INTRODUCTION
Protozoan parasites cause a wide range of infectious diseases of medical and veterinary importance, and thus represent a major economic burden worldwide. Prevention and chemotherapy are two routes that are tracked to combat parasitic diseases. However, despite the fact that considerable efforts have been undertaken, not a single vaccine against a parasitic infection in humans has been marketed to date, and only a few have been commercialized for veterinary application, most of them live-attenuated vaccines. Thus, chemotherapeutic treatment still remains the sole option. Most drugs that are currently in use to combat parasitic infections were discovered decades ago, and development of resistance poses a major threat (Mäser et al. Reference Mäser, Wittlin, Rottmann, Wenzler, Kaiser and Brun2012). Chemotherapy is a viable choice, if potent, safe and inexpensive drugs are made available, which are also easily administered, preferentially interfering in more than just one stage of the parasite life cycle and exhibiting selective toxicity (Delves et al. Reference Delves, Plouffe, Scheurer, Meister, Wittlin, Winzeler, Sinden and Leroy2012). Thus, candidate compounds must be thoroughly characterized with regard to their mechanisms of action and potential targets.
Intracellular protozoan parasites, especially the trypanosomatids (e.g. Leishmania, Trypanosoma cruzi) and the apicomplexans (e.g. Plasmodium and Toxoplasma among others), have a significant socio-economic impact worldwide. Their pathogenesis is related to the intracellular stages of their life cycle. This means that any compound targeted to kill these parasites must first cross the host cell membrane, then the parasitophorous vacuole membrane (relevant for most, but not all species), followed by the parasite plasmalemma, and finally also membranes of the organelle containing the relevant target(s). If the pathogen infects the CNS, the blood–brain barrier represents a further obstacle. Not surprisingly, the treatment options for diseases caused by these parasites are still limited, despite recent advances in some areas (Mäser et al. Reference Mäser, Wittlin, Rottmann, Wenzler, Kaiser and Brun2012).
In this review, we will focus on the design, synthesis, pharmacology and the mechanisms of action of amidine compounds and analogues (essentially derivatives of the broad-spectrum drug pentamidine) upon a range of intracellular protozoan parasites causing important diseases in animals and man, including Leishmania, T. cruzi, Plasmodium, Babesia, Toxoplasma, Neospora and Besnoitia.
DESIGN AND SYNTHESIS
Investigations into the antiprotozoan activity of classical aromatic diamidines (dicationic molecules – Figs. 1–6) began over 7 decades ago with the pioneering work of Warrington Yorke (King et al. Reference King, Lourie and Yorke1937). The early work led to the discovery that pentamidine (Fig. 1) and other aromatic diamidines were effective against human African trypanosomes (HAT) and some species of Leishmania (Ashley et al. Reference Ashley, Barber, Ewins, Newbery and Self1942). Despite decades of extensive investigation into this class of compounds, pentamidine remains the only diamidine with significant clinical value and it is still used to treat antimony-resistant leishmaniasis, early stage Trypanosoma brucei gambiense HAT and Pneumocystis jiroveci infections (formerly Pneumocystis carinii) (Sands et al. Reference Sands, Kron and Brown1985; Goa and Campoli-Richards, Reference Goa and Campoli-Richards1987; Barrett et al. Reference Barrett, Burchmore, Stich, Lazzari, Frasch and Cazzulo2003, Reference Barrett, Boykin, Brun and Tidwell2007; Werbovetz, Reference Werbovetz2006; Singh et al. Reference Singh, Kumar and Singh2012). A limitation of pentamidine (and most diamidines) is that it must be administered parenterally due to its poor bioavailability attributable, in large part, to the high pK a of the amidine functional group (10–12).
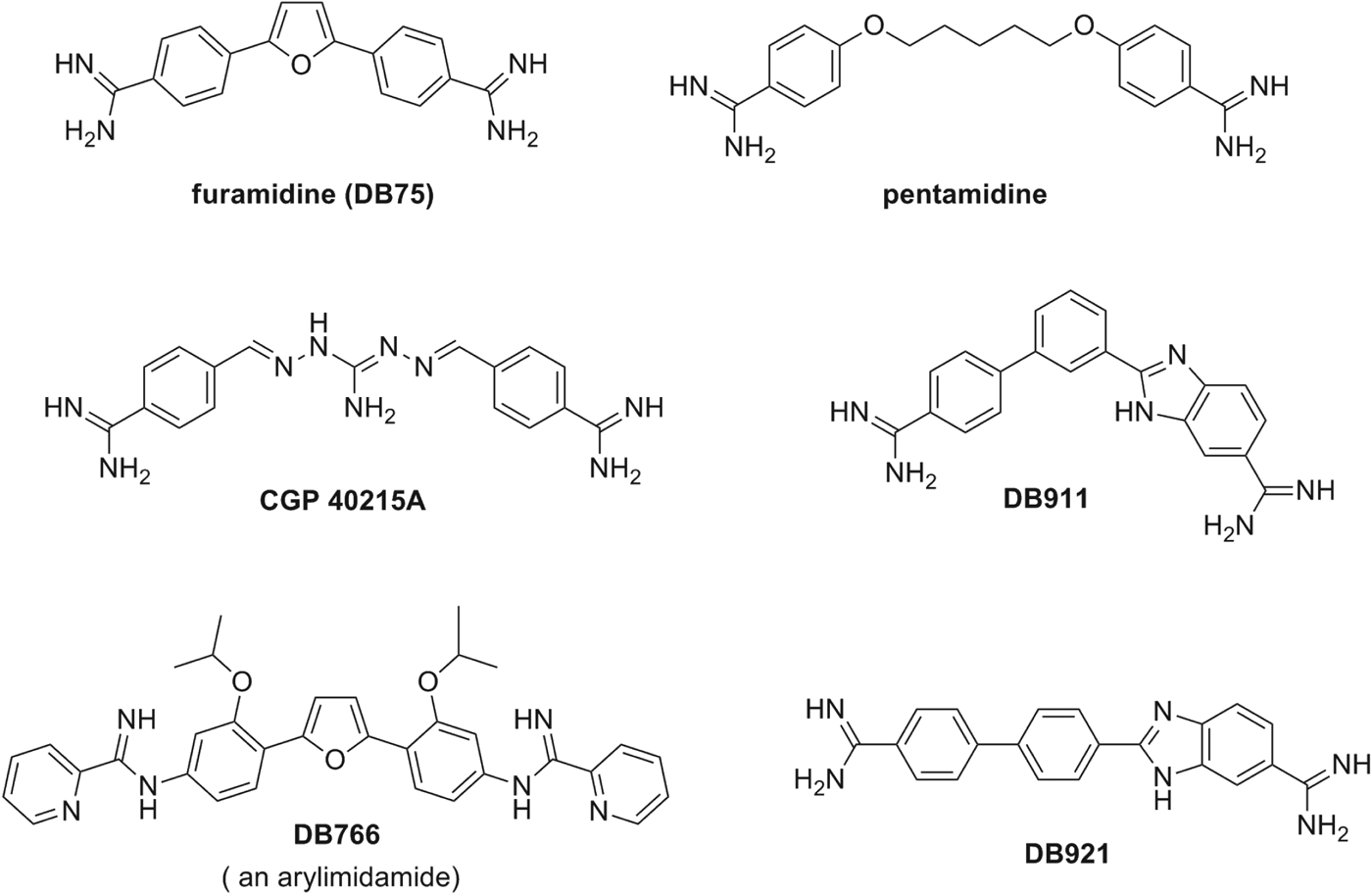
Fig. 1. Chemical structures of aromatic diamidines and arylimidamides.

Fig. 2. Fluorescent microscopy of aromatic diamidine DB1582 (A) and DB1651 (B) staining of bloodstream trypomastigotes (A) and intracellular forms (B) of T. cruzi (Y strain). Parasites and infected-cardiac cell cultures were incubated for 60 min with10 μg mL−1 DB1582 and DB1651. Note the dicationic compound in the parasite KDNA (arrow), nuclei (star) and within several non-DNA-containing organelles (arrowhead) distributed within the cytoplasm of intracellular amastigotes (acidocalcisomes-like structures). See host cell nuclei also labelled (N).

Fig. 3. Representative examples of diamidines, amidoximes, methamidoximes with some known pharmacokinetic properties.

Fig. 4. Representative examples of AIAs tested against intracellular Leishmania. Panel A – Compounds possessing potent antileishmanial activity (IC50 values <1 μ m). Panel B – Compounds that are inactive (IC50 values >10 μ m).

Fig. 5. Representative examples of amidines tested against Trypanosoma cruzi in in vitro and in vivo studies.

Fig. 6. Transmission electron microscopy of Neospora caninum tachyzoites cultured in the absence (A, B) and presence (C–E) of the arylimidamide DB745. (A) and (B) show control cultures at 48 h post-infection. Tachyzoites are situated within the cytoplasm and proliferate within a parasitophorous vacuole, surrounded by a parasitophorous vacuole membrane (arrows in (B)). Rhoptries (rop), micronemes (mic), dense granules (dg), mitochondria (mito) and the nucleus (nuc) are visible. Arrows in (A) pointed to the conoid at the apical end of the parasites, while arrows in (B) indicate the parasitophorous vacuole membrane. The nuclei (nuc) contain electron-dense chromatin, and white arrows point to the intact nuclear membrane. Hcmito=host cell mitochondria, hcnuc=host cell nucleus, pvtn=parasitophorous vacuole tubular network forming the matrix of the vacuole. Bar in (A)=0·84 μm; bar in (B)=0·55 μm. (C–E) represent images of tachyzoites after treatment with 1 μ m DB745 for 24 h (C) and 48 h (D–E). In (C), separated nuclear membranes are pointed out with white arrows. Cytoplasmic vacuoles (v) are partially filled with membranous and/or electron-dense material of unknown origin. Bar=0·65 μm. (D) Tachyzoites after 48 h of culture but still fused together, showing increased vacuolization (v) and large numbers of dense granules (dg). (E) Higher magnification view of the fused contact sites shown in (D). Parasites are clearly separated by two distinct inner membranes, but held together by the outer plasma membrane. The black arrow indicates the blind ending of the inner membrane of the upper tachyzoite, the white arrow shows the contact site with the outer membrane fusing the parasites together. Bars in (D)=0·4 μm; (E)=0·25 μm.
