle layers are situated beneath the longitudinal muscle layer and are connected to their respective muscle fibres by short cytoplasmic processes. Myosin immunoreactivity was demonstrated in the somatic muscle layers and in the muscle layers surrounding various organ systems within the fluke. Double labelling for actin and myosin confirmed the co-localization of the 2 muscle proteins in the muscle fibres of the ventral sucker. Muscle fibres from the somatic muscle layers and the ventral sucker have been isolated and images obtained with phase-contrast microscopy and scanning electron microscopy. The muscle fibres contain actin and myosin, but lack a nucleus, the connection with the cell body having been broken during the isolation procedure.
A well-developed muscular system is essential for the survival of both endo- and ecto-parasitic flatworms. This is not just required for the purposes of locomotion and attachment, the latter process being aided by the development of specialized attachment organs. A high degree of muscular co-ordination is also required for many aspects of biology, including feeding and reproduction (egg laying, for example).
The somatic musculature is organized into 3 main layers: an outer circular layer immediately below the tegument or epidermis, beneath which is a relatively thick layer of longitudinal muscle. A third layer of more oblique or diagonal muscle fibres underlies the longitudinal muscle layer. Relatively few ultrastructural studies have been carried out on the fine structure of the muscle fibres and their associated cell bodies (Lumsden & Byram, 1967; Silk & Spence, 1969; Webb, 1977, 1987; Rieger et al. 1991 b; Willms et al. 2003). The studies have demonstrated that the somatic muscles are smooth in type, though more specialized types have been identified in attachment organs and the tail of trematode cercariae, for example (Lumsden & Foor, 1968; Chapman, 1973; Rees, 1975; Reger, 1976; Ward, Allen & McKerr, 1986). While the muscle cell body is often described as being at some distance from its associated contractile component, the spatial relationship between the cell body and the main layers of muscle is unknown, and the number of fibres associated with each cell body and vice versa is poorly understood (see Discussion section). The muscle fibres are believed to communicate with each other by means of gap junctions (for references, see Discussion section). The nature of muscle innervation is another issue that has not been fully clarified, although in a number of organisms cytoplasmic extensions from the contractile component make contact with nerve processes (Chien & Koopowitz, 1972; Webb, 1977, 1987; Rieger et al. 1991 a).
A number of muscle-associated proteins have been demonstrated in turbellarians, trematodes and cestodes and their genes isolated. They are, actin (Matsumoto et al. 1988; Pascolini et al. 1992 a,b; da Silva et al. 1993; Oliveira & Kemp, 1995; Wahlberg, 1997); myosin (Grossman et al. 1990; Ambrosio et al. 1997; Cebria et al. 1997; Kobayashi et al. 1998); paramyosin (Ishii & Sano, 1980; Laclette et al. 1987, 1995; Matsumoto et al. 1988; Grossman et al. 1990; Landa et al. 1993; Muhlschlegel et al. 1993; Becker et al. 1995; Schmidt et al. 1996; Gobert et al. 1997; Vargas-Parada & Laclette, 2003); and tropomyosin (Xu et al. 1989; Dissous et al. 1990; MacGregor & Shore, 1990). Phalloidin staining of actin elements has been used to determine the gross organization of the muscle layers (Rieger et al. 1994; Mair et al. 1998, 2000; Wahlberg, 1998; Hooge & Tyler, 1999). As impressive as the resulting images are, they merely confirm previous histological observations, although the organization of the muscle layers has proved to be a useful tool for evaluating taxonomic relationships between turbellarians (Cebria et al. 1997; Tyler & Hyra, 1998; Hooge & Tyler, 1999). The images do not provide data on the relationship between muscle cell bodies and their associated fibres or on the distribution of cell bodies relative to the main muscle layers. While the cell body does not appear to be required for muscle contraction, its absence is likely to be important for cell survival, turnover of cytoskeletal proteins and the position of receptors, for example. Moreover, the organization of contractile units is important for the integration between muscle cells and of overall muscle activity (see Discussion section).
Initial experiments on muscle physiology involved the use of intact organisms (Carolei, Margotta & Palladini, 1975; Fetterer, Pax & Bennett, 1977; Tomosky-Sykes et al. 1977; Fairweather, Holmes & Threadgold, 1984; Ward et al. 1986; Venturini et al. 1989; Blair & Anderson, 1994). Such preparations are difficult to study pharmacologically because the tegument or epidermis restricts access of drugs to the muscles. Muscle strip preparations have been used: particularly for polar compounds, they enable more direct interaction of drugs with receptors on muscle (Paasonen & Vartiainen, 1958; Aleksandryuk, 1964; Ward et al. 1986; Graham, Fairweather & McGeown, 1997, 2000; Tembe et al. 1993; Graham, McGeown & Fairweather, 1999). More recently, a number of workers have attempted to isolate muscle ‘cells’ (Blair et al. 1991; Day et al. 1993; Blair & Anderson, 1994) and muscle ‘fibres’ (Day et al. 1995; Johnston et al. 1996; Miller et al. 1996; Moneypenny et al. 2001) for use in pharmacological and electrophysiological experiments. Since many of the isolated elements do not possess a cell body and their morphology is variable, it is not at all clear what these preparations represent.
It is evident, then, from the foregoing discussion that a number of issues remain to be resolved in relation to muscle organization, structure and physiology. With the move towards the use of muscle preparations for physiological experiments, it seems particularly timely to re-examine a number of these aspects. The present study focusses on the liver fluke, Fasciola hepatica and has employed a range of preparative and technical approaches. A number of novel findings are presented, including the direct visualization of the muscle layers in situ. The location of the muscle cell bodies in relation to the muscle layers has been determined and the relationship between the muscle cell body and its associated contractile element established. Muscle fibres enzymatically isolated from the fluke have been characterised in terms of their morphology and contractile proteins. Finally, myosin II has been demonstrated in extracts and tissues of F. hepatica for the first time.
Experimental infections of Fasciola hepatica were maintained in male albino Sprague-Dawley rats. Each rat was infected with 20 metacercariae. Juvenile flukes were recovered from the liver parenchyma 3 weeks post-infection in warm (37 °C) Hédon-Fleig saline and maintained in this solution prior to the treatments outlined below. Adult flukes (at least 12 weeks old) were collected from the bile duct of infected rats. Infections of Taenia crassiceps were maintained in female BKW Swiss T0 mice and cysticerci recovered from the abdominal cavity in warm (37 °C) Hanks' balanced salt solution and maintained in this solution prior to the experiments described below.
