Introduction
The date moth [Apomyelois (Ectomyelois) ceratoniae, Zeller], also known as the carob moth is a serious pest of the many fruits from a wide range of plant families as well as dried fruits during storage (Gothilf, Reference Gothilf1984; Warner, Reference Warner1988). This cosmopolite polyphagous pest causes significant damage to various crops throughout the world, which varies by region, host plant and plant variety. For instance, while it causes infestation on the date palm (Phoenix dactylifera L.) in Tunisia and Algeria, it is a major pest of the pomegranate (Punica granatum L.) in Iran and Turkey (Norouzi et al., Reference Norouzi, Talebi and Fathipour2008; Idder et al., Reference Idder, Idder-Ighili, Saggou and Pinturea2009; Öztürk and Ulusoy, Reference Öztürk and Ulusoy2009; Zouba et al., Reference Zouba, Khoualdia, Diaferia, Rosito, Bouabidi and Chermiti2009; Öztop et al., Reference Öztop, Keçeci and Kıvradım2010). Besides these crops, there are lots of records of its damage on other host plants such as pistachio, Pistacia vera L. (Dhouibi, Reference Dhouibi1982; Mehrnejad, Reference Mehrnejad1993), carob, Ceratonia siliqua (Gothilf, Reference Gothilf1964), almond, Prunus dulcis (Mill.) (Gothilf, Reference Gothilf1984), fig, Ficus carica L. (Shakeri, Reference Shakeri1993), walnut, Juglans nigra L. (Balachowsky, Reference Balachowski and Masson1975), dried fruits and nuts.
The struggle of the date moth is also varied, as are the plant species in which it causes damage. In Iran, this pest is controlled by collecting and burning infected pomegranate fruits at the end of the growing season that reduces overwintering sites (Behdad, Reference Behdad1991). However, the date moth is widely controlled with different pesticides like methyl bromide which is highly toxic and poses several hazards to animals and humans (Hallier et al., Reference Hallier, Deutschmann, Reichel, Bolt and Peter1990). Using chemicals against pests affects the environment and non-target organisms negatively, and these effects have led to new approaches, especially in the last quarter, to identify the natural pathogens of pests (Bekircan et al., Reference Bekircan, Bülbül, Güler and Becnel2017; Bekircan, Reference Bekircan2020).
Microsporidia (Opisthokonta) phylum, is a very special group that infect the diverse Animalia taxa, especially Insecta (Solter et al., Reference Solter, Becnel, David, Vega and Kaya2012). This phylum has 200 genera and more than 1300 species, it consists of intracellular pathogens that cause various abnormalities on their hosts (Becnel et al., Reference Becnel, Takvorian, Cali, Weiss and Becnel2014). Especially, entomopathogenic microsporidia have detrimental effects on insects including reduced longevity and fecundity (Hajek and Delalibera, Reference Hajek and Delalibera2010). Because of these effects, microsporidia can be used as natural regulators against certain pest insect species.
In this study, the microsporidian pathogens of A ceratoniae were investigated and a new isolate of the Nosema fumiferanae (Nosema fumiferanae TY61) complete description was done for the first time based on morphological and molecular data.
Materials and methods
Insect samples and light microscopy
Apomyelois ceratoniae individuals were collected from October to December 2017–2019 in Trabzon, Turkey. The larvae and adult members, which collected from nuts storages, were placed in separate plastic boxes and transported laboratory as soon as possible. The internal organs of thorax and abdomen for each specimen were excised and examined for microsporidiosis by light microscopy according to Yaman et al. (Reference Yaman, Bekircan, Radek and Linde2014). Microsporidia positive slides were fixed with methanol for 5 min after air-dried and stained for approximately 10 hours in freshly prepared 5% solution of Giemsa stain (Undeen and Vávra, Reference Undeen, Vávra and Lacey1997). Microsporidian spores and life cycle stages were photographed with a Zeiss AXIO microscope combined with Axiocam ERc5s digital camera. Spore measurements were taken using ZEN 2.3 Elements imaging software.
Electron (TEM) microscopy
For transmission electron microscopy (TEM), infected tissues were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 1–2 h, washed with cacodylate buffer and postfixed in 1% aqueous OsO4 for 2 h. After postfixation, the tissues were washed with cacodylate buffer and dehydrated through an ascending alcohol series and acetone before embedding in Spurr's resin (Spurr, Reference Spurr1969; Baki and Bekircan, Reference Baki and Bekircan2018). Thin sections were taken with Leica EM UC7 ultramicrotome and mounted on Pioloform-coated copper grids which were then stained with saturated uranyl acetate and Reynolds’ lead citrate (Reynolds, Reference Reynolds1963). The samples were examined and photographed with a HITACHI HT7800 transmission electron microscope.