In the last decade, pafuramidine (DB289, Fig. 3), an orally effective pro-drug of the diamidine furamidine (Fig. 1), was found to be effective against first-stage HAT in a Phase III trial. Unfortunately, in a concurrent extended safety trial (Phase I) delayed renal toxicity was observed and the development of DB 289 was discontinued (Paine et al. Reference Paine, Wang, Generaux, Boykin, Wilson, De Koning, Olson, Pohlig, Burri, Brun, Murilla, Thuita, Barrett and Tidwell2010). Later (as will be detailed in the following sections), another class of amidines, initially described as ‘reversed amidines’ but now referred to as arylimidamides (Figs 1, 4 and 5), demonstrated high potency against several parasites like T. cruzi, Leishmania sp. (Stephens et al. Reference Stephens, Brun, Salem, Werbovetz, Tanious, Wilson and Boykin2003; Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010; Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012), Neospora caninum (Debache et al. Reference Debache, Guionaud, Kropf, Boykin, Stephens and Hemphill2011; Schorer et al. Reference Schorer, Debache, Barna, Monney, Boykin, Stephens and Hemphill2012), Besnoitia besnoiti (Cortes et al. Reference Cortes, Müller, Boykin, Stephens and Hemphill2011) and Echinococcus multilocularis (Stadelmann et al. Reference Stadelmann, Kuster, Scholl, Barna, Kropf, Keiser, Boykin, Stephens and Hemphill2011). Arylimidamides (AIAs) have lower pK a values (7–8) than classical aromatic amidines and therefore generally exhibit better bioavailability (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). The mode of action of both classes of amidines remains to be rigorously determined (as will be further discussed in see section ‘Mechanisms of antiparasitic action’). The two strands of the DNA double helix wrapped around each other creates two helical grooves, a wider, the major groove and a narrow one, the minor groove. The effectiveness of classical aromatic diamidines in recognizing the minor groove (space between the strands) of AT-rich sequences of DNA has led to a useful strategy for the design of new potential antiparasitic agents (Tidwell and Boykin Reference Tidwell, Boykin, Demeunynck, Bailly and Wilson2003; Wilson et al. Reference Wilson, Nguyen, Tanious, Mathis, Hall, Stephens and Boykin2005, Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008; Nguyen et al. Reference Nguyen, Boykin, Wilson, Lee and Strekowski2007). The beginning of a design paradigm for the targeting of the minor groove of DNA was clearly presented as early as the mid 1980s (Goodsell and Dickerson, Reference Goodsell and Dickerson1986). Based on crystal structure data for known minor-groove binders such as netropsin, distamycin, Hoechst 33 258 and related compounds, these authors concluded that the shape of the small molecule should be isohelical with the groove to which it was binding. Furthermore, the hydrogen bond acceptor and donors needed to be properly spaced to optimize the hydrogen-bonding interactions. Using these ideas J. W. Lown and co-workers developed a number of libraries of lexitropsin DNA minor-groove binders that were quite effective (Lown, Reference Lown1994). These isohelical design ideas were expanded to aromatic diamidine analogues, including pentamidine, and were correlated with estimated radii of curvature for the small molecules (Cory et al. Reference Cory, Tidwell and Fairly1992). Thus for a number of years it was believed that a crescent-shaped small molecule was necessary to achieve higher binding affinity (Tidwell and Boykin, Reference Tidwell, Boykin, Demeunynck, Bailly and Wilson2003). The near linear potent antitrypanosomal compound CGP 40215A (Fig. 1) was found to be a very strong minor-groove binder and the structure for a co-crystal with d(CGCGAATTCGCG)2 showed that 2 water molecules participate in an indirect hydrogen bond between one of the amidine groups and a thymine carbonyl group (Nguyen et al. Reference Nguygen, Lee, Hamelberg, Joubert, Bailly, Brun, Neidle and Wilson2002, Reference Nguygen, Hamelberg, Bailly, Colson, Stanek, Brun, Neidle and Wilson2004). A study of the antitrypanosomal isomeric dicationic biphenylbenzimidazoles DB911 and DB921 (Fig. 1) provided another case to evaluate the necessity of small molecule-DNA groove shape complementarities (Ismail et al. Reference Ismail, Brun, Wenzler, Tanious, Wilson and Boykin2004, Reference Ismail, Batista-Parra, Miao, Wilson, Wenzler, Brun and Boykin2005; Miao et al. Reference Miao, Lee, Batista-Parra, Ismail, Neidle, Boykin and Wilson2005). In this pair DB911 has the classical crescent shape associated with the DNA minor groove whereas DB921 is a near-linear molecule, therefore it was a surprise that the DNA binding affinity to an A/T site for DB921 was 10-fold higher than that of DB911 (Miao et al. Reference Miao, Lee, Batista-Parra, Ismail, Neidle, Boykin and Wilson2005). The structure of a DB921-d(CGCGAATTCGCG)2 co-crystal clearly showed that 1 water molecule forms a hydrogen bond array between the benzamidine group of DB921 and an adenine N atom (Miao et al. Reference Miao, Lee, Batista-Parra, Ismail, Neidle, Boykin and Wilson2005). These examples show that water can mediate the interaction between linear dications and the DNA minor groove thus in effect providing an isohelical complex. Since water can serve as hydrogen bond isohelical mediators for linear dicationic molecules, such interactions should be taken into account in the design of potential minor-groove binders. For a more detailed treatment of this subject see the review by Nguyen et al. (Reference Nguyen, Boykin, Wilson, Lee and Strekowski2007).
Aromatic diamidines are usually prepared from their corresponding bis-nitriles. In this review 3 major methods for achieving this conversion will be presented along with illustrations from the literature. The most venerable method for the nitrile to amidine conversion is known as the Pinner amidine synthesis which was first described by Pinner in 1877 (Pinner and Klein, Reference Pinner and Klein1877). This method, which is arguably the most widely used, has been reviewed by several authors (Shriner and Neumann, Reference Shriner and Neumann1944; Roger and Neilson, Reference Roger and Neilson1961; Eloy and Lenaers, Reference Eloy and Lenaers1962; Aly and Nour-El-Din, Reference Aly and Nour-El-Din2008). Scheme 1 illustrates this 2-step process which was used in the synthesis of furamidine (Fig. 1) (Das and Boykin, Reference Das and Boykin1977). The first step involves acid-catalysed addition of ethanol to the nitrile groups to form an imidate ester. The second step results in the formation of the amidine by the addition of ammonia or a wide array of amines; the latter gives this approach great versatility by allowing the generation of libraries of N-substituted amidines (Boykin et al. Reference Boykin, Kumar, Spychala, Zhou, Lombardy, Wilson, Dykstra, Jones, Hall, Tidwell, Laughton, Nunn and Neidle1995). A major limitation of this method is that water must be rigorously excluded. A further complication is that aromatic bis-nitriles are frequently quite insoluble, which necessitates large volumes of solvent or long reaction times, either of which increase the possibility for exposure to water.

Scheme 1. Synthesis of furamidine by the Pinner method.
The second important method involves the intermediacy of amidoximes and is outlined in Scheme 2 (Judkins et al. Reference Judkins, Allen, Cook, Evans and Sardharwala1996). This methodology was employed for the synthesis of highly active imidazo[1,2-a]pyridines (Ismail et al. Reference Ismail, Brun, Wenzler, Tanious, Wilson and Boykin2004) and has been effectively employed in several other systems (Ismail et al. Reference Ismail, Arafa, Reto, Wenzler, Miao, Wilson, Generaux, Bridges, Hall and Boykin2006, Reference Ismail, Arafa, Wenzler, Brun, Tanious, Wilson and Boykin2008, Reference Ismail, El Bialy, Brun, Wenzler, Nanjunda, Wilson and Boykin2011). This process formally involves 3 steps; however, in practice the acetoxy analogue is not isolated, so functionally it is a 2-step process. This method has the advantage that rigorous water exclusion is not necessary. Unlike the Pinner process, N-substituted amidines cannot be made using this sequence. The presence of functional groups which can be reduced or cleaved under catalytic hydrogenation conditions and compounds that include sulphur-containing groups, which may poison the catalyst, reduce the flexibility of this method.

Scheme 2. Synthesis of an aromatic diamidine via a diamidoxime.
The third important method is clearly the most elegant and direct approach for conversion of nitriles into amidines and is shown in Scheme 3 (Boere et al. Reference Boere, Oakley and Reed1987). This approach was utilized in the preparation of the highly active naphthalene-biphenyl diamidine presented in Scheme 3 (Chackal-Catoen et al. Reference Chackal-Catoen, Mao, Wilson, Wenzler, Brun and Boykin2006) and has been extensively used in other systems (Hu et al. Reference Hu, Arafa, Ismail, Wenzler, Brun, Munde, Wilson, Nzimiro, Samyesudhas, Werbovetz and Boykin2008, Reference Hu, Arafa, Ismail, Patel, Munde, Wilson, Wenzler, Brun and Boykin2009; Farahat et al. Reference Farahat, Kumar, Say, Barghash, Goda, Eisa, Wenzler, Brun, Liu, Mickelson, Wilson and Boykin2010, Reference Farahat, Paliakov, Kumar, Barghash, Goda, Eisa, Wenzler, Brun, Yang Liu, Wilson and Boykin2011; Branowska et al. Reference Branowska, Farahat, Kumar, Wenzler, Brun, Liu, Wilson and Boykin2010; Ismail et al. Reference Ismail, El Bialy, Brun, Wenzler, Nanjunda, Wilson and Boykin2011). This process involves the nucleophilic addition of a silyl protected amide anion to the nitrile group. The reaction is worked up with HCl/EtOH which both de-protects the nitrogen atom and forms the hydrochloride salt of the amidine. This approach is limited to preparation of unsubstituted amidines. If acidic hydrogen is present in a molecule that on de-protonation yields an anion that is in conjugation with the nitrile group, deactivation of the system to nucleophilic attack occurs, thus formation of the amidine often fails.

Scheme 3. Synthesis of an aromatic diamidine using LiN(TMS)2.
Bis-arylimidamides are readily prepared from bis-nitro analogues as illustrated in Scheme 4 (Stephens et al. Reference Stephens, Tanious, Kim, Wilson, Schell, Perfect, Franzblau and Boykin2001; Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). The iso-propoxy compound DB766 (Fig. 1) and its analogues were proved to be highly effective in animal models for intracellular obligatory parasites (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010; Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeirob; Da Silva et al. Reference Da Silva, Junqueira, Lima, Romanha, Sales Junior, Stephens, Som, Boykin and Soeiro2011a, Reference Da Silva, Daliry, Silva, Akay, Banerjee, Farahat, Mary, Fisher, Hu, Kumar, Liu, Stephens, Boykin and Soeirob, Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012) The bis-nitro compounds can be reduced to the corresponding bis-amino analogues by either of two standard methods; catalytic hydrogenation or stannous chloride reduction. The target AIAs are readily obtained by reaction of the bis-amine with S-(2-naphthylmethyl)pyridine-2-carbimidothioate. The major limitation of this method is that a relatively basic amine is required as a substrate since bis-amines containing strong electron withdrawing groups fail to react (Stephens et al. Reference Stephens, Tanious, Kim, Wilson, Schell, Perfect, Franzblau and Boykin2001). Other synthetic approaches to these type AIAs have been reported (Hu and Boykin, Reference Hu and Boykin2009). Both classical diamidines and bis-arylimidamides are attractive candidates for use against various parasitic diseases; however for one to become useful it is essential that it is economical to synthesize. The synthesis of either class by one or more of the methods outlined in Schemes 1–4 are likely to be at a reasonable cost. The cost of synthesis for the required bis-nitrile or bis-nitro precursor, however, can be quite variable and consideration should be given to linker selection early in the drug development process.

Scheme 4. Synthesis of the arylimidamide DB766.
MECHANISMS OF ANTIPARASITIC ACTION
In spite of many years of study by a number of laboratories, it has proven surprisingly difficult to definitively establish a single mechanism of antiparasitic action of dications, and various targets have been suggested including both mitochondrial and nuclear DNA, microtubules, acidocalcisomes, and a range of enzymes (Tidwell and Boykin, Reference Tidwell, Boykin, Demeunynck, Bailly and Wilson2003; Arafa et al. Reference Arafa, Brun, Wenzler, Tanious, Wilson, Stephens and Boykin2005; Soeiro et al. Reference Soeiro, De Souza, Stephens and Boykin2005; Wilson et al. Reference Wilson, Nguyen, Tanious, Mathis, Hall, Stephens and Boykin2005, Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008; Werbovetz, Reference Werbovetz2006; Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeirob; Paine et al. Reference Paine, Wang, Generaux, Boykin, Wilson, De Koning, Olson, Pohlig, Burri, Brun, Murilla, Thuita, Barrett and Tidwell2010; Ward et al. Reference Ward, Burgess, Burchmore, Barrett and de Koning2010). These compounds have typically been shown to selectively target AT-rich DNA and to form a complex in the minor groove of the double helix. This observation has led to considerable research to determine whether DNA is at least one of their cellular targets. Even for these fairly simple but structurally diverse compounds, however, it now seems certain that there is more than 1 bioreceptor in parasitic microorganisms. Both the mitochondrial and nuclear DNAs are well established as dication targets and the strongest evidence indicates that DNA functional destruction is related to their antiparasitic action (Wang and Englund, Reference Wang and Englund2001; Soeiro et al. Reference Soeiro, De Souza, Stephens and Boykin2005, Reference Soeiro, de Castro, de Souza, Batista, Silva and Boykin2008; Wilson et al. Reference Wilson, Nguyen, Tanious, Mathis, Hall, Stephens and Boykin2005, Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008; Werbovetz, Reference Werbovetz2006; Soeiro and De Castro, Reference Soeiro and de Castro2009; Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). In trypanosomes, Leishmania and related organisms, the mitochondrial kinetoplast DNA (kDNA) network typically contains DNA minicircles that encode for guide RNAs that edit transcripts from the maxicircle DNA, which corresponds to the mitochondrial genome (Shapiro and Englund, Reference Shapiro and Englund1995). The thousands of DNA minicircles are topologically linked in a planar disc array (Shapiro and Englund, Reference Shapiro and Englund1995; Klingbeil et al. Reference Klingbeil, Drew, Liu, Morris, Motyka, Saxowsky, Wang and Englund2001), exhibiting, as does the genomic DNA of Plasmodium, greater AT content (>75%), with extensive, closely spaced, sequences that act as strong binding sites and selective therapeutic target sites for these dicationic compounds (Carlton, Reference Carlton2003; Purfield et al. Reference Purfield, Tidwell and Meshnick2009).