The ventral sucker was carefully dissected from 3–4 adult flukes and stirred continuously with a magnetic bar for 20 min at 37 °C in nominally calcium-free Hédon-Fleig saline containing 0·1% (w/v) bovine serum albumin (BSA), 5 mM dithiothreitol (DTT) and 0·2% (w/v) papain. The suckers were then washed 3 times in nominally calcium-free Hédon-Fleig saline containing 0·1% (w/v) BSA and 5 mM DTT and stirred for a further 40 min at 37 °C in nominally calcium-free Hédon-Fleig saline containing 0·1% (w/v) BSA, 5 mM DTT and 0·2% (w/v) trypsin. Following this, the suckers were again washed 3 times in nominally calcium-free Hédon-Fleig saline and stirred for 30 min at 37 °C in nominally calcium-free Hédon-Fleig saline lacking BSA, DTT and enzyme(s). Finally, the sucker preparation was gently triturated (30–40 times) through a 0·5 mm-mouth Pasteur pipette to isolate the muscle fibres. For preparations showing incomplete dispersal of the muscle fibres, the procedure was stopped after 3–4 triturations.
For the isolation of somatic muscle fibres, individual flukes were chopped into small pieces, approximately 1 mm cubed, and the tissue pieces subjected to the same isolation protocol as described above.
BSA, DTT and enzymes were obtained from Sigma-Aldrich Co. Ltd, Poole, Dorset, UK.
Adult flukes were initially lightly flat-fixed for 30 min at room temperature in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4). The flukes were subsequently sliced transversely into thin strips and the strips were free-fixed in fresh fixative for a further 3 h at 4 °C. The tissue strips were then washed thoroughly in 0·1 M sodium cacodylate buffer (pH 7·4), dehydrated in an ascending series of ethanols and embedded in JB-4 resin (Agar Scientific, Stansted, Essex, UK). Sections, approximately 4 μm in thickness, were cut on a pyramitome, mounted on clean glass slides and stained with 1% (w/v) aqueous toluidine blue for general morphology. Isolated muscle fibres were viewed by phase-contrast microscopy.
For the observation of muscle fibres in situ, involving the removal of the tegument, a modification of the method of Murakumo et al. (1995) was used. Whole flukes were lightly flat-fixed for 4 h at room temperature in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose. The flukes were then treated with 30% (w/v) aqueous potassium hydroxide at 60 °C for 1 h with intermittent shaking. This was followed by washing for 1 h in 0·1 M phosphate-buffered saline (PBS, pH 7·4), incubation for 1 h at 37 °C with continuous shaking in Hédon-Fleig saline containing 0·2% (w/v) collagenase (Type 1A, Sigma-Aldrich Co. Ltd, Poole, Dorset, UK) and then washing in 0·1 M PBS (pH 7·4) for 1 h. The flukes were then treated with a 2% (w/v) aqueous solution of tannic acid for 1 h at room temperature, rinsed thoroughly in distilled water and fixed in 1% aqueous osmium tetroxide overnight at room temperature. Following washing in double-distilled water, the specimens were treated with 0·5% (w/v) uranyl acetate for 1 h, washed again in double-distilled water, dehydrated through an ascending series of acetones, critical-point dried in liquid carbon dioxide, fixed onto aluminium stubs and coated with gold-palladium in a Polaron E.5000 sputter-coating unit. The specimens were viewed in a Jeol 35-CF scanning electron microscope operating at an accelerating voltage of 10 keV.
For the observation of muscle fibres isolated from the ventral sucker, a modification of the technique of Lincks et al. (1998) was used. Following isolation, muscle fibres were transferred to glass cover-slips and allowed to settle, initially for 5 min at room temperature, then for 30 min at 4 °C. They were then fixed for 30 min at room temperature in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose. Following fixation, the fibres were washed several times in 0·1 M sodium cacodylate buffer (pH 7·4), fixed in 1% osmium tetroxide in 0·1 M sodium cacodylate buffer (pH 7·4) for 30 min at room temperature, then washed thoroughly in double-distilled water. The fibres were processed further for scanning electron microscopy (SEM) as described above.
Partially digested ventral sucker specimens were prepared as described above, fixed in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4) and processed for SEM as described above. Transverse and longitudinal sections of the fluke, approximately 0·5 mm in thickness, were fixed in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4) and processed for SEM as described above.
For transmission electron microscopy (TEM), thin transverse sections were removed from the midbody region of adult flukes. The material was fixed for 4 h at 4 °C in 4% (w/v) glutaraldehyde in 0·1 M sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose. After fixation, the material was washed in 0·1 M sodium cacodylate buffer (pH 7·4) containing 3% (w/v) sucrose overnight. This was followed by post-fixation for 1 h in 1% aqueous osmium tetroxide, dehydration through an ascending series of ethanols and embedding in Agar 100 epoxy resin (Polaron Equipment Ltd, Watford, UK). Ultrathin sections, 60–70 nm in thickness, were cut on an LKB Ultramicrotome IV, mounted on uncoated copper grids and double-stained with alcoholic uranyl acetate (15 min) and aqueous lead citrate (10 min). The sections were viewed in a Jeol 100-CX transmission electron microscope operated at an accelerating voltage of 100 keV.
For the demonstration of actin, direct fluorescence was used involving fluorescein isothiocyanate-or tetramethylrhodamine B isothiocyanate-conjugated phalloidin (FITC-phalloidin or TRITC-phalloidin, respectively; Sigma-Aldrich Co. Ltd, Poole, Dorset, UK). Three-week-old juvenile flukes were fixed for 4 h at room temperature in 4% (w/v) paraformaldehyde (PFA) in 0·1 M phosphate-buffered saline (PBS, pH 7·4). The flukes were then incubated for 24 h at 4 °C in 0·1 M PBS containing 0·5% (v/v) Triton X-100, 0·1% (w/v) BSA and 0·1% (w/v) sodium azide. Following this, the flukes were placed in FITC-phalloidin or TRITC-phalloidin at a concentration of 200 ng/ml and left for 24 h at 4 °C. The flukes were subsequently washed in 0·1 M PBS (pH 7·4) and mounted in PBS[ratio ]glycerol (1[ratio ]9) and viewed in a Leitz epifluorescence microscope or a confocal scanning laser microscope (MRA-1, Bio-Rad Ltd, Abingdon, Oxfordshire, UK). The excitation/emission wavelengths for fluorescein were 488/530 nm and for tetramethylrhodamine were 514/570 nm.