Molecular studies
Mature spores were obtained from infected tissues which were collected in sterile 1.5 ml Eppendorf tubes and homogenized in Ringer's solution with a micropestle. The suspensions were filtered with cheesecloth and then centrifuged for 2 min at 300 rpm (Chen et al., Reference Chen, Shen, Zhu, Guan, Hou, Zhang, Xu, Tang and Xu2012). Further, 1 mL of distilled water was used to rinse the spore pellet. Purified spores were stored at −20°C until DNA extraction (Martín- Hernández et al., Reference Martín-Hernández, Meana, Prieto, Salvador, Garrido-Bailón and Higes2007).
Microsporidian DNA was extracted from purified spores using a slightly modified protocol of Higes et al. (Reference Higes, Martin and Meana2006). Purified spores were placed in a 0.5 ml microfuge tube with equal volumes 0.3% hydrogen peroxide (H2O2) and kept at room temperature for 15 minutes to stimulate spore wall disruption. An approximately 0.1 g of glass beads (0.425–0.600 μm) were added into the same tube and vigorously shaken for 2 min at maximum speed on the vortex (Hylis et al., Reference Hylis, Weiser, Oborník and Vávra2005). The DNA extraction was then performed with the QIAGEN DNA Isolation Kit, No. 69504 according to the manufacturer's guidelines. To amplify the small subunit rRNA (SSU rRNA) and the largest subunit of RNA polymerase II (RPB1), the Qiagen Multiplex PCR Kit (QIAGEN, Cat. no. 206143) was used. The 18F/1537R primer set was used to amplify the SSU rRNA gene (18F/1537R: 5′-CACCA GGTTG ATTCT GCC-3′/5′-TTATG ATCCT GCTAA TGGTT C-3′) and the primers for the RPB1 gene were newly designed (Yıldırım and Bekircan, Reference Yıldırım and Bekircan2020). For sequencing, bidirectional readings were made and after the necessary examinations, a consensus sequence was established and loaded GenBank. The polymerase chain reaction (PCR) reaction (94°C for 15 min; 45 cycles of 94°C for 30 s; 61°C for 90 s; 72°C for 90 s; and 72°C for 10 min) was processed in a total volume of 50 μL. After the amplification, the 16S SSU rRNA and RPB1 gene base sequences were determined by the Macrogen Inc. Company, The Netherlands.
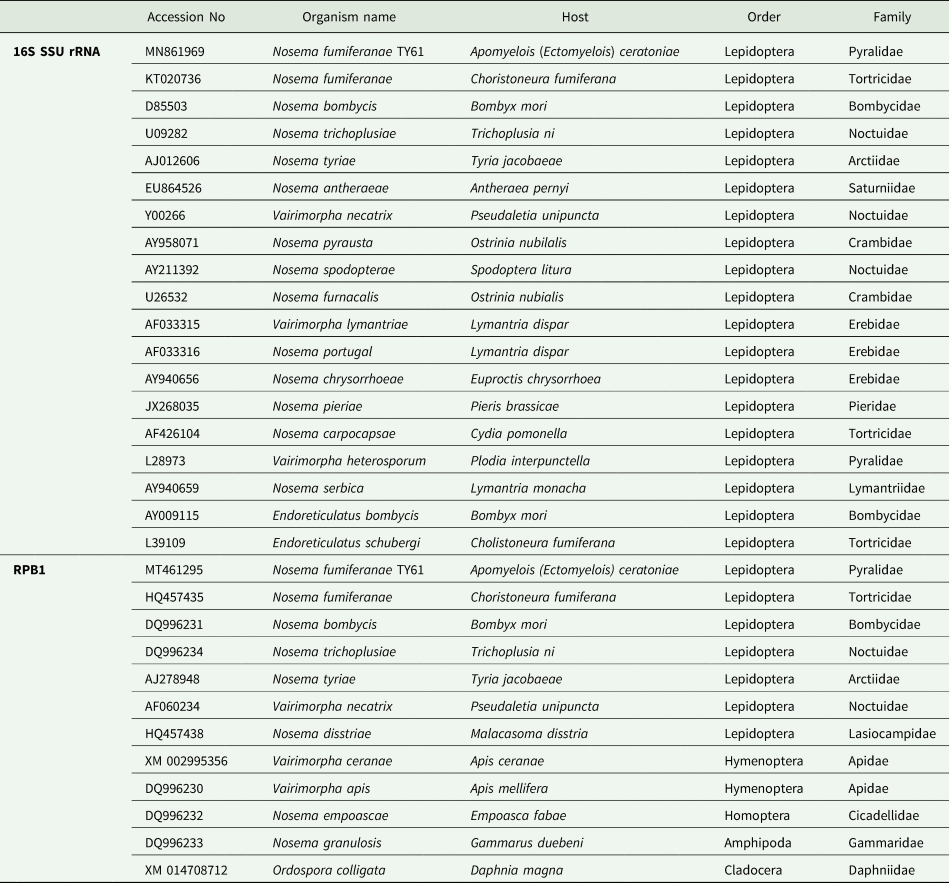
The microsporidian base sequences were aligned with the closely related species mostly from the genus Nosema (Table 1). While Endoreticulatus bombycis and Endoreticulatus schubergi (Microsporidia: Encephalitozoonidae) were included as outgroup species for 16S SSU rRNA, Ordospora colligata (Microsporidia: Ordosporidae) were included for RPB1. Datasets were aligned using BioEdit and CLUSTAL_W programs. Phylogenetical analyses were performed using either the Maximum Parsimony algorithm with PAUP 4.0a or MEGA 10. The GC content of the base sequences of the current microsporidium and other sequences were analysed with the FastPCR program.