The heterocyclic dications typically have absorption bands near 350–400 nm and emission bands in solution in the blue region near 450 nm (Wilson et al. Reference Wilson, Tanious, Buczak, Venkatramanan, Das, Boykin, Pulman and Jortner1990). By fluorescence (Fig. 2) and transmission electron microscopy analysis (Fig. 6), it is possible to follow the compound distribution and evaluate their ultrastructural effect on the treated parasites (Stewart et al. Reference Stewart, Krishna, Burchmore, Brun, de Koning, Boykin, Tidwell, Hall and Barrett2005; Lanteri et al. Reference Lanteri, Stewart, Brock, Alibu, Meshnick, Tidwell and Barrett2006; Werbovetz, Reference Werbovetz2006; Hu et al. Reference Hu, Arafa, Ismail, Wenzler, Brun, Munde, Wilson, Nzimiro, Samyesudhas, Werbovetz and Boykin2008; Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeirob). Results for a number of dications showed that they are first preferentially located in the kinetoplast and secondarily in the nucleus of Trypanosoma brucei, T. cruzi and Leishmania (Wang and Englund, Reference Wang and Englund2001; Lanteri et al. Reference Lanteri, Stewart, Brock, Alibu, Meshnick, Tidwell and Barrett2006; Mathis et al. Reference Mathis, Holman, Sturk, Ismail, Boykin, Tidwell and Hall2006, Reference Mathis, Bridges, Ismail, Kumar, Francesconi, Anbazhagan, Hu, Tanious, Wenzler, Saulter, Wilson, Brun, Boykin, Tidwell and Hall2007; Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeiro2010b; Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). The kDNA and kinetoplast itself are extensively modified shortly after treatment and the latter structure is generally destroyed about 24 h after diamidine exposure, leading to parasite death. In an important study, the Barrett group demonstrated that pentamidine targets the kinetoplast in Leishmania mexicana and that its concentration in the nucleus, cytoplasm and other cell compartments is very low (Basselin et al. Reference Basselin, Denise, Coombs and Barrett2002). In a strain of pentamidine-resistant L. mexicana organisms, this amidine (Fig. 1) passed the plasma membrane as usual, but did not efficiently achieve access to the mitochondrion, thus not being able to destroy the kDNA. As no other significant biological target was detected in those parasites, these data indicated that the antiparasitic activity of amidines, like pentamidine requires their uptake and delivery to the mitochondrion, allowing binding to the kDNA. As described above, considerable current literature data support kDNA as a primary antitrypanosomal biological target of these dications and there is also new evidence for their mechanism of action in T. brucei. Results from Englund´s laboratory showed that blockage of kinetoplast replication through RNA interference of topoisomerase II production led to the death of bloodstream parasites (Wang and Englund, Reference Wang and Englund2001). These findings clearly demonstrate that the kinetoplast is essential for these parasite forms. More recent research from the same group has definitively shown that in T. brucei compounds that act as kDNA binders destroy the kinetoplast structure and cause parasite death (Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). The same group reported in T. brucei that death triggered by ethidium bromide (EB – an intercalating agent also used as a fluorescent marker for DNA) was linked to its effect on parasite kDNA (Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). However, the existence of viable parasites lacking kDNA (dyskinetoplastic) has been a source of a long-lasting controversy about the role of the kinetoplast as target and whether kDNA loss could be in fact the mechanism of killing. Additional studies indicated that kDNA is essential in bloodstream forms of T. brucei and that dyskinetoplastic parasites only survive due to a compensating mutation in the nuclear genome (Schnaufer et al. Reference Schnaufer, Clark-Walker, Steinberg and Stuart2005; Lai et al. Reference Lai, Hashimi, Lun, Ayala and Lukes2008). These important observations led the Englund group to re-study the question of kDNA as a drug target. They found that binding of EB to covalently closed kDNA minicircles released from the network during replication caused extensive DNA super-twisting, helix distortion, inhibition of replication, and resulted in kDNA loss, culminating in parasite death. Interestingly, although EB could also kill dyskinetoplastic trypanosomes by targeting nuclear DNA, death occurred at higher concentrations through inhibition of nuclear DNA replication. The effect on kDNA occurred at around a 10-fold lower drug concentration as compared with the effect on the nuclear DNA, indicating the kinetoplast as the primary target but that the effect on nuclear replication can also contribute to cell killing (Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). While the studies performed by Englund's laboratory were performed with EB, these data may contribute to the knowledge regarding the mechanism of action of other compounds that act in the kinetoplast structure. Evidence obtained by transmission electron microscopy showed that some aromatic dications also target the mitochondrial kinetoplast in Leishmania (Hu et al. Reference Hu, Arafa, Ismail, Wenzler, Brun, Munde, Wilson, Nzimiro, Samyesudhas, Werbovetz and Boykin2008) and T. cruzi (De Souza et al. Reference De Souza, Menna-Barreto, Araújo-Jorge, Kumar, Hu, Boykin and Soeiro2006), demonstrating that kDNA targeting of these compounds holds for, at least, pathogenic kinetoplastids. Clearly, additional studies are needed to provide definitive proof regarding the mechanism of action of these dicationic compounds on different organisms.
It should be noted that binding to DNA may be only the initial step in the biological activity of most of these dications and other mechanisms are yet to be defined. It is likely that the functions of DNA-targeted control proteins or enzymes are directly or indirectly modified by dication binding to DNA for antiparasitic activity to occur. For this reason some optimum level of DNA binding is required, but a direct correlation of DNA binding constants and biological activity is not generally observed (Daliry et al. Reference Daliry, Pires, Silva, Pacheco, Munde, Stephens, Kumar, Ismail, Liu, Farahat, Akay, Som, Hu, Boykin, Wilson, De Castro and Soeiro2011). The experimental results obtained so far in trypanosomes and Leishmania led to a general concept that diamidines act on these parasites by targeting their kinetoplast (Hu et al. Reference Hu, Arafa, Ismail, Wenzler, Brun, Munde, Wilson, Nzimiro, Samyesudhas, Werbovetz and Boykin2008; Wilson et al. Reference Wilson, Tanious, Mathis, Tevis, Hall and Boykin2008). The destruction of the kinetoplast by diamidines could occur, for example, by compound interference with topoisomerase II-catalysed opening and rejoining of kDNA minicircles. In agreement with this model, Shapiro and Englund (Reference Shapiro and Englund1990) showed in a fundamental set of experiments that pentamidine, berenil and other antitrypanosomal drugs bind to DNA and selectively target kinetoplast topoisomerase II leading to its linearization. There are other DNA replication steps in the large, catenated kDNA network where drug binding could also cause replication errors and DNA degradation (Chowdhury et al. Reference Chowdhury, Bakshi, Wang, Yildirir, Liu, Pappas-Brown, Tolun, Griffith, Shapiro, Jensen and Englund2010). Additionally, the antiparasitic activity and relatively low host toxicity of dications suggest that the intricate structure of the catenated kDNA network must be also considered. Since there are thousands of DNA minicircles in the network, each with multiple closely spaced AT sequences, a very small compound-induced error percentage in the normal biological functions of the kinetoplast could be enough to cause destruction of the network, kinetoplast disintegration and cell death. This structure is unique to kinetoplastid organisms and this fact may account for their generally low cytoxicity to mammalian cells. Minor topological effects of the compounds at the repeated AT sequences of the kDNA network could act in synergy, leading to a biologically catastrophic effect in the treated parasites.
It seems likely that mitochondrial functions in African trypanosomes are much more complex than previously realized. RNA editing, for example, a key kinetoplast function, has been demonstrated to be essential for survival of bloodstream forms of T. brucei (Schnaufer et al. Reference Schnaufer, Panigrahi, Panicucci, Igo, Wirtz, Salavati and Stuart2001). Given the normal close coupling of kDNA, nuclear DNA and cell division; it is also possible that the loss of the kinetoplast disrupts normal cell division (Schnaufer et al. Reference Schnaufer, Domingo and Stuart2002). An interesting additional aspect of the alterations induced by diamidines on the kinetoplast topology and physiology is the occurrence of parasites expressing programmed cell death characteristics (Schnaufer et al. Reference Schnaufer, Domingo and Stuart2002; De Souza et al. Reference De Souza, Menna-Barreto, Araújo-Jorge, Kumar, Hu, Boykin and Soeiro2006). Clearly, the kinetoplast is essential for trypanosomes and its destruction by diamidine treatment can yield the observed biological activity. In summary, 3 clear statements can be made with regard to the antiparasitic mechanisms of dicationic amidines. The kDNA (in kinetoplastid organisms) is a primary target of these dications which generally cause destruction of the kinetoplast. Nuclear DNA is a secondary target in these organisms while it is at least one of the main targets in other parasites such as Plasmodium (Purfield et al. Reference Purfield, Tidwell and Meshnick2009), although the evidence for this mode of action is less definitive. The nuclear mechanism is dominant in dyskinetoplastic organisms and this secondary mechanism accounts for the long held, but completely incorrect, view among some scientists that the kinetoplast could not be the primary target of any antiparasitic drug. Third, there are certainly other mechanisms of action of some compounds in this large and diverse group of molecules, but the evidence for any other specific mechanism is very limited and certainly not definitively established at present. An obvious corollary to these statements is that much more research on the antiparasitic mechanisms of this important set of compounds is urgently needed.
PHARMACOKINETICS
Absorption
As briefly presented above, the classical antiparasitic diamidines, e.g. pentamidine and furamidine (Fig. 1), are not well-absorbed through the gastrointestinal (GI) tract after oral administration, and hence are given by injection to achieve the desired bioavailability. The in vitro permeability of furamidine measured in Caco-2 cell monolayers was low, similar to that of the paracellular permeability marker mannitol, and it was modulated by the absence of Ca2+, suggesting a paracellular route for traversing epithelial membranes (Zhou et al. Reference Zhou, Lee, Thaker, Boykin, Tidwell and Hall2002). As stated in the section ‘Design and synthesis’, the high pK a values and the resulting positive charge at physiological pH preclude diamidine molecules from passively diffusing across lipid membranes, thus carrier-mediated transport is required to achieve cellular uptake. To overcome the absorption limitation of cationic diamidines, pro-drug strategies and novel structural designs have been employed to enhance oral absorption. The acid dissociation constant of the amidine group can be modulated by N-derivatization to form the amidoxime (e.g. DB290, DB821 and DB840) and alkyloxy prodrugs (e.g. pafuramidine, DB844 and DB868) (Fig. 3). The measured pK a values for DB290 and pafuramidine were 5·2 and 4·1, respectively, significantly lower than that of furamidine (pK a=11·8). This lowered pK a resulted in an 85-fold increase of the permeability of pafuramidine relative to furamidine (Zhou et al. Reference Zhou, Lee, Thaker, Boykin, Tidwell and Hall2002) although no increase was detected for DB290 due to extensive metabolism within the cells (Saulter, Reference Saulter2005). In addition to the pro-drug approach, amidine groups were modified to form AIAs by attaching the imino group of the amidine to an anilino nitrogen (e.g. DB745 and DB766; Figs 1 and 4). This modification lowered the pK a to 7·1 and 7·4 for DB745 and DB766, respectively, and contributed to their oral activity against Leishmania and T. cruzi in mice and hamsters (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010, Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, Reference Batista, Pacheco, Kumar, Branowska, Ismail, Hu, Boykin and Soeirob; Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012).