Transverse and longitudinal thick tissue sections of adult flukes (more than 12 weeks old) were fixed and processed for phalloidin staining as described above. Partially digested ventral suckers of adult flukes were prepared as described previously, then fixed and processed for phalloidin staining. Muscle fibres, isolated by the method described previously, were allowed to adhere to clean glass slides, then fixed for 20 min at room temperature in 4% (w/v) PFA in 0·1 M PBS (pH 7·4), incubated for 2 h at room temperature in 0·1 M PBS containing 0·5% (v/v) Triton X-100, 0·1% (w/v) BSA and 0·1% (w/v) sodium azide, and processed further for phalloidin staining as described above. For cryostat sectioning, the oral cone region of the fluke was snap-frozen in 2-methylbutane using liquid nitrogen and embedded in tissue embedding medium (Cryo-M-Bed, Bright Instruments Co. Ltd, UK), placed on a stub and quickly frozen with quick-freezing spray (Surgipath Frostbite, Surgical Medical Industries Inc., USA). Sections measuring 6–7 μm in thickness were cut on a Leica CM 1900-1-1 cryostat, collected on clean glass slides, air-dried, then fixed for 30 min at room temperature in 4% (w/v) PFA in 0·1 M PBS (pH 7·4) and subsequently processed for phalloidin staining as described above.
For the demonstration of myosin, the indirect immunofluorescence technique of Coons, Leduc & Connolly (1955) was used. Cryostat sections of adult flukes and isolated ventral sucker muscle fibres were prepared as described above, fixed in methanol for 10 min at −20 °C, washed in 0·1 M PBS (pH 7·4), then incubated for 3 h at room temperature in primary antiserum raised in rabbit against semi-purified native type II myosin of Taenia solium at a dilution of 1[ratio ]1000. The sections were subsequently washed in 0·1 M PBS (pH 7·4), incubated for 3 h at room temperature in an appropriate secondary antiserum conjugated to FITC at a dilution of 1[ratio ]50 and washed again prior to mounting and examination. (FITC was obtained from Dako Ltd, High Wycombe, Bucks., UK). Controls involved omission of the primary antibody and substitution of the primary antibody with non-immune rabbit serum (Dako Ltd, High Wycombe, Bucks., UK). Fresh-frozen cryostat sections and methanol post-fixed cryosections of the cysticerci of T. crassiceps were also incubated in the T. solium myosin antibody and the incubation run in parallel with sections from the liver fluke. Sections of F. hepatica and T. crassiceps were also incubated in a mammalian (bovine uterus) anti-myosin antibody; control sections from rat duodenum were incubated in parallel with the parasite sections.
For the double-labelling of actin and myosin in cryostat sections of adult flukes, PFA and methanol post-fixation could not be used. Instead, fresh-frozen cryostat sections were used, employing the protocols for actin and myosin described above. Sections were labelled first for actin and then for myosin and vice versa.
For the localization of the muscle cell bodies, a number of preparations were used: whole juvenile flukes; thick tissue sections and cryostat sections of adult flukes; partially digested ventral sucker preparation from adult flukes; and isolated ventral sucker muscle fibres from adult flukes. The preparations were fixed in PFA and incubated overnight in PBS containing Triton X-100, BSA and sodium azide as described previously. The preparations were then double-labelled, first with FITC-conjugated phalloidin (for actin) and then incubated in propidium iodide at a concentration of 10 μg/ml and left for 30 min to overnight. Following washing, the preparations were mounted and observed in an epifluorescence microscope or confocal microscope, as described above. Propidium iodide binds to DNA and RNA and emits a red fluorescence (Crissman & Steinkamp, 1973; Krishnan, 1975). The excitation/emission wavelengths for propidium iodide were 536/600 nm.
The antibody to T. solium myosin was raised in New Zealand rabbits. The rabbits were immunized on 4 occasions (at 14-day intervals) by subcutaneous injection of myosin from T. solium cysticerci or Taenia saginata adults. For this, 500 μg of a protein extract was separated by electrophoresis in SDS–PAGE gels (5%) with 8 M urea and the band corresponding to 200 kDa (myosin heavy chain) was cut, minced and mixed with complete Freund's adjuvant for the first injection and with incomplete adjuvant for the subsequent injections. Hyperimmune serum was obtained 7 days after the last immunization (Harlow & Lane, 1988).
Following recovery, flukes were homogenized in PBS containing the protease inhibitors ethylenediaminetetraacetic acid (sodium salt, EDTA), phenylmethylsulfonyl fluoride (PMSF) and p-hydroxymercuribenzoic acid (sodium salt, PCMB) and the homogenate lyophilized. For analysis, the powder was hydrated (1 mg/300 μl) using either high-quality water (MilliQ grade) or a filamentous protein extraction solution containing 0·04 M potassium chloride and 1 mM magnesium chloride in 6·7 mM phosphate buffer, pH 7·4.
SDS–PAGE electrophoresis was carried out using the mini-PROTEAN 3 Electrophoresis Cell and PowerPac 3000 Power Supply (Bio-Rad Mexico). The fluke samples were mixed with Laemmli buffer (1[ratio ]1) and boiled in the presence of beta-mercaptoethanol and 8 M urea for 5 min. Samples were added to the wells of miniprecast gels, subjected to 4–20% Tris–HCl gradient SDS–PAGE, and fractionated at 50 V for the upper part and 100 V for the final fractionation until bromophenol blue arrived at the front of the gel. Gels were stained and destained with Coomassie blue and 10% (v/v) acetic acid–50% (v/v) methanol, respectively.
For Western blotting, PVDF membranes were previously activated using absolute methanol for 15 sec, washed with high-quality water for 2 min and incubated, as for the SDS–PAGE gels with fractionated proteins, for 15 min in transfer buffer (48 mM Tris-base, 39 mM glycine, 0·0375% (w/v) glycine and 20% (v/v) methanol, pH 9·2). Blotting was performed on PVDF membranes using a semi-dry chamber (Trans-Blot SD Semi-Dry Transfer Cell, Bio-Rad Mexico) and an initial time of 10 min at 15 V and a final time of 15 min at 25 V. For antibody–antigen detection, membranes were incubated for 1 h at room temperature in the presence of anti-native type II myosin of T. solium cysticerci as primary antibody (dilution 1[ratio ]500) and 30 min with goat anti-rabbit IgG conjugated to peroxidase (dilution 1[ratio ]1500) (Zymed, San Francisco, California, USA) as secondary antibody. All antibodies were previously solubilized in PBS–0·3% (v/v) Tween complemented with 1% (w/v) BSA. After each incubation, membranes were given 3×5 min washes in PBS–Tween. Prior to the chemiluminescent treatment, membranes were washed for 24 h in alternating steps in the presence of PBS or PBS–0·1% (v/v) Tween at room temperature. Enzymatic activity was developed using the Supersignal West Pico Chemiluminescence Kit (Pierce Chemical Co., Rockford, Illinois, USA) and the chemiluminescent signal was registered for 45 sec using Kodalith MP II film. (All reagents were from Sigma unless otherwise specified.)
