INTRODUCTION
Paleoparasitological examination provides an important source of information about past parasite–host associations that contribute to the understanding of the features/processes that modelled the structure and composition of parasite communities. The increasing paleoparasitological information recovered from humans and from fauna remains of Patagonia (Fugassa, Reference Fugassa2005, Reference Fugassa2007; Fugassa et al. Reference Fugassa, Denegri, Sardella, Araújo, Guichón, Martinez, Civalero and Aschero2006; Fugassa et al. Reference Fugassa, Bayer and Sardella2007a , Reference Fugassa, Sardella and Denegri b ; Sardella and Fugassa, Reference Sardella and Fugassa2009a , Reference Sardella and Fugassa b ; Sardella et al. Reference Sardella, Fugassa, Rindel and Goñi2010; Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015, among others) improved paleoepidemiology inferences and defined the role of caves and shelters in the transmission of zoonoses. Among archaeological fauna, South American camelids are the native artiodactyls of major size in South America (Franklin, Reference Franklin, Eisenberg and Kleiman1983). They originated in North America plains about 11–9 million years ago (Wheeler, Reference Wheeler1995), and in their diversification and dispersion into South America, they likely carried some parasites and lost others (Navone and Merino, Reference Navone and Merino1989; Taglioretti, Reference Taglioretti2015; Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015). Previous paleoparasitological examination of coprolites of camelids from Patagonia (Fugassa, Reference Fugassa, Bayer and Sardella2007; Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015) shed light on the importance of this kind of research in order to understand their parasite biogeography and on the necessity to expand the paleoparasitological information of camelids for a better comprehension of the processes that contributed to the establishment of the present camelids–parasites association in the studied area. Moreover, some parasites could remain viable for long time periods and as camelid coprolites were collected from sites occupied by humans, their paleoparasitological examination is an important source of evidence of the potential zoonoses that humans could be exposed in the past (Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015).
Alero Destacamento Guardaparque (ADG) is an archaeological site located in Perito Moreno National Park (PMNP; Santa Cruz Province, 47°57′S 72°05′W, Southern Patagonia) with evidence of camelid occupation since middle (7000 years BF) to late Holocene, including the Historic period (ca. 4000–100 years BP) (Rindel, Reference Rindel, Civalero, Fernández and Guraieb2004, Reference Rindel2008). This period is characterized by an increased climate variability including, during the final late Holocene, a trend towards the increase of aridity with a maximum recorded from 1150 to 600 years BP (Stine and Stine, Reference Stine and Stine1990; Mancini et al. Reference Mancini, Paez and Prieto2002; Mancini, Reference Mancini2007). ADG exhibits, in addition, archaeological layers with evidence of the introduction of European livestock to Patagonia (Rindel, Reference Rindel, Civalero, Fernández and Guraieb2004, Reference Rindel2008). A combination of both kind of factors, the climate variability and introduction of exotic fauna, which probably represented ecological perturbations to the local faunal assembly are often critical determinants of parasite diversification (Hoberg and Brooks, Reference Hoberg and Brooks2015). As resource specialists, parasites have restricted host ranges, but shifts to relatively unrelated hosts commonly occur during their phylogenetic diversification and can be found in real time. This paradox is explained by Hoberg and Brooks (Reference Hoberg and Brooks2015) within the framework of the Stockholm Paradigm. The present research represents an opportunity to add information of the influence of these factors on the diversification of parasite–host assemblages of American fauna.
The aim of the present study was to examine the parasite remains present in camelid coprolites collected from the archaeological site ADG and to discuss the paleoparasitological findings in a biogeographical and paleoecological context. This is the first paleoparasitological examination of camelid coprolites from archaeological layers, which exhibits evidence of the introduction of European livestock to the studied area.
MATERIALS AND METHODS
Studied area
The archaeological site ADG is located in the southern portion of the Cordillera de Los Andes, in PMNP, Santa Cruz Province (47°40′S and 72°30′W), close to (10 km) the archaeological site Cerro Casa de Piedra (CCP) (Fig. 1). ADG is a large shelter (250 m length) situated on a low hill oriented towards southwest. The site consists of nine archaeological layers dated from middle to late Holocene, including layers with evidence of the presence of introduced livestock (Rindel, Reference Rindel, Civalero, Fernández and Guraieb2004, Reference Rindel2008).