Distribution
Once absorbed, the cationic amidines as well as AIAs exhibit extensive distribution into various tissues. After a single oral dose (10 mg kg−1) of radio-isotope labelled pafuramidine to rats, concentrations of radioactivity in nearly all tissues exceeded those in blood (Midgley et al. Reference Midgley, Fitzpatrick, Taylor, Houchen, Henderson, Wright, Cybulski, John, McBurney, Boykin and Trendler2007). Liver was the major organ of drug accumulation, where 13–21% of radioactivity was found 7 days post-dose and essentially all was in the form of the active compound furamidine. Other tissues, with significant concentrations of radioactivity included: kidney, adrenal, nasal mucosa, intestine and pancreas. Concentrations of radioactivity in the brain were similar to those in plasma, whereas they were below the limit of detection in the cerebrospinal fluid. The limited CNS exposure is consistent with the lack of cures for pafuramidine in the mouse and monkey models of second-stage HAT (Mdachi et al. Reference Mdachi, Thuita, Kagira, Ngotho, Murilla, Ndung'u, Tidwell, Hall and Brun2009; Wenzler et al. Reference Wenzler, Boykin, Ismail, Hall, Tidwell and Brun2009). More recently, novel aza-analogues of furamidine (DB820 and DB829, Fig. 3) and their methoxy pro-drugs (DB844 and DB868) (Fig. 3) have cured animals in the second-stage mouse (100%) and monkey (43–100%) models of HAT (Wenzler et al. Reference Wenzler, Boykin, Ismail, Hall, Tidwell and Brun2009; Thuita et al. Reference Thuita, Wang, Kagira, Denton, Paine, Mdachi, Murilla, Ching, Boykin, Tidwell, Hall and Brun2012; J. K. Thuita and R. Brun, personal communication), suggesting an improved brain penetration by the new aza-analogues. The AIA DB766 accumulated preferentially to the antileishmanial target organs (i.e. liver and spleen), as well as kidneys, with maximum concentrations up to 26-fold higher than those in plasma (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). The compound did not appear to get into the brain efficiently relative to the above tissues, with concentrations generally close to or below the corresponding plasma concentrations. Studies to delineate the mechanisms of the differential brain distribution of cationic diamidines are warranted and are currently underway.
Metabolism
Berger and coworkers demonstrated that pentamidine was extensively metabolized in the perfused rat livers with only 15% of recovered radioactivity as pentamidine after 4 h incubation (Berger et al. Reference Berger, Naiman, Hall, Peggins, Brewer and Tidwell1992). Two major metabolites were the alkyl linker oxidation products, accounting for approximately 62% of recovered radioactivity. Amidoxime formation through N-hydroxylation of pentamidine was a minor reaction pathway, accounting for <1% of the recovered radioactivity. Unlike pentamidine, little metabolism has been observed for diamidines with a furan linker (e.g. furamidine, DB820 and DB829, Figs 1 and 3) replacing the alkyl chain in the pentamidine. As discussed in the section ‘Design and synthesis’, various pro-drugs of diamidines have been designed to improve oral bioavailability, by masking the positive charge of the amidine moiety using hydroxy, alkyloxy, acetoxy and amino acids (Clement and Raether, Reference Clement and Raether1985; Boykin et al. Reference Boykin, Kumar, Hall, Bender and Tidwell1996; Ismail et al. Reference Ismail, Brun, Easterbrook, Tanious, Wilson and Boykin2003; Kotthaus et al. Reference Kotthaus, Schade, Schwering, Hungeling, Muller-Fielitz, Raasch and Clement2011). Biotransformation of pafuramidine (Fig. 3), the methoxy pro-drug of furamidine, to the active furamidine is complex, involving multiple steps of sequential oxidative O-demethylation and reductive N-dehydroxylation reactions that are catalysed by cytochrome P450 enzymes and the cytochrome b5/NADH b5 reductase system, respectively (Saulter et al. Reference Saulter, Kurian, Trepanier, Tidwell, Bridges, Boykin, Stephens, Anbazhagan and Hall2005; Wang et al. Reference Wang, Saulter, Usuki, Cheung, Hall, Bridges, Loewen, Parkinson, Stephens, Allen, Zeldin, Boykin, Tidwell, Parkinson, Paine and Hall2006, Reference Wang, Wu, Bridges, Zeldin, Kornbluth, Tidwell, Hall and Paine2007). Similar enzymatic reactions have been shown to be involved in the biotransformation of aza-analogues of pafuramidine (DB844 and DB868) to their respective active diamidines (DB820 and DB829) (Ansede et al. Reference Ansede, Voyksner, Ismail, Boykin, Tidwell and Hall2005; Generaux et al. Reference Generaux, Ainslie, Bridges, Ismail, Boykin, Tidwell, Thakker and Paine2013). Metabolism studies on AIAs are limited due to their early stage in drug development. Previous metabolic stability results using liver microsomes indicate that mice and hamsters metabolize AIAs more extensively than rats and humans (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). However, no information is available on the metabolite identity.
Excretion
Following oral administration in rats, pafuramidine was excreted predominantly in the feces (approximately 62% of dose) as the original pro-drug (20% of dose), furamidine (22% of dose) and an intermediate mono-amidine metabolite (10% of dose) (Midgley et al. Reference Midgley, Fitzpatrick, Taylor, Houchen, Henderson, Wright, Cybulski, John, McBurney, Boykin and Trendler2007). Urinary excretion accounted for less than 5% of dose. A considerable portion of the dose (6–21%) was retained in the body (mainly in the liver as furamidine) at 7 days post-dose and was slowly eliminated. Biliary excretion accounted for 14–26% of dose in the first 48 h post-dose. In general, methoxy pro-drugs of diamidines are eliminated rapidly (plasma t 1/2 ranging from 1 to 18 h in rats and monkeys), whereas the active diamidines exhibit extensive peripheral distribution (plasma t 1/2 ranging from days to weeks) and are often detectable in accumulating tissues (e.g. liver) several months post-dose (Donnelly et al. Reference Donnelly, Bernard, Rothkotter, Gold and Armstrong1988; Midgley et al. Reference Midgley, Fitzpatrick, Taylor, Houchen, Henderson, Wright, Cybulski, John, McBurney, Boykin and Trendler2007; Thuita et al. Reference Thuita, Wang, Kagira, Denton, Paine, Mdachi, Murilla, Ching, Boykin, Tidwell, Hall and Brun2012). Experimental excretion data for other diamidines/prodrugs and AIAs are scarce, but presumably follow a similar pattern.
As reviewed above, the most successes in improving dicationic amidines against trypanosomiasis infection like HAT are those that optimize pharmacokinetic properties in terms of oral bioavailability and/or target-tissue penetration, as pentamidine remains among the most active trypanocidal diamidines against HAT. Greater membrane permeability also contributed to the potent activity of AIAs against intracellular Leishmania and T. cruzi parasites. Future efforts should consider investigating mechanisms of tissue uptake of the diamidines to reduce unwanted high tissue accumulation, especially in the liver and kidney. The aza analogue DB829 represents such a new diamidine, which accumulated less in the rat liver and kidney (by approximately 2- and 5-fold, respectively), and yet showed twice more systemic exposure than furamidine following intravenous administration (Paine et al. Reference Paine, Wang, Generaux, Boykin, Wilson, De Koning, Olson, Pohlig, Burri, Brun, Murilla, Thuita, Barrett and Tidwell2010). DB868 (Fig. 3), the methoxy pro-drug of DB829, was also better tolerated in vervet monkeys than its predecessors and cured the monkeys in the acute and CNS model of HAT after oral administration (J. K. Thuita and R. Brun, personal communication).
EFFICACY AND SELECTIVITY OF AMIDINES AND THEIR DERIVATIVES AGAINST LEISHMANIA
Leishmania parasites cause a spectrum of disease ranging from cutaneous lesions to potentially fatal visceral infections of the mononuclear phagocyte system depending on the species of infecting organism. These parasites alternate between an elongated promastigote form that develops in the sandfly vector and a smaller, sessile amastigote form that exists within the macrophages of an infected host. Recent epidemiological studies estimate that between 0·7 and 1·2 million cases of cutaneous leishmaniasis occur annually, while anywhere from 0·2 to 0·4 million cases of visceral disease and between 20 000 and 40 000 deaths due to visceral leishmaniasis occur each year (Alvar et al. Reference Alvar, Vélez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). Chemotherapy for leishmaniasis has traditionally relied on the pentavalent antimonial drugs sodium stibogluconate and meglumine antimoniate; treatment for both visceral and cutaneous disease still depends on these injectable antimonials in most areas. However, resistance to pentavalent antimonials on the Indian subcontinent, a focal point for visceral leishmaniasis, has led to the increased use of the parenteral drugs amphotericin B and paromomycin as well as miltefosine, the first oral drug for the treatment of this disease (Picado et al. Reference Picado, Rijal, Sundar and Boelaert2012). Unfortunately, all of these agents have one or more weaknesses, including adverse effects, high cost, the requirement of a long course of treatment, and fears due to favourable conditions for the development of drug-resistant parasites (Croft and Olliaro, Reference Croft and Olliaro2011). A safe, effective and inexpensive oral treatment would be a welcome addition for the treatment of leishmaniasis, either as a component of combination therapy in areas where drug resistance has been reported or as a convenient form of monotherapy in regions where resistance has not been documented. Pentamidine (Fig. 1) was used in the past for the treatment of antimony-resistant visceral leishmaniasis infections, but response rates have decreased with time (Olliaro et al. Reference Olliaro, Guerin, Gerstl, Haaskjold, Rottingen and Sundar2005) as illustrated by a clinical study published in 2009 showing that pentamidine was inferior to amphotericin B in treating antimony-resistant visceral leishmaniasis in Bihar, India (Das et al. Reference Das, Siddiqui, Pandey, Singh, Topno, Singh, Verma, Ranjan, Sinha and Das2009). Pentamidine is currently the treatment of choice for cutaneous leishmaniasis caused by Leishmania guyanensis (van der Meide et al. Reference van der Meide, Sabajo, Jensema, Peekel, Faber, Schallig and Fat2009; Hu et al. Reference Hu, Kent, Adams, van der Veer, Sabajo, Mans, de Vries, Schallig and Fat2012) and is also a recommended drug against Leishmania panamensis (World Health Organization, 2010) despite the fact that the clinical antileishmanial efficacy of pentamidine is relatively low. For example, while the administration of three 4 mg kg−1 doses of pentamidine by the intramuscular route was as effective as higher doses of meglumine antimoniate against cutaneous leishmaniasis caused by L. guyanensis, the cure rate after 6 months was only 58·1% (Neves et al. Reference Neves, Talhari, Gadelha, Silva Júnior, Guerra, Ferreira and Talhari2011).