A laboratory imaging and analysis system (UVP) was used to capture the electrophoresis and Western blot images and the images were processed using LabWorks Analysis Software v.3.0.02.00 and Adobe Photoshop v.6.0.
Treatment of whole flukes with potassium hydroxide followed by collagenase resulted in the complete removal of the tegument and partial to total removal of the basal lamina. SEM images revealed the muscle layers beneath the basal lamina, the outer circular muscle layer lying over the longitudinal muscle layer beneath (Fig. 1A). The outer circular muscle layer was organized into flat strips or ribbons of muscle fibres 3·5–4·3 μm in width that were fairly tightly grouped together, with a narrow separation between the individual muscle bundles (Fig. 1A). The muscle fibres of the longitudinal muscle layer were also arranged in strips, but the latter were wider (4·0–6·0 μm in width) and more widely spaced apart than those in the circular muscle layer. The muscle strips in the longitudinal muscle layer were orientated almost at right angles to the muscle fibres in the circular muscle layer (Fig. 1A). At higher magnification, the muscle strips took the form of flat, ribbon-like sheets of muscle fibres, each strip being composed of up to 10 muscle fibres (Fig. 1B). The ends of the muscle strips overlapped with adjacent muscle strips and formed a fairly tight association with the latter (Fig. 1C).

Fig. 1. Scanning electron micrographs (SEMs) of adult fluke preparations. (A) Potassium hydroxide/collagenase treatment, showing removal of the tegument and basal lamina to expose the outer circular muscle bundles (CM) and underlying longitudinal muscle layer (LM). A portion of the basal lamina (BL) remains and an empty spine socket (arrow) is visible. (B) Potassium hydroxide/collagenase treatment, showing muscle bundles in the longitudinal muscle layer. Each bundle takes the form of a flat strip or ribbon of individual muscle fibres. (C) Potassium hydroxide/collagenase treatment, showing the end of one muscle strip (large arrow) where it overlaps with another muscle strip (small arrow). (D) Thick tissue section showing the circular muscle layer (CM) beneath the tegument (T). Immediately below the circular layer is the longitudinal muscle layer (LM) and beneath the latter bundles of diagonal or oblique muscle fibres (DM) are also evident. (E) SEM of 2 somatic muscle fibres butt-joined to each other at their ends (large arrow), giving the impression of an abnormally elongated fibre. A possible cell body (small arrow) is attached to one of the muscle fibres. (F) SEM of a somatic muscle fibre that is tapered at one end and rounded at the other. A possible cell body (arrow) is attached to the fibre. (G) SEM of 2 somatic muscle fibres entwined around each other to give the impression of a bifurcated muscle fibre. (H) SEM of a clump of incompletely separated muscle fibres.
In thick tissue sections of adult flukes, the circular muscle layer was seen to lie beneath the basal lamina (Fig. 1D). Immediately below the circular muscle layer, there was a regular spacing of longitudinal muscle fibres, orientated at right angles to the circular muscle layer; however, a number of the fibres appeared to have been lost during processing (Fig. 1D). Below the 2 main muscle layers, oblique or diagonal muscle fibres were evident. Again, the fibres were grouped into bundles of individual muscle fibres (Fig. 1D).
Different images of isolated somatic muscle fibres were seen with scanning electron microscopy (SEM). Generally, the fibres appeared elongated and tapered at both ends. They were approximately 60 μm in length (range 40–78 μm). Occasionally they appeared longer than normal, where cell isolation was incomplete and 2 muscle fibres remained butt-joined at their ends (Fig. 1E). A number of muscle fibres were tapered at one end and rounded at the other (Fig. 1F). Possible cell bodies attached to the muscle fibres were occasionally observed (Fig. 1E and F). They were 4·7×3·1 μm and 1·6×1·1 μm, respectively, but the small size of the latter probably precludes it from being a cell body. Some muscle fibres appeared bifurcated at first sight, but on closer examination were seen to be 2 separate fibres coiled around each other (Fig. 1G). Other images were seen of possible ‘frayed’ muscle fibres, but they probably represent a clump of incompletely separated fibres following digestion (Fig. 1H).
The fine structure of the somatic musculature of F. hepatica, as viewed by TEM, conformed to that described by Fairweather, Threadgold & Hanna (1999) and will not be repeated in detail here. The muscle fibres are composed of thick and thin myofilaments and are separated from each other by a narrow zone of interstitial material (Fig. 2A). The end of one fibre is closely apposed to an adjacent muscle fibre (Fig. 2A).

Fig. 2. (A) Transmission electron micrograph of muscle fibres (large arrows). The fibres are composed of thick and thin myofilaments and are separated from each other by interstitial material. The end of one muscle muscle fibre is seen to be closely apposed to an adjacent muscle fibre (small arrows). (B) Phase-contrast image of an isolated somatic muscle fibre, showing its slightly-flexed, spindle-like shape, with tapering at both ends. (C) Phase-contrast image of an isolated somatic muscle fibre, showing its elongated, spindle-like shape. (D) Light micrograph of a section stained with toluidine blue, showing a muscle cell body (arrow) connected to a muscle fibre belonging to a muscle bundle in the diagonal muscle layer.
Typical phase-contrast images of isolated somatic muscle fibres are shown in Fig. 2B and C. The muscle fibres have a spindle-like shape and are tapered at both ends. They can appear more squat and shorter at times, depending on the degree of contraction (Fig. 2B and C). They measured approximately 40 μm long×8–10 μm wide.
In a section stained with toluidine blue and observed under the light microscope, a muscle cell body can be seen to be connected to a muscle fibre belonging to a muscle bundle in the diagonal muscle layer (Fig. 2D). The connection measured approximately 6 μm in length.
Following partial digestion of the ventral sucker, the lining was seen to be made up of a circumferential arrangement of muscle bundles (Fig. 3A). The individual muscle bundles were composed of several muscle fibres (Fig. 3B and C). Muscle cell bodies (measuring approximately 5·5±1·4×4·0±1·0 μm: n=10) were seen to be connected to individual muscle fibres belonging to the muscle bundles (Fig. 3B, C). Muscle fibres isolated from the full isolation digestion protocol were either elongated in shape, up to 55 μm in length and tapered at both ends (Fig. 3D), or were shorter (approximately 30 μm long), with a more spindle-like shape (Fig. 3E). Possible cell bodies attached to the muscle fibres were occasionally observed (Fig. 3D).