Fig. 1. Archaeological sites located in the PMNP. ADG, Alero Destacamento Guardaparque; CCP, Cerro Casa de Piedra; PMNP, Perito Moreno National Park.
Late Holocene (ca. 4000–100 years BP) is characterized by increased climate variability in the short term. It has been mainly related to water availability associated with temperature and precipitation (Stine and Stine, Reference Stine and Stine1990; Mancini et al. Reference Mancini, Paez and Prieto2002; Mancini, Reference Mancini2007). During the final late Holocene, a trend towards increasing aridity with a maximum record between 1150 and 600 years BP occurred, during the weather phenomenon known as the Medieval Climate Anomaly (MCA) (Stine and Stine, Reference Stine and Stine1990).
Material studied
Twenty pellets assigned to camelid coprolites, collected from six archaeological layers were examined for parasites (Table 1). Samples came from the middle and late Holocene, including the historic period, a time span of approximately 7000 years.
Table 1. Radiocarbon dating and archaeological layers of ADG from which coprolites were recovered and concentration techniques applied

ADG, Alero Destacamento Guardaparque; SSed, spontaneous sedimentation; FZn, flotation-centrifugation with zinc chloride; Fsac, sucrose flotation-centrifugation; FPol, sodium polytungstate flotation-centrifugation techniques.
Each coprolite was fully processed by rehydration in a 0·5% water solution of tris-sodium phosphate for a week and then two concentration techniques were applied in series: first spontaneous sedimentation (Lutz, Reference Lutz1919) and then flotation-centrifugation with zinc chloride, according to Taglioretti et al. (Reference Taglioretti, Sardella and Fugassa2014a ). In some cases, sucrose flotation-centrifugation and sodium polytungstate flotation-centrifugation techniques (Taglioretti, Reference Taglioretti2015) were also applied to recover gastrointestinal parasites (Table 1).
Similarity in the species composition among parasite communities found from different archaeological layers was estimated, since the different archaeological layers represent different time periods. To elucidate if the nearest archaeological layers shared more parasitic species than more distant archaeological layers, a one-way PERMANOVA (considering the factor ‘Period’ and type I error) and a Non-metric Multi-dimensional Scaling (NMDS) were performed using Jaccard's similarity matrix (Underwood, Reference Underwood1998; Anderson et al. Reference Anderson, Gorley and Clarke2008).
Integration with previous findings
To contribute to the knowledge of some of the patterns that could model the parasitic communities of Patagonian camelids in the past, it was important to integrate the paleoparasitological findings in the region. For this purpose, previous information of parasite that infected pre-Hispanic camelids from the archaeological site CCP located in PMNP (Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015), close to (10 km) the archaeological site ADG, was included in the analysis. Paleoparasitological records obtain from CCP7 (Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015) dated from Pleistocene-Holocene transition (9640 ± 190 years 14C BP) to late Holocene (3920 ± 80 years 14C BP).
For this purpose, the similarity in species composition was estimated between parasite communities of the archaeological site CCP7 (Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015) and ADG (present study), using Jaccard's index. One-way PERMANOVA (considering the factor ‘Site’ and type I error) and a NMDS analysis were performed using Jaccard's similarity matrix (Underwood, Reference Underwood1998; Anderson et al. Reference Anderson, Gorley and Clarke2008).
RESULTS
Paleoparasitological examination of ADG
Three representatives of the superfamily Trichostrongyloidea were found in samples of ADG (Table 2), including large eggs [n = 8, 175·6 ± 12·7 µm in length (155–190) and 80·4 ± 8·8 (67·5–90) μm in width] compatible with Nematodirus lamae or Lamanema chavezi (Fig. 2A), three eggs with slightly sharp ends (205 µm in length and 92 µm in width) with third larvae stage (L3) inside (Fig. 2B) attributed to Nematodirus spathiger and seven oval eggs [155 ± 5·7 (145–160) μm in length by 72·5 ± 4·2 (67·5–77·5) μm in width] thin-walled (about 3 µm of thick) with larvae of the first stage of development (L1) inside. First larvae stage with cephalic end-rounded and sharp caudal end, forming a slightly conical tail with rounded end, had dark granules inside and measured approximately 559 ± 26·6 µm in length and 20·5 µm in width (Fig. 2C). These Trichostrongylidae eggs are compatible with eggs of the genus Dictyocaulus, possibly D. filaria (superfamily Trichostrongyloidea, family Dictyocaulidae, subfamily Dictyocaulinae, genus Dictyocaulus). Eggs of Dictyocaulus sp. and those compatible with N. spathiger were only recorded in sample 532 (Table 2).