Pentamidine possesses good in vitro activity against extracellular Leishmania, but does not completely clear Leishmania-infected macrophages at concentrations that are not toxic to the host cell (Berman and Wyler, Reference Berman and Wyler1980; Gebre-Hiwot et al. Reference Gebre-Hiwot, Tadesse, Croft and Frommel1992; X. Zhu, unpublished observations). Many other diamidine-containing molecules also possess in vitro activity against Leishmania (Mayence et al. Reference Mayence, Vanden Eynde, LeCour, Walker, Tekwani and Huang2004; Huang et al. Reference Huang, Vanden Eynde, Mayence, Donkor, Khan and Tekwani2006; Bakunova et al. Reference Bakunova, Bakunov, Wenzler, Barscz, Werbovetz, Brun, Hall and Tidwell2007, Reference Bakunova, Bakunov, Wenzler, Barszcz, Werbovetz, Brun and Tidwell2009, Bakunov et al. Reference Bakunov, Bakunova, Ghebru, Werbovetz, Brun and Tidwell2010), but the potency of these agents against intracellular Leishmania and their efficacy in animal models of leishmaniasis was not reported. Earlier papers indicated that diamidines possess limited activity in animal models of leishmaniasis (Hanson et al. Reference Hanson, Chapman and Kinnamon1977; Steck et al. Reference Steck, Kinnamon, Rane and Hanson1981; Macharia et al. Reference Macharia, Bourdichon and Gicheru2004), an observation that may also restrict the clinical utility of pentamidine and other diamidines for treating cutaneous and visceral leishmaniasis. The low in vivo efficacy of diamidines may be a result of the dicationic nature of these compounds precluding oral bioavailability and also limiting the passive diffusion of these molecules across membranes (see details in the sections ‘Introduction’ and ‘Mechanisms of antiparasitic action’). This may be a major problem in the treatment of intracellular parasites such as Leishmania, where a drug is required to cross at least 3 sets of membranes (host cell membrane, parasitophorous vacuole membrane, parasite cell membrane) to enter the intracellular organism.
In 2003, Stephens et al. showed that AIAs possess potent activity against both extracellular and intracellular Leishmania donovani, with the potency being higher against intracellular organisms (Stephens et al. Reference Stephens, Brun, Salem, Werbovetz, Tanious, Wilson and Boykin2003). With their lower pK a values (∼7) and greater lipophilicity compared with pentamidine (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010), AIAs may exhibit superior activity against intracellular Leishmania due to improved membrane permeability. Several different structural variants of bis-AIAs (compounds containing 2 terminal amidine groups attached to a heterocycle) have now been prepared. The initial paper reporting the potent activity of AIAs against intracellular Leishmania described the synthesis and evaluation of a series of 8 symmetrical bis-AIAs containing different functional groups on the phenyl rings of the diphenylfuran linker. The potency of these compounds against intracellular L. donovani ranged from 150–3600 nm (Stephens et al. Reference Stephens, Brun, Salem, Werbovetz, Tanious, Wilson and Boykin2003). A broader series of over 50 symmetrical bis-AIAs was reported by Collar et al. in 2011; these compounds were tested for their ability to reduce intracellular Leishmania amazonensis parasite burdens. Compounds with 2-pyridyl terminal groups and fairly large hydrophobic substitutions on the diphenylfuran linker showed potent activity. However, when the substitutions became too large (i.e. benzyloxy or isobutoxy), potency was diminished. In terms of the terminal groups, the 2-pyridyl terminal substitution was superior to other heterocyclic substitutions such as 2-pyrimidyl, 2-pyrazinyl and 2-pyrrolyl, although a compound with a 2-methyl-5-thiazolyl terminal group retained potent intracellular activity (Collar et al. Reference Collar, Zhu, Werbovetz, Boykin and Wilson2011). Symmetrical bis-AIAs containing different linker groups have also been synthesized and evaluated against intracellular Leishmania. Compounds with 1,4-phenyl and 1,4-phenylenediamine linkers showed dramatically reduced antileishmanial potency, while compounds with 4,4′-biphenyl, 4,4′-oxydianiline and 4,4′-oxybis(methylene)dianiline linkers retained antiparasitic activity (Banerjee et al. Reference Banerjee, Farahat, Kumar, Wenzler, Brun, Munde, Wilson, Zhu, Werbovetz and Boykin2012). Bis-AIAs containing unsymmetrical substitutions on the diphenylfuran linker also retain potent activity against intracellular Leishmania; an AIA bearing an isopropoxy substitution on one of the 2 phenyl rings of the diphenylfuran linker displayed an IC50 value of 5·3 nm against L. donovani and 93 nm against L. amazonensis (Reid et al. Reference Reid, Farahat, Zhu, Pandharkar, Boykin and Werbovetz2012). A structure-activity map summarizing the in vitro antileishmanial potency of bis-AIAs was recently published (Reid et al. Reference Reid, Farahat, Zhu, Pandharkar, Boykin and Werbovetz2012) and examples of active and inactive bis-AIAs against intracellular Leishmania are given in Fig. 4. The most promising AIAs in terms of their in vivo antileishmanial potential are 2,5-bis[2-(2-propoxy)-4-(2-pyridylimino)aminophenyl]furan and the closely related 2,5-bis[2-(2-cyclopentyloxy)-4-(2-pyridylimino)aminophenyl]furan. When prepared as its dihydrochloride salt form, the 2-propoxy compound is known as DB766, while as its dimesylate, it is termed DB1960. The cyclopentyloxy compound dihydrochloride salt has the code name DB1852, while the corresponding dimesylate is DB1955 (Figs 1 and 4). DB766 displayed IC50 values of 36, 87 and 14 nm against intracellular L. donovani, L. amazonensis and Leishmania major amastigotes, respectively, comparable to the in vitro potency of the clinically applied antileishmanial drug amphotericin B. When tested orally in animal models of visceral leishmaniasis, DB766 reduced L. donovani parasite burdens in the liver by 71% and 89% when administered to infected BALB/c mice and golden hamsters at 100 mg kg−1 day−1×5; the compound was not mutagenic in the Ames test (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). DB1852 was less active than DB766 against intracellular Leishmania in vitro, but showed in vivo potency similar to that of DB766 in the murine visceral leishmaniasis model. The corresponding mesylate salts of DB766 and DB1852, DB1960 and DB1955, respectively, were 5·3-fold and 3·1-fold more soluble than their hydrochloride salt counterparts, allowing for repeat-dose toxicology studies to be performed at doses up to 500 mg kg−1 day−1. The maximum tolerated dose of both mesylate salts was found to be <100 mg kg−1 day−1 by the oral route, with liver, kidney and haematological toxicity observed at high doses and microscopic findings in the GI tract observed at lower doses. DB1960 was more toxic than DB1955, but neither compound exhibited a sufficient therapeutic window to move forward with pre-clinical development (Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012). Unfortunately, other bis-AIAs that display potent in vitro antileishmanial activity are more toxic than DB766, DB1955 and DB1960 in vivo (Banerjee et al. Reference Banerjee, Farahat, Kumar, Wenzler, Brun, Munde, Wilson, Zhu, Werbovetz and Boykin2012; Reid et al. Reference Reid, Farahat, Zhu, Pandharkar, Boykin and Werbovetz2012 and X. Zhu, unpublished observations). Bis-AIAs are extremely potent against intracellular L. donovani (IC50 values in the mid to low nanomolar range), but toxicity issues have precluded the further development of the class as candidates against visceral leishmaniasis. Since the mechanism of action of the AIAs is unknown, better understanding of the target(s) of the AIAs could lead to the design of more selective agents with a greater therapeutic window. While diamidines cause dilation of the Leishmania mitochondrion (Croft and Brazil, Reference Croft and Brazil1982; Hu et al. Reference Hu, Arafa, Ismail, Wenzler, Brun, Munde, Wilson, Nzimiro, Samyesudhas, Werbovetz and Boykin2008), ultrastructural investigation of the effects of DB766 in L. donovani axenic amastigotes reveals little effect on this parasite organelle (T. Pandharkar, unpublished observations). The results of further mechanistic investigations with AIAs will hopefully benefit future antileishmanial drug discovery efforts.
EFFICACY AND SELECTIVITY OF AMIDINES AND THEIR DERIVATIVES AGAINST T. CRUZI
The obligatory intracellular protozoan, T. cruzi is the aetiological agent of Chagas disease, an important and neglected tropical disease, which affects more than 10 million people in endemic areas of Latin America (Dias, Reference Dias2009). Chagas disease has 2 phases: the acute, which appears shortly after the infection, and the chronic phase that develops in about 20–30% of the infected individuals, after a silent period of years or decades, called the indeterminate phase (Rocha et al. Reference Rocha, Teixeira and Ribeiro2007). In both clinical stages, tissue damage is directly induced by the parasite, or indirectly, by parasite-driven immune modulation (Coura and Borges-Pereira, Reference Coura and Borges-Pereira2012). The main clinical manifestations of Chagas disease are cardiac and/or digestive alterations but the mechanisms that govern this pathogenesis are not fully understood (Marin-Neto and Rassi, Reference Marin-Neto and Rassi2009). Due to the well-known undesirable side effects, and the occurrence of parasite strains naturally resistant to both nitro-heterocyclic compounds (Benznidazole and Nifurtimox) associated with their poor efficacy in the later chronic disease stage, the identification of novel anti-T. cruzi agents are justified (Romanha et al. Reference Romanha, Castro, Soeiro, Lannes-Vieira, Ribeiro, Talvani, Bourdin, Blum, Olivieri, Zani, Spadafora, Chiari, Chatelain, Chaves, Calzada, Bustamante, Freitas-Junior, Romero, Bahia, Lotrowska, Soares, Andrade, Armstrong, Degrave and Andrade2010). However, as a consequence of the low interest of most pharmaceutical companies and the lack of standardized/proper experimental protocols and animal models for drug screening, very few compounds moved to clinical trials, including 2 triazole inhibitors of the parasite's C14α sterol demethylase: posaconazole (Schering-Plough Research Institute) and a pro-drug of ravuconazole (E1224 (Eisai Company)) (De Castro et al. Reference De Castro, Batista, Batista, Batista, Daliry, de Souza, Menna-Barreto, Oliveira, Salomão, Silva, Silva and Soeiro2011). However, these azoles have important limitations mainly related to their high costs and the potential to induce parasite resistance as they enhance T. cruzi sterol 14alpha-demethylase gene expression and require increasing doses to maintain constant cellular growth inhibition (Lepesheva and Waterman, Reference Lepesheva and Waterman2011). Thus, the identification of other promising trypanocidal candidates is urgently needed. In recent years, a considerable amount of data has been collected regarding the effect of amidine-containing molecules against T. cruzi (Soeiro and De Castro, Reference Soeiro and de Castro2011). While pentamidine does not display potent activity (>32 μ m) on bloodstream trypomastigotes of T. cruzi (Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012), other aromatic diamidines show considerable in vitro effects (De Souza et al. 2004, Pacheco et al. Reference Pacheco, da Silva, de Souza, Batista, da Silva, Kumar, Stephens, Boykin and Soeiro2009; Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a; Daliry et al. Reference Daliry, Pires, Silva, Pacheco, Munde, Stephens, Kumar, Ismail, Liu, Farahat, Akay, Som, Hu, Boykin, Wilson, De Castro and Soeiro2011; Da Silva et al. Reference Da Silva, Junqueira, Lima, Romanha, Sales Junior, Stephens, Som, Boykin and Soeiro2011a). For instance, 3-bromo-4-methyl-2,5-bis(4-amidinophenyl)thiophene (DB1362 – see Fig. 5) induced a dose-dependent trypanocidal activity giving an IC50 value of 6 μ m, and provided a partial reduction of the parasitaemia levels in an experimental mouse model of acute infection using the Y strain of T. cruzi (Da Silva et al. Reference Da Silva, Batista, Batista, de Souza, da Silva, de Oliveira, Meuser, Shareef, Boykin and Soeiro2008). DB569, an N-phenyl derivative of furamidine (DB75), exhibited a dose- and time-dependent activity towards both bloodstream (IC50=2 μ m) and amastigotes (>4 μ m) of different T. cruzi strains (De Souza et al. Reference De Souza, Lansiaux, Bailly, Wilson, Hu, Boykin, Batista, Araújo-Jorge and Soeiro2004). In vivo, DB569 significantly reduced the cardiac parasite burden in mice, partially increased survival rates, and lowered the levels of alanine aminotransferase and creatinine, indicating a protective role against renal and hepatic lesions induced by infection (De Souza et al. Reference De Souza, Oliveira and Soeiro2007). DB569 also protected infected mice from electric cardiac alterations due to T. cruzi invasion possibly due to the reduced parasitism associated with the modulation of an aggressive host immune response, since this amidine (but not benznidazole) decreased CD8+ T cells levels at the inflamed hearts of the infected and treated mice (De Souza et al. Reference De Souza, Oliveira and Soeiro2007). Interestingly, diamidines like DB569 control intracellular parasitism more efficiently when professional phagocyte cells such as macrophages are used as host cells as compared with non-professional phagocytes such as cardiac cells (De Souza et al. Reference De Souza, Lansiaux, Bailly, Wilson, Hu, Boykin, Batista, Araújo-Jorge and Soeiro2004). These results suggest that depending on the host cell type, different levels of trypanocidal events may be triggered directly by these aromatic compounds in mammalian cells, contributing to the control of parasite proliferation. In fact, E. M. De Souza and M. N. C. Soeiro (unpublished data) demonstrate the ability of amidines like DB569 to induce nitric oxide production by mammalian cells giving strong support that not only the antiparasitic mechanism of action of these compounds should be more thoroughly investigated but also, most importantly, their potential effect on host cells metabolism and activation of trypanocidal responses that could also limit the parasite burden.