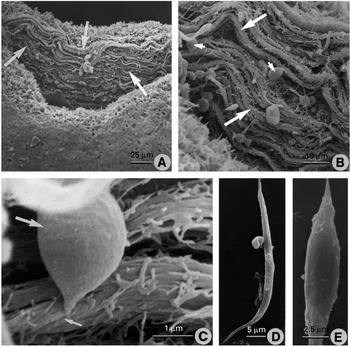
Fig. 3. Scanning electron micrographs (SEMs) of muscle preparations from the ventral sucker. (A) SEM of the ventral sucker following partial digestion. The muscle bundles (arrows) are seen to have a circumferential organization around the inner lining of the sucker. (B) Higher power SEM of the muscle bundles (large arrows), showing them to be composed of a number of muscle fibres. Cell bodies (small arrows) are associated with the muscle bundles. (C) High-power SEM of a muscle cell body (large arrow), showing its connection with its muscle fibre (small arrow), which forms part of a muscle bundle. (D) SEM of an isolated muscle fibre showing its elongated, tapered shape. (E) SEM of an isolated muscle fibre showing a more squat, spindle-like shape.
Individual (5), alternate images from a Z-series taken at 1 μm intervals through a juvenile fluke are shown in Fig. 4A–E. In these images, the muscle layers are shown in green (following FITC-phalloidin staining) and the cell bodies (stained with propidium iodide) shown in red. The images show the circular, longitudinal and diagonal muscle layers. In the circular muscle layer, the muscle fibres are organized into muscle strips that run parallel to each other and are closely packed together (Fig. 4A). The muscle strips of the longitudinal muscle layer are orientated at right angles to those of the circular muscle layer. They are wider than those in the circular muscle layer, are more widely spaced apart and do not necessarily lie parallel to each other (Fig. 4B). In the diagonal muscle layer, the muscle bundles run obliquely in comparison with the longitudinal muscle layer and criss-cross each other. The individual muscle bundles are quite widely separated and generally are more narrow than the strips in the other layers (Fig. 4C). No cell bodies were present at the level of the circular (Fig. 4A) or longitudinal (Fig. 4B) muscle layers. The cell bodies begin to appear beneath the longitudinal muscle layer (Fig. 4C and D). From a number of cell bodies, short cytoplasmic extensions were seen to merge with individual muscle fibres belonging to the muscle bundles (Fig. 4E). On occasion, it appears that more than 1 muscle fibre emanates from a single cell body (Fig. 4F), but it was not possible to determine the exact number of muscle fibres per cell body. The cell bodies measured approximately 9·8±3·0×6·9±1·5 μm (n=10).

Fig. 4. (A–E) CSLM images from a series taken at 1 μm intervals of a 3-week-old juvenile fluke double-stained with FITC-phalloidin (green) for actin and propidium iodide (red) for cell bodies. (A) Mainly shows muscle bundles belonging to the circular muscle layer. (B) Mainly shows muscle bundles belonging to the longitudinal muscle layer. (C) Image taken just below the longitudinal muscle layer. Muscle bundles of the diagonal muscle layer (large arrows) are evident and a number of muscle cell bodies (small arrow) are present. (D) Muscle bundles belonging to the diagonal muscle layer (large arrows) are evident, together with a larger number of cell bodies (small arrows) at this greater depth in the fluke. (E) Shows a large number of cell bodies interspersed with diagonal muscle bundles. From the muscle cell body emerges a short extension (small arrow) that connects with muscle fibres (large arrow) belonging to a muscle bundle. (F) Confocal scanning laser micrograph (CSLM) of a 3-week-old juvenile fluke double-stained with FITC-phalloidin (green) and propidium iodide (red). Labelling for propidium iodide is confined to the cell bodies (small arrows). Muscle fibres stained for actin (large arrows) extend away from the cell bodies. On occasion (arrowhead), it appears that more than 1 muscle fibre is associated with a single cell body. (G) CSLM of partially digested thick tissue section of an adult fluke, double-stained with FITC-phalloidin and propidium iodide. Note absence of cell bodies. (H) CSLM of an isolated somatic muscle fibre (small arrow) stained with FITC-phalloidin to show labelling for actin. Alongside the fibre is a much larger and apparently ‘frayed’ or ‘bifurcated’ muscle fibre (large arrow). It probably represents a group of incompletely digested muscle fibres.
The double-labelled images presented in Fig. 4C–F were obtained from specimens that had not been subjected to the digestion process for the isolation of muscle fibres. In these images cell bodies associated with the muscle layers are clearly present. In a thick tissue section following partial digestion, cell bodies are absent, indicating that they have been removed during the digestion process (Fig. 4G). The muscle bundles appear to be more widely spaced and more disorganized than normal. Following complete isolation of the muscle fibres and labelling with FITC-phalloidin, a typical muscle fibre is seen to have a characteristic spindle-like shape and is 30 μm in length (Fig. 4H). By the side of this fibre is a much larger and apparently ‘frayed’ or ‘bifurcated’ muscle fibre, 78 μm long (Fig. 4H). However, it probably represents a number of fibres remaining together as a result of incomplete digestion.
Using an antibody raised against type II myosin of T. solium, immunoreactivity (IR) for myosin was observed in the muscle belonging to the oral sucker and uterus (Fig. 5A and B) and in the 2 main somatic muscle layers (outer circular and inner longitudinal), together with the diagonal muscle layers (Fig. 5C and D). Myosin-IR was also localized in the muscle tissue surrounding the ovary (Fig. 5E) and the gut caeca (Fig. 5F). Immunolabelling for myosin occurred in isolated somatic and ventral sucker muscle fibres; the fibres had a similar appearance and size to that described above.

Fig. 5. Light micrographs of myosin immunostaining in cryostat sections of an adult fluke. (A) Section through the oral cone region showing myosin-immunoreactivity (IR) in the oral sucker (OS), uterus (U) and subtegumental muscle layers (arrows). (B) Section through the oral sucker showing myosin-IR in the muscle fibres (arrows) belonging to the sucker. (C) Micrograph showing myosin-IR in the 2 main subtegumental muscle layers (outer circular and inner longitudinal) (large arrows) and in the muscle bundles belonging to the diagonal muscle layers (small arrows). (D) High-power micrograph of myosin-IR in the outer circular (CM) and inner longitudinal (LM) muscle layers. Immunolabelling for myosin is also evident in the diagonal muscle fibres (DM). (E) Section showing myosin-IR in the muscle tissue (arrows) surrounding the ovary (OV). (F) Section showing myosin-IR in the muscle tissue (arrows) surrounding the base of the gut (G).