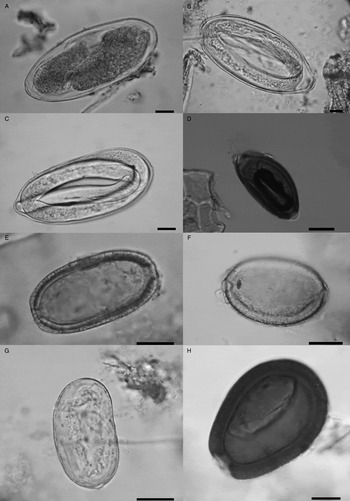
Fig. 2. (A) Egg of Trichostrongylidae, compatible with Nematodirus Lamae or Lamanema chavezi. (B) Egg attributed to Nematodirus spathiger. (C) Eggs compatible with Dictyocaulus sp. (D) Trichuris sp. egg. (E) Egg of unidentified capillariids, morphotype I. (F) Egg of unidentified capillariid, morphotype III. (G) Strongylus-type egg. (H) Oocyst of Eimeria macusaniensis. Scale bars: 20 µm.
Table 2. Parasitic distribution in archaeological samples from ADG

Archaeological layer (L), archaeological sample (S).
Among representatives of the superfamily Trichinelloidea, eggs of Trichuris sp. (Fig. 2D) of 63·25 (60–67·5) μm in length and 34 (33·75–35) μm in width, were found in 3/6 samples examined (Table 2). Two unidentified representatives of the family Capillariidae including 116 eggs similar to Calodium sp. [66·62 ± 3·4 (57·5–77·5) μm in length by 38·42 ± 2·57 (32·5–50) μm in width], named capillariid morphotype I (Fig. 2E) and 10 eggs of another unidentified capillariid [64·95 ± 2·3 (61·25–67·5) μm in length by 42·25 ± 3·2 (37·5–47·5) μm in width] with globular appearance and thicker walls than capillariid type I, named capillariid morphotype III, were found in 5/6 and 2/6 samples, respectively (Fig. 2F, Table 2). Capillariid type I was the parasite most represented and abundant in the paleoparasitological record of ADG (Table 2).
Two Strongylus-type eggs were also found in one sample (Fig. 2G, Table 2).
Regarding protozoa, oocysts of Eimeria macusaniensis [86·75 (82·5–92·5) × 62·8 (58·75–67·5) μm] were found in 2/6 samples examined (Fig. 2H, Table 2).
There were no differences in parasitic species composition between different time periods (P = 0·8), as it can be observed in Fig. 3. Archaeological layers of the same time period were not necessarily grouped closely in the NMDS plot (Fig. 3).

Fig. 3. NMDS ordination diagram of samples based on Jaccard's similarity matrix (stress = 0·0). Numbers identify archaeological layers and symbols represent different time periods. Black square: middle Holocene. Grey circles: late Holocene. Closeness of archaeological layers in the bidimentional space indicates that they have similar parasite communities. NMDS, Non-metric Multi-dimensional Scaling.
Integrating paleoparasitological results of camelids from the PNPM – Southern Patagonia
Results indicated that there were no significant differences in parasite species composition among different time periods or archaeological sites (P = 0·13). Samples from both archaeological sites were arbitrarily mixed in the NMDS ordination plot (Fig. 4).

Fig. 4. NMDS ordination plot considering samples from both archaeological sites (CCP7 and ADG) based on Jaccard's similarity matrix (stress = 0·09). Grey circles: CCP7. Black square: ADG. ADG, Alero Destacamento Guardaparque; CCP, Cerro Casa de Piedra; NMDS, Non-metric Multi-dimensional Scaling.