As described for Leishmania, AIAs (especially bis-AIAs – see the section ‘Efficacy and selectivity of amidines and their derivatives against Leishmania’) are highly potent against T. cruzi, with many of them displaying superior efficacy as compared with classical aromatic diamidines (e.g. DB75 and pentamidine) (De Souza et al. Reference De Souza, Lansiaux, Bailly, Wilson, Hu, Boykin, Batista, Araújo-Jorge and Soeiro2004; Da Silva et al. 2007; Pacheco et al. Reference Pacheco, da Silva, de Souza, Batista, da Silva, Kumar, Stephens, Boykin and Soeiro2009). Studies with bloodstream forms demonstrated that the AIA DB766 (IC50=60 nm, Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a) is about 260-fold more potent than DB75 (IC50=16 000 nm, De Souza et al. Reference De Souza, Oliveira and Soeiro2007), showing that minor modifications of the chemical structure of amidine-containing molecules can result in higher selectivity and efficacy. As also discussed for Leishmania in the section ‘Efficacy and selectivity of amidines and their derivatives against Leishmania’ of the present review, the superior activity of AIAs compared with amidine compounds may be related to their lower pK a values and greater lipophilic character, contributing to their efficient entrance into host and parasite cell membranes. In fact, despite the fact that T. cruzi disrupts the parasitophorous vacuole membrane and proliferates freely within the cytoplasm of the host cells, in order to be active against this protozoan infection, especially during the late chronic phase, a desirable drug candidate must cross both T. cruzi and host plasmalemmas to reach the parasite intracellular milieu and act on its cellular targets. DB766 exhibited strong activity (in the nanomolar range) and excellent selectivity on intracellular amastigotes, showing striking effects upon a large panel of T. cruzi strains including parasite isolates that circulate in peridomiciliar and sylvatic ecotopes from different regions from Brazil (Ceará State and Amazon region), and those that are naturally resistant to benznidazole and nifurtimox (e.g. Colombiana and YuYu), with higher efficacy in vitro than the reference drugs (Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a). DB766 as well as several other AIAs (such as DB745, DB1831 and DB1868 – Figs 4 and 5) keep a high activity at 4 °C in the presence of 96% mouse blood, being at least 30·6-fold more effective than gentian violet, the reference drug for blood therapy in Chagas disease (Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a, 2011; Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012). These results demonstrate the potential application of AIAs in the prophylaxis of T. cruzi infection acquired through contaminated blood stored in blood banks. On the other hand, other AIAs (like DB1852 – Fig. 5) showed decreased activity when incubated at 4 °C even in the absence of blood, suggesting that such a decrease may be related to impairment of drug uptake due to a transport-mediated mechanism and/or to decreased cellular target metabolism (Da Silva et al. Reference Da Silva, Junqueira, Lima, Romanha, Sales Junior, Stephens, Som, Boykin and Soeiro2011a, Reference Da Silva, Daliry, Silva, Akay, Banerjee, Farahat, Mary, Fisher, Hu, Kumar, Liu, Stephens, Boykin and Soeirob).
Due to the high efficacy and selectivity of novel bis-AIAs, some compounds such as DB766 (selectivity index (SI) >533) were assessed with in vivo models (Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a). DB766 suppressed the parasite load in the blood and cardiac tissues (80–>99% reduction), presented similar efficacy as benznidazole in mouse models of T. cruzi-infection employing Y (moderated resistant) and Colombiana (high resistant) strains, using oral and intraperitoneal (i.p.) doses up to 100 mg kg−1 day−1 (given after the establishment of parasite infection), and provided 90–100% protection against mortality (Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a). The effectiveness of this AIA in eradicating intracellular parasites (as we observed in cardiac cells during in vitro and in vivo infection, Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a) may be related to its pharmacokinetic properties (see section on ‘Pharmacokinetics’) including long half-lives and large volumes of distribution (Wang et al. Reference Wang, Zhu, Srivastava, Liu, Sweat, Pandharkar, Stephens, Riccio, Parman, Munde, Mandal, Madhubala, Tidwell, Wilson, Boykin, Hall, Kyle and Werbovetz2010). This issue is highly relevant, since the poor activity of benznidazole during the chronic stages of CD has been attributed, at least in part, to its poor pharmacokinetic properties, such a relatively short half-life and limited tissue penetration (Soeiro and De Castro, Reference Soeiro and de Castro2009). Although it is not a pro-drug, DB766 showed good activity upon oral administration (dose 100 mg kg−1 day−1) (Batista et al. Reference Batista, Batista, Oliveira, Amaral, Lannes-Vieira, Britto, Junqueira, Lima, Romanha, Sales Junior, Stephens, Boykin and Soeiro2010a). However, using a similar mouse model of acute T. cruzi infection (Y strain), the mesylate salt of DB766 (DB1960) only decreased the parasitaemia levels by 46% when given orally at 100 mg kg−1 day−1×5 (Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012). It is possible that the increased solubility of DB1960 may result in decreased permeability and therefore lesser drug accumulation in parasitized organs, resulting in lower efficacy (Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012).
Another highly effective AIA that has been screened against T. cruzi and showed high activity is DB1852, exhibiting IC50 values of 60 nm against bloodstream trypomastigotes (Y strain) (Da Silva et al. 2011b). When assayed in vivo, the mesylate salt of DB1852 (DB1955) had no effect on parasitaemia when administered using the same dosing regimen as DB1960 and DB766 in a similar mouse model of acute infection (Y strain). The lower in vivo efficacy of DB1960 and DB1955 may be due to their lower accumulation in the heart and plasma of infected mice, which are important sites of T. cruzi infection (Zhu et al. Reference Zhu, Liu, Yang, Parman, Green, Mirsalis, Soeiro, de Souza, da Batista, da Gama Jaen Batista, Stephens, Banerjee, Abdelbasset Farahat, Munde, Wilson, Boykin, Wang and Werbovetz2012). Additionally, another point of discussion is the fact that DB766, DB1955 and DB1852 displayed similar effects upon bloodstream forms (60–70 nm), but the former AIA was much more potent against intracellular forms (IC50=25, 180 and 538 nm for DB 766, DB 1955 and DB1852, respectively). Although a direct correlation between in vitro and in vivo is not always found, cumulative data suggest that the activity against intracellular amastigotes, which are the reproductive forms in the vertebrate host, represents a more reliable determinant in the selection of effective compounds to move from in vitro to in vivo assays (De Castro et al. Reference De Castro, Batista, Batista, Batista, Daliry, de Souza, Menna-Barreto, Oliveira, Salomão, Silva, Silva and Soeiro2011). The excellent activity of DB766 motivated the design and synthesis of novel structurally related compounds like the AIA – DB1831 (hydrochloride salt) and its mesylate salt form (DB1965) (Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012). DB1831 displayed excellent activity against bloodstream trypomastigotes (IC50=20 nm), and, as did DB766, retained excellent efficacy at 4 °C (IC50 values of 80 and 24 nm with or without mouse blood, respectively). When assessed against intracellular amastigotes grown in cardiac cell cultures, DB1831 exhibited an outstanding IC50 of 5 nm already at 48 h after the initiation of treatment, as well as very high SI values (2900). Due to its poor solubility, a methanesulfonic acid salt (DB1965) was obtained for further in vivo analysis. DB1965 administered i.p. for 5 daily consecutive doses (starting at the onset of parasitaemia) reduced parasitaemia levels by 93 and 99% when administered at doses of 12·5 and 25 mg kg−1, respectively, similar to the reference drug (Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012). Although treatment with 12·5 mg kg−1 of DB1965 for 10 days suppressed the parasitaemia and gave 90% protection against mortality, longer periods of therapy were not performed due to undesirable side effects (like hyperactivity) and a combined treatment of 5 mg kg−1 DB1965+50 mg kg−1 benznidazole (suboptimal dose) was chosen following the same protocol previously carried out with DB766, under a scheme of 20 daily consecutive doses (Batista et al. Reference Batista, Batista, de Oliveira, Britto, Rodrigues, Stephens, Boykin and Soeiro2011). However, despite the promising trypanocidal effects of AIAs in combination with benznidazole, neither DB766, DB1965 nor the reference drug induced parasitological cure (Batista et al. 2011b; Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012), possibly due to the highly stringent protocol employed (male mice, only 20 days of compound administration, starting treatment at the onset of parasitaemia, using only positive infected animals). In fact, previous studies performed in T. cruzi-infected mouse models reporting high parasitological cure rates (e.g. benznidazole) employed different protocols using female mice (instead of male mice as used for AIAs), longer periods of treatment (⩾40 days) or abortive scheme (starting therapy 24 h after parasite inoculation) (Urbina et al. Reference Urbina, Payares, Contreras, Liendo, Sanoja, Molina, Piras, Piras, Perez, Wincker and Loebenberg1998; Molina et al. Reference Molina, Martins-Filho, Brener, Romanha, Loebenberg and Urbina2000; Bustamante et al. Reference Bustamante, Bixby and Tarleton2008). Although displaying quite similar results as benznidazole, DB1965 was not as active in vivo as DB766, especially by p.o. administration and was also less well tolerated according to acute toxicity studies (Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2012). Since the only difference in structure between DB766 and DB1831 concerns the terminal groups (pyridine and pyrimidine, respectively), differences in toxicity and efficacy between DB766 and DB1965 may also be attributed to this minor modification. Thus, further investigations are required to sort out this effect, allowing the design of novel AIAs bearing pyrimidine and pyridine units since they seem to be advantageous for retaining activity against T. cruzi. When other T. cruzi candidates that have moved (e.g. ravuconazole with IC50=1 nm, Urbina et al. Reference Urbina, Payares, Sanoja, Lira and Romanha2003) or are expected to move soon to clinical trials (protease inhibitors such as K11777 – McKerrow et al. Reference McKerrow, Doyle, Engel, Podust, Robertson, Ferreira, Saxton, Arkin, Kerr, Brinen and Craik2009) are compared with DB 766 (IC50=25 nm), similar efficacy is found against intracellular amastigotes, meriting further investigation on AIAs against parasitic infection on intracellular microorganisms. In fact, the biological activity of 7 novel AIAs was recently assayed for 48 and 24 h against intracellular parasites and bloodstream forms (Y strain), respectively. The data demonstrated the outstanding trypanocidal effect of AIAs against T. cruzi, especially DB1853, DB1862, DB1867 and DB1868 (Fig. 6), giving IC50 values ranging between 16 and 70 nm against both parasite forms. All AIAs presented superior potency and some, such as DB1868, also exhibited promising activity as candidates for blood prophylaxis (Da Silva et al. 2011a, b). The lower activity of DB1850 may be due to the markedly different terminal groups (5-membered ring thiazoles for DB1850 and 6-membered ring pyridine or pyrimidine for the others). Also, in this study, the higher activity against BT forms suggests that a range of variations in the O-alkyl groups (OCH2CF3 to O-c-pentyl) is tolerated but additional experimental assays are needed to further evaluate this hypothesis.