Table 1. 16S Small subunit (SSU) ribosomal RNA and RNA polymerase II largest subunit (RPB1) gene sequences used for phylogenetic analyses
Results
Light microscopy
Between 2017 and 2019, 202 larvae and 45 adults of A. ceratoniae were dissected and observed with the light microscope. During the examinations, 19 infected larvae (9.4%) and 7 infected adults (15.5%) were determined (total infection rate 10.5%). Examination by light microscopy showed that the infection was confined to the gut and hemolymph of the host (Fig. 1). Fresh spores were oval in shape and measured 3.29 ± 0.23 μm (4.18–3.03 μm, n = 200) in length and 1.91 ± 0.23 μm (2.98–1.66 μm, n = 200) in width. During the examinations on Giemsa-stained smears, mature spores and intracellular life stages were observed at the same time. The binucleate spores were in direct contact with the host cell cytoplasm and showed a disporoblastic (Nosema type) development. Binucleate meronts are usually spherical and measure 4.30 ± 0.66 μm in diameter (n = 20) (Fig. 2A). The spherical binucleate sporonts produced sporoblasts via binary fission Spherical sporonts measured 3.30 ± 0.50 μm in diameter (Fig. 2B). Sporoblasts were elongated and measured 5.91 × 3.60 μm (Fig. 2C). After the Giemsa staining, stained mature spores measured as 3.11 ± 0.31 μm (3.72–2.41 μm, n = 150) in length and 1.76 ± 0.23 μm (2.16–1.25 μm, n = 150) in width (Fig. 2D).

Fig. 1. Light micrographs of spore stages of Nosema fumiferanae TY61 from Apomyelois (Ectomyelois) ceratoniae, in wet mount

Fig. 2. Light micrographs of the life stages of Nosema fumiferanae TY61 from Apomyelois (Ectomyelois) ceratoniae, in Giemsa-stained smears A – diplokaryotic meront (gut); B – diplokaryotic sporonts (gut); C – early sporoblast (gut); D – fresh spores (gut).
Electron (TEM) microscopy
The binucleate mature spores were oval in shape (2.85 × 1.43 μm) (Fig. 3). Electron microscopic observations confirmed that the oval spores contained two nuclei in diplokaryotic arrangement with spherical nuclei measuring 375–560 nm in diameter (Fig. 3). The spore wall was thick and measured 106–203 nm, additionally, it had a clear endospore thickness of 64–142 nm and an electron-dense wrinkled exospore thickness of 31–93 nm (Fig. 3). The polar filament was isofilar and had 10–12 polar filament coils (Fig. 3) with a diameter of 80–102 nm. The last coils were immature and hence thinner (Fig. 3). They contained a central core surrounded by four concentric layers (Fig. 3). The developmental stages and spores were in direct contact with the host cell cytoplasm (Fig. 3). A sporophorous vesicle was not observed during the light and electron microscopical observations.

Fig. 3. TEM photographs of Nosema fumiferanae TY61 in Apomyelois (Ectomyelois) ceratoniae gut tissue. Diplokaryotic microsporidian spore with a thick wall consisting of a thin exospore (ex) and a thick electron-lucent endospore (en), showing 10–12 coils of the polar filament (pf), a clearly visible diplokaryon (n), regular meshes of endoplasmic reticulum (er) are arranged on both side of diplokaryon and section of the anterior portion of a spore showing an anchoring disc (ad) attenuated apically to the endospore. Bars: A-100nm, B-500nm B, C,D-1 μm.