Positive immunolabelling for myosin was observed in sections of T. crassiceps incubated in the T. solium myosin antibody. This preparation was used as a positive control for the antibody (Ambrosio et al. 1997). No IR was evident in any of the control incubations involving F. hepatica and T. crassiceps. No immunostaining was observed in sections of F. hepatica and T. crassiceps following incubation in the mammalian smooth muscle (uterus) myosin antibody. In these experiments, myosin-IR was present in sections of rat duodenum used as positive control. Furthermore, F. hepatica did not react with a commercial rabbit anti-mouse skeletal muscle anti-myosin antibody (Sigma-Aldrich Co. Ltd, Poole, Dorset, UK).
Double labelling for actin and myosin was carried out on cryostat sections of the ventral sucker of adult F. hepatica. TRITC-phalloidin labelling for actin is present in the muscle fibres belonging to the ventral sucker (Fig. 6A). Immunostaining for myosin also occurred in the muscle fibres following incubation in the T. solium myosin antibody and an FITC-conjugated secondary antibody (Fig. 6B). Double labelling with TRITC-phalloidin and FITC-myosin confirmed the co-localization of the 2 muscle proteins in the same muscle fibres of the ventral sucker (Fig. 6C). Controls to exclude bleed-through in the detection system were carried out separately. This involved demonstrating that negligible fluorescence was detected when a specimen strongly stained using TRITC alone was imaged using the FITC excitation/emission wavelengths, and vice versa.

Fig. 6. Confocal scanning laser micrographs (CSLMs) of a cryostat section of the ventral sucker of an adult fluke. The section was double-labelled with TRITC-phalloidin for actin and anti-myosin antibody which was visualized using FITC-labelled secondary antibody. The micrographs were taken at the same magnification. (A) TRITC-phalloidin staining of actin in the muscle fibres (arrows) belonging to the ventral sucker. (B) Myosin-IR in the same muscle fibres (arrows). (C) Co-localization of actin- and myosin-immunostaining in the muscle fibres (arrows) belonging to the ventral sucker.
Myosin was recovered from lyophilized extracts of F. hepatica only when the filamentous protein extraction solution was used (Fig. 7, lane 2). The myosin was recognized in Western blots using the anti-T. solium myosin type II antibody (Fig. 7, lane 3).

Fig. 7. Detection of myosin in extracts of Fasciola hepatica. Parasite samples were analysed using SDS–PAGE electrophoresis and Coomassie blue staining (lane 2) and Western blot chemiluminescence treatment (lane 3). Lyophilized powder was dissolved in filamentous protein extraction solution (lane 2). Recovered myosin was recognized by the anti-Taenia solium myosin antibody (arrow, lane 3). Pre-stained molecular weight standards are in lane 1.
The present results have made a number of valuable contributions to the study of flatworm muscle. For example, they have provided a direct visualization (by SEM) of the organization of the muscle fibres that form the muscle bundles belonging to the main muscle layers and to the ventral sucker. The positioning of the muscle cell bodies beneath the longitudinal muscle layer and their connections with the muscle layers have also been determined. The present study has characterized the isolated muscle fibres, not just in terms of their morphology, but in terms of the presence of 2 contractile proteins, actin and myosin. The presence and distribution of myosin-immunoreactivity has also been demonstrated.
Each muscle fibre within a muscle bundle, or fascicle, is surrounded by finely fibrillar filamentous connective tissue and, in turn, each muscle bundle is surrounded by a connective tissue sheath (MacRae, 1965; Morita, 1965; Lumsden & Byram, 1967; Silk & Spence, 1969; Pedersen, 1972; Rieger et al. 1994). The connective tissue is believed to be a form of collagen (MacRae, 1965; Pedersen, 1972; Nordwig & Hayduk, 1969; Torre-Blanco & Toledo, 1981). The potassium hydroxide–collagenase method of Murakumo et al. (1995) removes collagen and basal laminae, thus exposing the muscle bundles and individual muscle fibres and permitting their visualization in situ. The muscle bundles were seen to consist of up to 10 muscle fibres. The greater resolution afforded by SEM provides a more distinct view of the complement of individual muscle fibres than that given by confocal microscopy.
Double labelling with propidium iodide and FITC-phalloidin was used in the present study to examine the relationship between the muscle fibres and bundles belonging to the main muscle layers and their cell bodies. The cell bodies were seen to lie beneath the 2 main muscle layers and are interspersed between the tegumental cell bodies. This arrangement is typical of trematode and cestode muscle, but in the turbellarian, Convoluta the cell bodies of the circular muscle layer lie above the muscle layer, between it and the epidermis, while the cell bodies of the longitudinal muscle layer lie beneath it (Tyler & Rieger, 1999). The observation that the muscle cell bodies lie at some distance from their associated contractile element is consistent with previous work on turbellarian, cestode and trematode muscle (MacRae, 1963; Lumsden & Byram, 1967; Reissig & Colucci, 1968; Chien & Koopowitz, 1972; Baguna & Romero, 1981). The resolution of confocal microscopy was not sufficient to determine with an absolute degree of certainty the number of muscle fibres emanating from a single muscle cell body. Typically, there was one, but there may be more.
With respect to the identity of the isolated muscle ‘cells’ or ‘fibres’, the typical image observed in the present study was that of an elongated, spindle-like muscle fibre, lacking a cell body. Possible cell bodies were seen occasionally, but the presence of a cell body could not be confirmed with propidium iodide staining. This is because no cell bodies attached to isolated muscle fibres were evident following double labelling with FITC-phalloidin and propidium iodide. Images of possible cell bodies attached to isolated muscle fibres from the turbellarian, Bdelloura have been described by Blair & Anderson (1994) and by Johnston et al. (1996). In this organism, retention of the cell body is a common, but not universal, phenomenon. In contrast, loss of the cell body during the isolation process has been reported in studies on Schistosoma mansoni (Blair et al. 1991; Day et al. 1993) and Procerodes littoralis (Moneypenny et al. 2001).
Previous studies have indicated that more than 1 type of muscle fibre is present in flatworms. For example, ‘frayed’ muscle fibres have been described in previous studies on flatworm muscle (Day et al. 1993; Johnston et al. 1996; Moneypenny et al. 2001). Similar muscle fibres were observed in the present study, but we consider that they represent incompletely separated fibres following digestion. ‘Bifurcated’ muscle fibres have been described by Day et al. (1993) and Blair & Anderson (1994). Apparent bifurcated muscle fibres were observed in the present study, but when viewed at high power by SEM, they were resolved as 2 separate fibres coiled around each other. Previous descriptions of bifurcated muscle fibres may have been based on misleading data from low-power microscopical images. So the present study casts doubt on previous classifications of muscle fibre types. On the basis of the present results, it appears that there is only 1 type of somatic muscle fibre present in F. hepatica, but the possibility that there may be more than 1 type cannot be excluded. Isolated muscle fibres from the ventral sucker may represent a more homogeneous population of muscle fibres than fibres isolated from a whole fluke digest.