Another interesting point is that some of these AIAs, such as DB745, can be considered fast-acting compounds. Time-point studies using 10 μg mL−1 of each compound demonstrated that after 5 min of incubation, DB745 induced 51% of trypomastigote lysis whereas the diamidine DB569 and the AIA DB766 displayed only 16 and 27% of parasite death, respectively (Da Silva et al. 2011a). However, longer periods of incubation (60 min) resulted in a similar trypanocidal effect for both AIAs (>96%), while DB569 resulted in about 50% of parasite lysis (Da Silva et al. 2011a). The rapid toxicity of DB745 represents a desirable property for treating acute parasitic infections such as in Chagas disease, and further studies should be carried out to explore the possibility whether different transport mechanisms and/or cellular targets may be operating. Importantly, the different efficacies of some bis-AIAs (like DB1852 and DB1890) against infective and non-proliferative bloodstream forms compared with infective and highly multiplicative (intracellular) stages may be due to differences in uptake, mechanisms of action and/or different targets among these different parasite forms (Da Silva et al. Reference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeiro2011aReference Da Silva, Batista, Oliveira, De Souza, Hammer, Silva, Anissa Daliry, Siciliano, Britto, Rodrigues, Liu, Farahat, Kumar, Boykin and Soeirob47). In fact, striking differences have been found when the uptake of classical diamidines (like DB75) and AIAs (like DB766) has been evaluated on epimastigotes (non-infective but highly proliferative forms found in blood-sucking vectors) and bloodstream trypomastigotes: while DB75 (but not AIAs like DB766) is internalized by trypomastigotes via adenosine/adenine transporter systems, this is not observed in epimastigotes (E. Goulart and M. N. C. Soeiro, unpublished data). These data demonstrated that differences in sensitivity of dicationic compounds among the distinct T. cruzi forms may be due to (i) different transporters of diamidines and AIAs according to parasite evolutive stage and/or (ii) different cellular targets may be operating, and these must be further explored.
In summary, the excellent antitrypanosomal efficacy of these novel bis-AIAs against T. cruzi stimulates further in vivo studies and justifies the screening of new analogues with the goal of establishing a useful alternative therapy for Chagas disease.
EFFICACY AND SELECTIVITY OF AMIDINES AND THEIR DERIVATIVES AGAINST APICOMPLEXAN PARASITES
Apicomplexan parasites cause a wide range of highly relevant diseases in humans such as malaria, babesiosis, cryptosporidiosis and toxoplasmosis. Cryptosporidiosis, babesiosis and toxoplasmosis also have a high impact on animal health and are zoonotic diseases. Neosporosis (caused by Neospora caninum) and besnoitiosis (caused by Besnoitia besnoiti) are responsible for high economic losses in farm animal production worldwide (Müller and Hemphill, Reference Müller and Hemphill2013). The efficacies and interactions of amidine compounds with apicomplexans have been investigated in vitro and, to some extent, also in vivo.
Vector-borne apicomplexans: Plasmodium and Babesia
The genus Plasmodium causes malaria, and thus represents a major burden in tropical and subtropical countries with over 1 million deaths annually. The clinically most important agent is Plasmodium falciparum, responsible for malaria tropica, the most severe form of the disease. While a number of chemotherapeutically active antimalaria compounds have been developed and applied in the field, resistance has been spreading rapidly, and activity against drug-resistant isolates is a prerequisite for any lead compound to be developed (Mäser et al. Reference Mäser, Wittlin, Rottmann, Wenzler, Kaiser and Brun2012). In addition, other requirements include excellent oral bioavailability, low production costs, activity against all human–pathogenic Plasmodium species, and ideally cure by a single dose. Pentamidine-analogues demonstrated antimalarial potency (Bell et al. Reference Bell, Hall, Kyle, Grogl, Ohmeng, Allen and Tidwell1990; Stead et al. Reference Stead, Bray, Edwards, De Koning, Elford, Stocks and Ward2001; Bray et al. Reference Bray, Barret, Ward and De Koning2003), but with a low bioavailability since, as previously described, the capacity to pass through cellular membranes is hampered due to ionization of the 2 cationic moieties at physiological pH. Bakunova et al. (Reference Bakunova, Bakunov, Wenzler, Barscz, Werbovetz, Brun, Hall and Tidwell2007) synthesized 43 bisbenzofurans, of which 22 were more active against P. falciparum than pentamidine, and 7 dications were more effective than artemisinin. A series of pentamidine congeners bearing the benzofuran motif were synthesized and 7 compounds exhibited in vitro activities against P. falciparum that were superior to pentamidine (Bakunova et al. Reference Bakunova, Bakunov, Wenzler, Barszcz, Werbovetz, Brun and Tidwell2009). More recently, novel dicationic triazoles were shown to exhibit promising activities against a chloroquine-resistant P. falciparum in vitro, with IC50s ranging from 0·6 nm to 8·39 μ m (Bakunov et al. Reference Bakunov, Bakunova, Ghebru, Werbovetz, Brun and Tidwell2010). Eight of these analogues displayed better antiplasmodial activities in vitro than pentamidine (IC50=58 nm), and 4,4′-disubstituted diimidazoline was the most potent one, with an IC50 of 0·6 nm and an SI of >3 16 667 as determined by in vitro exposure of L6 rat myoblast cells. In comparison, the most effective antimalarial drug artemisinin exhibits an IC50 of 6 nm and a SI of 75 000 (Bakunov et al. Reference Bakunov, Bakunova, Ghebru, Werbovetz, Brun and Tidwell2010).
The genus Babesia comprised approximately 100 described species. Babesiosis has gained increased interest as a zoonotic disease that is transmitted by ticks, and affects a wide range of domestic and wild animals. The major economic impact is on the cattle industry, but infections also occur in horses, sheep, goats, pigs and dogs (Müller and Hemphill, Reference Müller and Hemphill2013). Babesia bovis and Babesia bigemina are widespread species affecting cattle in tropical and subtropical areas, while Babesia divergens infects cattle in Europe (de Waal and Combrink, Reference de Waal and Combrink2006). In ticks, the blood stages of the parasite are ingested during a bloodmeal and undergo development. Subsequent transmission of Babesia sporozoites to the host occurs when infected larvae, nymphs and/or adults feed on their hosts. Babesia parasites invade and proliferate within erythrocytes but, in contrast to Plasmodium spp., they do not develop in a parasitophorous vacuole; they divide by binary fission (not by schizogeny) and do not form hemozoin. In Europe, human cases of babesiosis are mostly caused by B. divergens, while in North America most human cases involve the rodent species Babesia microti (Kjemtrup and Conrad, Reference Kjemtrup and Conrad2000). Sporadic cases of human babesiosis have also been identified in Asia, Africa and South America. The standard drugs used against babesiosis in animals are atovaquone, imidocarb dipropionate and diminazene aceturate, of which only imidocarb acts consistently parasiticidal. In humans, Babesia infections are treated with combinations of quinine/clindamycin or atovaquone/azithromycin (Vial and Gorenflot, Reference Vial and Gorenflot2006). A recent study on the in vitro activities of novel diamidine compounds against 2 B. divergens strains showed that the most potent compound, a diphenyl furan, had an outstandingly low IC50 of 1·5 ng mL−1 (Nehrbass-Stüdli et al. Reference Nehrbass-Stüdli, Boykin, Tidwell and Brun2011). Several test compounds, including terphenyls, benzimidazoles, diphenyl furans, pentamidine and pentamidine analogues, were effective against B. microti in mice at a dosage of 12·5 and/or 25 mg kg−1 of body weight given by the subcutaneous route for 4 days.
Cryptosporidium
Cryptosporidiosis is a cause of diarrheal illness in immunocompetent hosts such as travellers and infants attending day care, and represents an important opportunistic diarrheal disease in immune-compromised humans. Cryptosporidiosis occurs worldwide, and also affects many young farm mammals as well as cats and dogs, some times with fatal outcomes. The only drug currently licensed for the treatment of Cryptosporidium parvum infection in humans is the nitro-thiazole nitazoxanide, but this compound is ineffective in immune-compromised patients (Hemphill et al. Reference Hemphill, Esposito and Müller2006), and its use in neonatal calves has been shown to be accompanied with severe adverse effects (Schnyder et al. Reference Schnyder, Kohler, Hemphill and Deplazes2009). Compounds such as halofuginone and decoquinate are reported to exhibit promising effects and the antibiotic paromomycin is frequently used (Lefay et al. Reference Lefay, Naciri, Poirier and Chermette2001; Lallemand et al. Reference Lallemand, Villeneuve, Belda and Dubreuil2006), but results are variable and none of these drugs are licensed for the treatment of cryptosporidiosis, neither in humans nor in food animals. Pentamidine analogues and aryl furans were investigated in vitro and in vivo employing C. parvum-infected Hsd/ICR suckling mice (Blagburn et al. Reference Blagburn, Sundemann, Lindsay, Hall and Tidwell1991, Reference Blagburn, Drain, Land, Moore, Kinard, Lindsay, Kumar, Shi, Boykin and Tidwell1998a), demonstrating that oral application of a diaryl furan by gavage at a dosage of 17·0 and 8·5 mg kg−1 for 5 days resulted in a reduction in oocyst shedding to 5·1% and 6·4% of levels, respectively, as compared with non-treated controls. In a subsequent study (Blagburn et al. Reference Blagburn, Drain, Land, Kinard, Hutton Moore, Lindsay, Patrick, Boykin and Tidwell1998b), application of 2,7-diamidinocarbazole dihydrochloride at dosages of 10 and 19 mg kg−1 resulted in oocyst outputs of only 0·3 and 0·8% respectively. In comparison, nitazoxanide applied orally at 100 mg kg−1 yielded a reduced oocyst output of 26%, and at 150 mg kg−1 the residual oocyst output was 4·3% compared with untreated neonatal mice (Blagburn et al. Reference Blagburn, Drain, Land, Kinard, Hutton Moore, Lindsay, Patrick, Boykin and Tidwell1998b). Paromomycin on the other hand, applied at 50 mg kg−1 exhibited a reduction of oocyst shedding to 1·2% compared with untreated control mice. In these experiments, drugs were applied already 4 h after infection, meaning that parasites did not establish substantial numbers of developmental stages prior to exposure to the compounds.