DISCUSSION
The parasite richness registered in ADG (eight species) constitutes the highest found for camelids coprolites at present. This richness compared with those documented in the archaeological site CCP7 (five species) (Taglioretti et al. Reference Taglioretti, Fugassa and Sardella2015) was due to the presence of N. spathiger and Dictyocaulus sp. in the sample 532, parasites that had not been previously found in the paleoparasitological record. Sample 532 was collected from the stratigraphic layer 3, corresponding to the late Holocene (post-1000 years BP) (Rindel, personal communication). The presence of N. spathiger and Dictyocaulus sp. in the present guanacos from Patagonia was associated with their contact with the introduced livestock (Beldoménico et al. Reference Beldoménico, Uhart, Bono, Marull, Baldi and Peralta2003). This is the first record of Dictyocaulus sp. and N. spathiger in archaeological samples. Rindel (Reference Rindel, Civalero, Fernández and Guraieb2004, Reference Rindel2008) reported the presence of sheep's remains in the upper archaeological layer (layer 2); so the presence of such parasites in layer 3 was therefore associated with the entry of European livestock into Patagonia and to the interaction with camelids, potentially facilitating host switching. Capillariids, mainly capillariid morphotype I, are commonly found in Patagonian archaeological sites (Fugassa et al. Reference Fugassa, Taglioretti, Gonçalves, Araújo, Sardella and Denegri2008; Taglioretti et al. Reference Taglioretti, Fugassa, Beltrme and Sardella2014b ). The finding of the capillariid morphotype III in the present research increases the capillariid biodiversity for camelids and also for the paleoparasitological records of Patagonia. Most capillariids are considered zoonotic. Their presence in camelid coprolites collected from archaeological sites brings information of the potential zoonotic infections that humans in the past may have been exposed by the ingestion of contaminated food or water with camelid dungs.
Enteric parasites of camelids had not changed since the Pleistocene-Holocene transition to late Holocene, up to the entry of introduced livestock in the PMNP, despite the climate fluctuations that were affecting Patagonia during this period (Mancini et al. Reference Mancini, Paez and Prieto2002; Mancini, Reference Mancini2007), indicating the high stability of camelids-parasite assemblages through time. The increase in parasite richness and also the differences in the composition of parasite communities observed up to the historic period (layer 3) could be interpreted as a result of the introduction of new hosts (as red deer or sheep) with similar physiological and ecological features that could facilitate the transmission of ‘new’ parasite species to native camelids. Moreover, as it is suggested by Hoberg and Brooks (Reference Hoberg and Brooks2015) within the framework of the Stockholm Paradigm, under the concept of ‘ecological fitting’, phenotypic flexibility of these parasites could provide opportunities for rapid host switching in changing environments, a scenario that had probably occurred in Patagonia during the introduction of European livestock. These findings provide the first paleoparasitological evidence supporting the hypothesis by Beldoménico et al. (Reference Beldoménico, Uhart, Bono, Marull, Baldi and Peralta2003) on the presence of N. spathiger and Dictyocaulus sp. in the present guanacos from Patagonia.
Similarity between parasitic compositions of both archaeological sites (CCP7 and ADG) could be explained by the interaction between camelid populations that had inhabited these sites. As archaeological sites are in the home range of the guanaco (up to 9 km) (Burgi, Reference Burgi2007), it is suggested that guanaco populations of ADG and CCP7 were in contact during the Holocene, enabling parasites transmission.
The present study provides information on the dispersion of some parasites in space and on the potential parasite host switching between sympatric species of herbivores that occupied the studied area. It also reinforces the hypothesis of the acquisition by the guanacos of new parasite species by the introduction of European livestock.
ACKNOWLEDGEMENTS
The authors gratefully thank the archaeologists Rafael Goñi and Diego Rindel, for bringing the archaeological samples.
FINANCIAL SUPPORT
This work was supported by the Universidad Nacional de Mar del Plata (UNMDP), Argentina (EXA 680/14); the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), Argentina (PIP 090) and the Fondo para la investigación y Tecnología (FONCyT), Argentina (PICT 2316).