The estimated lengths of the muscle fibres in F. hepatica (in the range 30–78 μm for somatic muscle fibres and 15–55 μm for sucker muscle fibres) are shorter than those reported for other flatworms. For example, in turbellarians, size ranges of 100–300 μm have been described for Notoplana (MacRae, 1965); 150–200 μm for Dugesia (Baguna & Romero, 1981); 20–500 μm for Bdelloura (Blair & Anderson, 1994; Johnston et al. 1996); and 60 μm for Procerodes (Moneypenny et al. 2001). Isolated muscle fibres of S. mansoni are in the ranges 15–200 μm (Day et al. 1993) and 3–30 μm (Blair et al. 1991). Muscle fibres from the miracidium of F. hepatica are of the order of 130 μm in length (Wilson, 1969). The data indicate that the lengths of muscle fibres are highly variable and that size per se may not be a very reliable criterion for establishing the identity of the fibres due to the limitations of the techniques used and the elasticity of the fibres. Previous identifications of muscle fibres were based on a similarity in structure and size to smooth muscle cells from other organisms (typically by the use of only a single microscopical method, in contrast to the present study) and the contractile nature of the fibres.
Typically, the nucleus of a muscle cell lies in a pocket of sarcoplasm that is connected to the muscle fibre by a narrow cytoplasmic process (MacRae, 1963; Lumsden & Byram, 1967; Reissig & Colucci, 1968; Chien & Koopowitz, 1972; Baguna & Romero, 1981; Moraczewski, 1981; Willms et al. 2003). The cell body may be several microns from the contractile process (Reissig & Colucci, 1968; Lumsden & Hildreth, 1983). The muscle cell is generally mononucleated and, at least in turbellarians, the nucleus is connected to the centre of the muscle fibre (Morita, 1965; Pedersen, 1972; Rieger et al. 1991 b). Most, if not all, of the muscle cells in trematodes and cestodes are mononucleated (Lumsden & Byram, 1967; Reger, 1976; Lumsden & Hildreth, 1983). In these organisms, the position of the nucleus with respect to the muscle fibre is less certain. In the present study on F. hepatica, the nucleus appears to lie at one end of the fibre rather than towards the middle (Figs 2D, 3C and 4E).
Generally, there is 1 muscle fibre per muscle cell, although exceptions are known (e.g. H. microstoma: Webb, 1977, 1987). Typically, the muscle cell is connected to the muscle fibre by a single process, although there can be more (Webb, 1987). An individual muscle fibre may be connected to more than one cell body (Lumsden & Hildreth, 1983). The individual muscle fibres are grouped together into bundles and are connected to each other via gap junctions, which will couple the cells both electrically and metabolically (Chien & Koopowitz, 1972; Lumsden & Hildreth, 1983; Webb, 1987; Rieger et al. 1991 a; Tyler & Rieger, 1999). In H. microstoma, Notoplana and Macrostomum, sarcoplasmic processes extend towards the nerves and make contact with axons, each process forming contacts with more than one axon. Moreover, several sarcoplasmic processes can form sarconeural junctions with a single axon and 1 axon can make contact with up to 4 sarcoplasmic processes, indicating a complex multineuronal, multiterminal pattern of innervation (Chien & Koopowitz, 1972; Webb, 1987; Rieger et al. 1991 a). The observations suggest that the muscular system functions in a syncytial fashion, forming motor units (Lumsden & Specian, 1980).
The present study has demonstrated the presence of 2 contractile proteins, actin and myosin, in the musculature of F. hepatica. The results for actin confirm previous studies on F. hepatica (Stitt et al. 1992; Mair et al. 1998). Phalloidin staining and actin antibodies have been used to label actin in the musculature of S. mansoni (Davis, Blanton & Klich, 1985; Abbas & Cain, 1987; Matsumoto et al. 1988; MacGregor & Shore, 1990; Mair et al. 2000) and Brachylaima (Ferrer, Gonzalez-Moreno & Gracenea, 2001); also in the turbellarian, Dugesia (Pascolini et al. 1992 a,b) and the cestode, Diphyllobothrium dendriticum (Wahlberg, 1998). The presence of multiple forms of actin is a common feature of eukaryotic organisms and flatworms are no exception (Abbas & Cain, 1989). Multiple actin genes have been isolated and sequenced from S. mansoni (Oliveira & Kemp, 1995) and the cestodes T. solium (Campos et al. 1990), Echinococcus granulosus (da Silva et al. 1993) and D. dendriticum (Wahlberg, Karlstedt & Paatero, 1994; Wahlberg & Johnson, 1997; Wahlberg, 1997). The spatial and temporal expression of the 6 actin genes in the plerocercoid larva and adult of D. dendriticum have also been studied by means of in situ hybridization (Wahlberg, 1997). To date 7–8 isoforms of actin have been detected in cysticerci of T. solium using 2-dimensional immobilized pH gradients (IPG) for isoelectric focusing and the isoforms show different tissue distributions in the parasite (Ambrosio, unpublished data).
This is the first localization of myosin immunoreactivity in F. hepatica and the third muscle-associated protein (along with actin and paramyosin) to be demonstrated in F. hepatica. Myosin immunostaining was present in the main subtegumental muscle layers, the sucker musculature and the muscular lining of the uterus, gut and ovary. The myosin antibody used was raised against the heavy chain of type II myosin from T. solium. Since the immunoreactivity was confined to muscle tissue, it is likely that the antibody is recognizing a common epitope that belongs to a type II myosin in F. hepatica. cDNA clones encoding myosin have been isolated and sequenced from S. mansoni (Newport et al. 1987; Grossman et al. 1990; Weston et al. 1993). Using an antibody raised against the native myosin, immunostaining was observed in the subtegumental and sucker musculature (Newport et al. 1987). In F. hepatica, myosin will be involved in effecting the contractile activity of muscle, thereby playing a role in the various muscular activities displayed by the fluke.
In conclusion, then, the present study has extended our understanding of the organization of the musculature in the liver fluke and the identity of the isolated muscle fibres. Having established the latter, it will be possible to carry out electrophysiological experiments on these preparations with greater confidence to characterize the nature of ion channels and the mechanisms underlying muscle contraction in the fluke. The knowledge derived from such experiments may help in the design of future fasciolicidal compounds. The muscle fibre preparations will be valuable as models to examine the potential muscle action of existing fasciolicides and to test the activity of novel compounds.
This research was supported by a Wellcome Travelling Research Fellowship (to D. Kumar) and DGAPA-UNAM grant IN-203900 (to J. R. A.). The authors would like to thank Mr G. Macartney for expert photographic assistance and M. Cruz-Rivera for production of the myosin antibodies.