Cyst-forming apicomplexans: Toxoplasma gondii, N. caninum and B. besnoiti
Toxoplasma gondii is the most successful of all apicomplexan parasites, infecting approximately 30–50% of the human population, and is highly prevalent in all warm-blooded animals, including birds. In an immunocompetent host, infection is normally benign and asymptomatic (Barratt et al. Reference Barratt, Harkness, Marriott, Ellis and Stark2010). Clinical problems can arise in individuals with an immature immune system or in immune-compromised individuals (e.g. AIDS patients), where infection can lead to toxoplasmic encephalitis or even death. Toxoplasmic encephalitis occurs as a result of the reactivation of brain cysts that encapsulate slowly proliferating bradyzoites, which then undergo bradyzoite-to-tachyzoite stage conversion (Henriquez et al. Reference Henriquez, Woods, Cong, McLeod and Roberts2010). Secondly, congenital toxoplasmosis poses a major health risk upon primary maternal infection during pregnancy, leading to placental or fetal infection and abortion or damage of the fetus (Feldman et al. Reference Feldman, Timms and Borgida2010). In addition, T. gondii is also a highly significant economic and veterinary medical concern in the livestock industry (Dubey, Reference Dubey2009). The chemotherapeutic options for the treatment of toxoplasmosis are limited. Synergistic combination therapies comprised of sulfadiazine/pyrimethamine, targeting the synthesis and the reduction of folic acid in tachyzoites, or clindamycin/pyrimethamine, are the most efficient options, but are prone to adverse effects, and the non-effectiveness of compounds against bradyzoites that are enclosed in tissue cysts represents a major problem (Barratt et al. Reference Barratt, Harkness, Marriott, Ellis and Stark2010). The in vitro efficacy of pentamidine and some pentamidine analogues against T. gondii has been investigated earlier (Lindsay et al. Reference Lindsay, Blagburn, Hall and Tidwell1991) and proliferation-inhibitory properties were reported at concentrations of around 10 μg ml−1. Leepin et al. (Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008) screened 19 dicationic compounds and demonstrated the in vitro efficacy of 4 derivatives of DB75 (furamidine), which are built symmetrically with the 2 core structure-benzene rings being altered. In the diamidine DB811, this occurs through the addition of 2 chloro atoms. In the AIA DB750, two hydroxy groups are added, and in the AIAs DB766 and DB786 iso-propoxy groups are added at different positions. These compounds exhibited IC50s between 0·16 and 0·66 μ m (0·14–0·5 μg ml−1). Neither pentamidine nor furamidine exhibited meaningful in vitro activity against T. gondii. Transmission electron microscopy demonstrated clear structural damage within drug-treated parasites, while host cells remained unaffected. However, since neither of these compounds exhibited detectable fluorescent properties, it was not possible to study in which cellular compartments these compounds localized (Leepin et al. Reference Leepin, Stüdli, Brun, Stephens, Boykin and Hemphill2008). More recently, Kropf et al. (Reference Kropf, Debache, Rampa, Barna, Schorer, Stephens, Ismail, Boykin and Hemphill2012) re-investigated the in vitro characteristics of the most promising compound, DB750, in the virulent T. gondii RH strain (genotype 1) and an avirulent strain, Me49 (genotype 2). Three-day-growth assays showed that DB750 inhibited the proliferation of tachyzoites of both strains with an IC50 of 0·11 and 0·13 μ m, respectively, but the drug did not affect extracellular parasites. Thus no inhibition of host cell invasion upon DB750 treatment was observed. In addition, fibroblast monolayers that were treated with 1 μ m DB750 for 12 h prior to infection were subsequently infected, and further culture of these infected fibroblasts in the absence of the DB750 for 3 days resulted in a pronounced inhibition of parasite proliferation, indicating that DB750 was actively taken up and accumulated within the host cells, causing a memory effect. However, upon continuous exposure to DB750 for 5–6 days, T. gondii tachyzoites adapted to the drug and resumed proliferation in the presence of a DB750 concentration of up to 1·2 μ m. This made it possible to generate DB750-adapted T. gondii strains. Kropf et al. (Reference Kropf, Debache, Rampa, Barna, Schorer, Stephens, Ismail, Boykin and Hemphill2012) also screened another set of compounds comprised of 10 bis-AIAs, 6 diamidines, 15 newly generated mono-arylimidamides, and 10 di-guanidino analogues against T. gondii, and the AIA DB745, exhibiting an IC50 of 0·03 μ m and most favourable selective toxicity was chosen for further studies. In DB745, each of the 2 phenyl rings is substituted by an O-ethyl-moiety. DB745 also efficiently inhibited the proliferation of DB750-adapted T. gondii (IC50=0·07 μ m) and, in contrast to DB750, DB745 had a profound toxic effect on extracellular T. gondii tachyzoites. Adaptation (or resistance) of T. gondii to DB745 (up to a concentration of 0·46 μ m) was difficult to achieve and feasible only by stepwise increasing the drug concentration over a period of 110 days. Overall, these investigations illustrated the astonishing capacity of T. gondii tachyzoites to adapt to environmental changes, at least under in vitro conditions, and open the door to further investigations on the mechanisms of action of these compounds in T. gondii (Kropf et al. Reference Kropf, Debache, Rampa, Barna, Schorer, Stephens, Ismail, Boykin and Hemphill2012).
Neospora caninum is closely related to T. gondii, but infection in humans has not been reported, and the parasite owes his importance due to the fact that it represents one of the most important causes of abortion in cattle worldwide (Monney et al. Reference Monney, Debache and Hemphill2011). Neospora isolates with different virulence have been obtained, such as Nc-Liverpool representing a highly virulent isolate, and Nc-1, which is an isolate with lower virulence. Screening for anti-Neospora activity involved the same compounds as for T. gondii, and not surprisingly, DB750 and DB745 also emerged as the most effective ones, albeit with differences in terms of IC50 values (e.g. IC50 of DB745=80 nm for N. caninum and 30 nm for T. gondii (RH) tachyzoites (Kropf et al. Reference Kropf, Debache, Rampa, Barna, Schorer, Stephens, Ismail, Boykin and Hemphill2012; Schorer et al. Reference Schorer, Debache, Barna, Monney, Boykin, Stephens and Hemphill2012). As for T. gondii, DB750 inhibited proliferation of N. caninum tachyzoites, but was not effective against extracellular parasites, while DB745 treatment resulted in inhibition of host cell invasion. A detailed TEM analysis of the effects of DB745 on intracellular N. caninum tachyzoites revealed (Fig. 6) that (i) the integrity of the nuclear membrane was altered in the drug-treated parasites and (ii) most nuclei of drug-treated parasites were devoid of a discernible nucleolus (Fig. 6C). This suggested that DB745 could interfere in the maintenance of the nuclear membrane integrity, possibly interfere in lipid metabolism, and/or in transcriptional activity of these parasites, the latter of which would be potentially in agreement with the postulated mode of action involving DNA-binding. In addition, DB745 treatment resulted in tachyzoites that were fused together at their outer membranes (Fig. 6D). Closer inspection of the contact sites at higher magnification revealed that these were tachyzoites caught in the process of endodyogeny, with the inner membrane layer surrounding both daughter cells and the inner membrane layer either fully present or partially interrupted (Fig. 6E; Schorer et al. Reference Schorer, Debache, Barna, Monney, Boykin, Stephens and Hemphill2012). These observations indicated that DB745 could potentially interfere in the formation/metabolism of membranous structures within these parasites. In this context it is interesting to note that a recent study by Ando et al. (Reference Ando, Kamei, Komagoe, Inoue, Yamada and Katsu2012) demonstrated that treatment with related compounds such as diminazene, pentamidine and 4′6′-diamidino-2-phenylindole (DAPI) increased the permeability of bacterial outer membranes.
Long-term growth assays in the presence of DB745 revealed that the drug had a parasiticidal action against the more virulent Nc-Liverpool isolate, while Nc-1 tachyzoites could adapt to increased concentrations of DB745 (up to 0·30 μ m within a timeframe of 69 days). In vivo studies were performed in mice that were experimentally infected with the Nc-1 isolate, showing that both DB745 and DB750 treatment by intraperitoneal injection of compounds at 2 mg kg−1 for 14 days resulted in a significantly increased number of survivors and significantly decreased cerebral parasite burden compared with non-treated mice (Debache et al. Reference Debache, Guionaud, Kropf, Boykin, Stephens and Hemphill2011; Schorer et al. Reference Schorer, Debache, Barna, Monney, Boykin, Stephens and Hemphill2012). In these experiments, treatments were started 14 days after infection, thus the parasites had already reached the CNS, and thus these compounds most likely were effective in crossing the blood–brain barrier. However, oral application of these two compounds was ineffective (unpublished findings). These initial in vivo results are promising, and point out the therapeutic potential of the AIAs DB745 and DB750, and the class of dicationic compounds as a whole, for the treatment of N. caninum infections. From the practical point of view on neosporosis in cattle, treatment of congenitally infected calves right after birth could potentially give rise to pathogen-free offspring (Härdi et al. Reference Härdi, Haessig, Sager, Greif, Staubli and Gottstein2006), provided an effective compound can be identified.
Dicationic compounds were also evaluated with respect to their activity against B. besnoiti tachyzoites grown in Vero cells. Besnoitia besnoiti is most closely related to N. caninum and T. gondii. The life cycle of B. besnoiti remains mysterious and its definitive host is unknown. Infection assays have shown that B. besnoiti infects a variety of potential hosts, but persists only in voles (Basso et al. Reference Basso, Schares, Gollnick, Rütten and Deplazes2011). Besnoitia besnoiti is transmitted to cattle by haematophagous insects and causes a systemic disease which, during the acute phase with a high parasitaemia, may be associated with fever and fever-associated signs, including abortion. Subsequently, in the chronic stage, the parasite shows a high tropism to the skin, leading to dramatic thickening, hardening and folding, always accompanied by hyperkeratosis, hyperpigmentation and alopecia, and a decrease of milk production is commonly observed. Furthermore, Besnoitia infection in bulls can cause irreversible aspermy and sterility (Jacquiet et al. Reference Jacquiet, Liénard and Franc2010). The rapidly proliferating B. besnoiti tachyzoites can be cultivated as described for N. caninum and T. gondii. By employing such cultures, drug-screening assays have been performed in order to identify compounds of potential interest for chemotherapeutic treatment, including AIAs (Cortes et al. Reference Cortes, Müller, Boykin, Stephens and Hemphill2011). Results were different compared with T. gondii and N. caninum. DB750 (IC50=0·59 μ m) acted only parasitostatic after 14 days of treatment at 1·7 μ m, while DB811 (IC50=0·079 μ m) was highly effective, and had a parasiticidal action in vitro (Cortes et al. Reference Cortes, Müller, Boykin, Stephens and Hemphill2011). Thus, B. besnoiti is susceptible to other dicationic compounds, implying that possibly the target(s) in B. besnoiti affected by these compounds are different or at least altered compared with T. gondii and N. caninum. However, the biggest challenge for drug development against these cyst-forming apicomplexans is still to generate compounds that affect the respective bradyzoite stages encapsulated within tissue cysts.
CONCLUDING REMARKS
In conclusion, amidine-containing compounds represent a versatile class of drugs, with a great potential for the further development of novel chemotherapies against intracellular parasitic protozoa. The hallmarks of the more recently generated compounds are oral bioavailability, metabolic stability, and an increased capacity to pass through biological membranes that makes them uniquely suited to reach parasites that reside and hide within host cells. Some structural modifications also aid in crossing the blood–brain barrier, which is relevant for infections that also affect the CNS. The use of in vitro culture systems has been instrumental for preliminary efficacy assessments and allowed identification of compounds with IC50s in the low nanomolar range, but care must be taken in the respective assessments, since the findings obtained in vitro cannot always be translated into high efficacy in vivo. Pentamidine and many of its derivatives have been designed to bind to the minor groove of DNA, and are found either within the kinetoplast in trypanosomatids or the nucleus in other parasites, but most likely other cellular targets may also be involved. Importantly, the higher potency of AIAs against intracellular agents (like T. cruzi and Leishmania) compared with classical dicationic amidines justify further screening studies also against other parasites like extra-erythrocytic Plasmodium species. The fact that the amidines act against a variety of species living an intracellular lifestyle, and that some are effective in many species while others affect only one, also argues for multiple modes of action. In the future, it will be important to understand the alterations induced by these drugs not only on morphology and ultrastructure, but also on the molecular level. It will be important to understand the molecular changes in gene expression, and to elucidate how these compounds affect the metabolism of their targeted parasites as well as mammalian host cells. This will help to understand their mode of action and to identify the relevant drug targets, information that can be exploited to generate novel and more effective compounds against parasitic diseases.
ACKNOWLEDGEMENTS
The authors thank Dr Denise da Gama Jaen Batista for providing the fluorescent images in Fig. 2 and Dr S.L. de Castro for revising the text.
FINANCIAL SUPPORT
The present study was supported by grants from PDTIS-Fiocruz, Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ: APQ1, Estudo de Doenças Negligenciadas Reemergentes-, PENSA RIO, e Apoio a Instituições de Ensino e Pesquisa Sediadas no Estado do Rio de Janeiro), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), CECAL/Fiocruz and PROEP/Fiocruz. Grant support from the National Institutes of Health, NIAID 064200 (W.D.W. and D.W.B.) is gratefully acknowledged. The Swiss National Science Foundation (grant No. 31003A-127374/1) is gratefully acknowledged.