Fig. 1. Scanning electron micrographs (SEMs) of adult fluke preparations. (A) Potassium hydroxide/collagenase treatment, showing removal of the tegument and basal lamina to expose the outer circular muscle bundles (CM) and underlying longitudinal muscle layer (LM). A portion of the basal lamina (BL) remains and an empty spine socket (arrow) is visible. (B) Potassium hydroxide/collagenase treatment, showing muscle bundles in the longitudinal muscle layer. Each bundle takes the form of a flat strip or ribbon of individual muscle fibres. (C) Potassium hydroxide/collagenase treatment, showing the end of one muscle strip (large arrow) where it overlaps with another muscle strip (small arrow). (D) Thick tissue section showing the circular muscle layer (CM) beneath the tegument (T). Immediately below the circular layer is the longitudinal muscle layer (LM) and beneath the latter bundles of diagonal or oblique muscle fibres (DM) are also evident. (E) SEM of 2 somatic muscle fibres butt-joined to each other at their ends (large arrow), giving the impression of an abnormally elongated fibre. A possible cell body (small arrow) is attached to one of the muscle fibres. (F) SEM of a somatic muscle fibre that is tapered at one end and rounded at the other. A possible cell body (arrow) is attached to the fibre. (G) SEM of 2 somatic muscle fibres entwined around each other to give the impression of a bifurcated muscle fibre. (H) SEM of a clump of incompletely separated muscle fibres.

Fig. 2. (A) Transmission electron micrograph of muscle fibres (large arrows). The fibres are composed of thick and thin myofilaments and are separated from each other by interstitial material. The end of one muscle muscle fibre is seen to be closely apposed to an adjacent muscle fibre (small arrows). (B) Phase-contrast image of an isolated somatic muscle fibre, showing its slightly-flexed, spindle-like shape, with tapering at both ends. (C) Phase-contrast image of an isolated somatic muscle fibre, showing its elongated, spindle-like shape. (D) Light micrograph of a section stained with toluidine blue, showing a muscle cell body (arrow) connected to a muscle fibre belonging to a muscle bundle in the diagonal muscle layer.

Fig. 3. Scanning electron micrographs (SEMs) of muscle preparations from the ventral sucker. (A) SEM of the ventral sucker following partial digestion. The muscle bundles (arrows) are seen to have a circumferential organization around the inner lining of the sucker. (B) Higher power SEM of the muscle bundles (large arrows), showing them to be composed of a number of muscle fibres. Cell bodies (small arrows) are associated with the muscle bundles. (C) High-power SEM of a muscle cell body (large arrow), showing its connection with its muscle fibre (small arrow), which forms part of a muscle bundle. (D) SEM of an isolated muscle fibre showing its elongated, tapered shape. (E) SEM of an isolated muscle fibre showing a more squat, spindle-like shape.

Fig. 4. (A–E) CSLM images from a series taken at 1 μm intervals of a 3-week-old juvenile fluke double-stained with FITC-phalloidin (green) for actin and propidium iodide (red) for cell bodies. (A) Mainly shows muscle bundles belonging to the circular muscle layer. (B) Mainly shows muscle bundles belonging to the longitudinal muscle layer. (C) Image taken just below the longitudinal muscle layer. Muscle bundles of the diagonal muscle layer (large arrows) are evident and a number of muscle cell bodies (small arrow) are present. (D) Muscle bundles belonging to the diagonal muscle layer (large arrows) are evident, together with a larger number of cell bodies (small arrows) at this greater depth in the fluke. (E) Shows a large number of cell bodies interspersed with diagonal muscle bundles. From the muscle cell body emerges a short extension (small arrow) that connects with muscle fibres (large arrow) belonging to a muscle bundle. (F) Confocal scanning laser micrograph (CSLM) of a 3-week-old juvenile fluke double-stained with FITC-phalloidin (green) and propidium iodide (red). Labelling for propidium iodide is confined to the cell bodies (small arrows). Muscle fibres stained for actin (large arrows) extend away from the cell bodies. On occasion (arrowhead), it appears that more than 1 muscle fibre is associated with a single cell body. (G) CSLM of partially digested thick tissue section of an adult fluke, double-stained with FITC-phalloidin and propidium iodide. Note absence of cell bodies. (H) CSLM of an isolated somatic muscle fibre (small arrow) stained with FITC-phalloidin to show labelling for actin. Alongside the fibre is a much larger and apparently ‘frayed’ or ‘bifurcated’ muscle fibre (large arrow). It probably represents a group of incompletely digested muscle fibres.

Fig. 5. Light micrographs of myosin immunostaining in cryostat sections of an adult fluke. (A) Section through the oral cone region showing myosin-immunoreactivity (IR) in the oral sucker (OS), uterus (U) and subtegumental muscle layers (arrows). (B) Section through the oral sucker showing myosin-IR in the muscle fibres (arrows) belonging to the sucker. (C) Micrograph showing myosin-IR in the 2 main subtegumental muscle layers (outer circular and inner longitudinal) (large arrows) and in the muscle bundles belonging to the diagonal muscle layers (small arrows). (D) High-power micrograph of myosin-IR in the outer circular (CM) and inner longitudinal (LM) muscle layers. Immunolabelling for myosin is also evident in the diagonal muscle fibres (DM). (E) Section showing myosin-IR in the muscle tissue (arrows) surrounding the ovary (OV). (F) Section showing myosin-IR in the muscle tissue (arrows) surrounding the base of the gut (G).

Fig. 6. Confocal scanning laser micrographs (CSLMs) of a cryostat section of the ventral sucker of an adult fluke. The section was double-labelled with TRITC-phalloidin for actin and anti-myosin antibody which was visualized using FITC-labelled secondary antibody. The micrographs were taken at the same magnification. (A) TRITC-phalloidin staining of actin in the muscle fibres (arrows) belonging to the ventral sucker. (B) Myosin-IR in the same muscle fibres (arrows). (C) Co-localization of actin- and myosin-immunostaining in the muscle fibres (arrows) belonging to the ventral sucker.
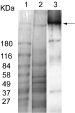
Fig. 7. Detection of myosin in extracts of Fasciola hepatica. Parasite samples were analysed using SDS–PAGE electrophoresis and Coomassie blue staining (lane 2) and Western blot chemiluminescence treatment (lane 3). Lyophilized powder was dissolved in filamentous protein extraction solution (lane 2). Recovered myosin was recognized by the anti-Taenia solium myosin antibody (arrow, lane 3). Pre-stained molecular weight standards are in lane 1.