Molecular studies
The 16S SSU rRNA sequence of the studied microsporidium that was 1110 bp, was deposited in GenBank (MN861969). The GC content of the current microsporidium was 33.5% (for other GC contents see Table 2). Pairwise phylogenetic distances between the current species and other species ranged from 0.0009 to 0.5671. Distances between the current microsporidium and the type species of the genera, Nosema bombycis (Nägeli, Reference Nägeli1857) and Vairimorpha necatrix (Pilley, Reference Pilley1976), were 0.0018 and 0.1646, respectively. The identities of 16S SSU rRNA sequences between current microsporidium and other species used in the phylogenetic analysis were 84.13–99.91% (Table 2). According to constructed maximum parsimonic tree, the current microsporidium settled the same branch with Nosema fumiferanae, a microsporidium from the Lepidopteran family Tortricidae (Fig. 4).
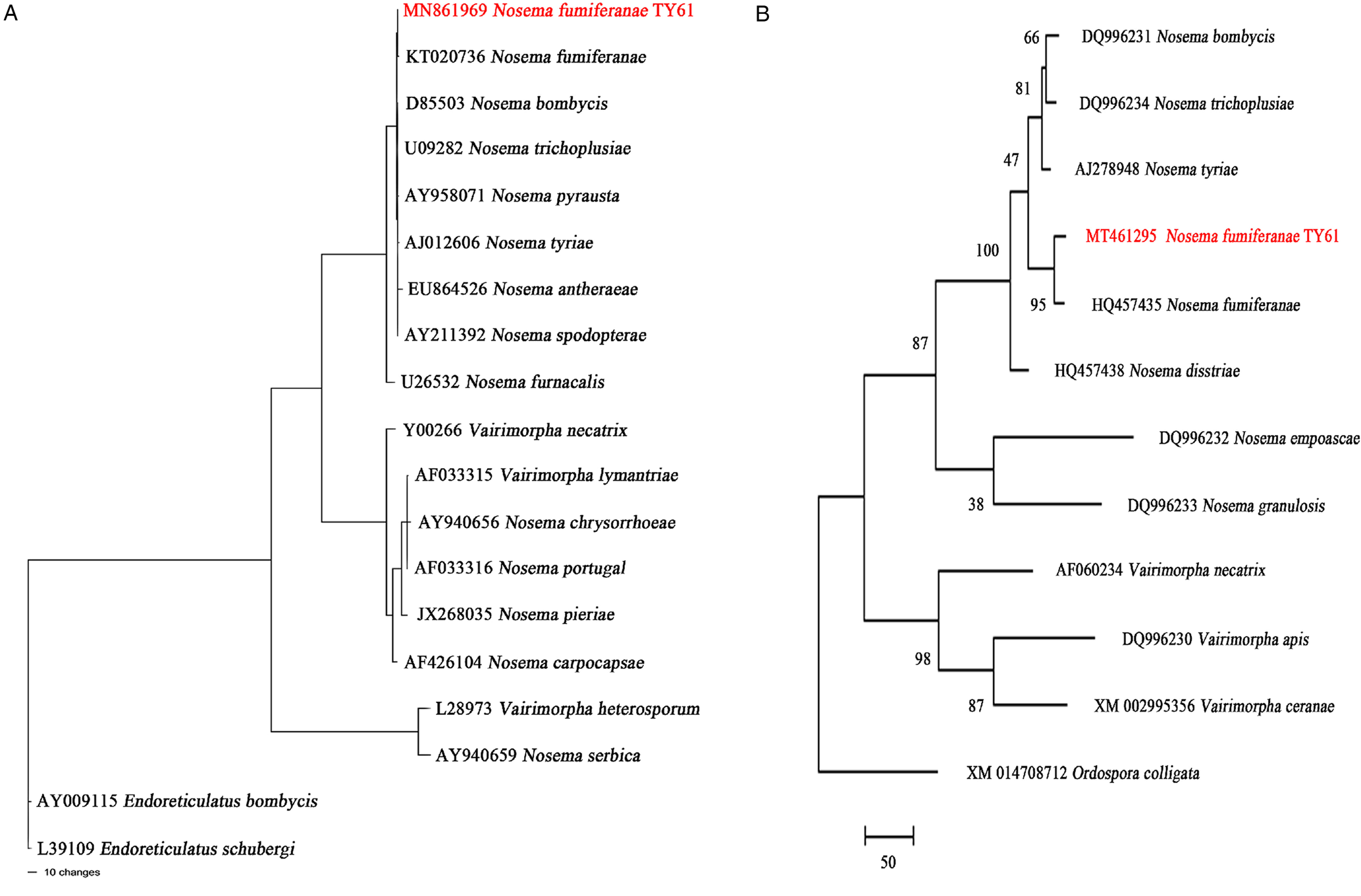
Fig. 4. Phylogeny inferred for Nosema fumiferanae TY61 and related taxa with GenBank accession numbers. Tree reconstructions based upon (A) the small subunit rRNA and (B) the largest subunit of RNA polymerase II gene alignments Numbers above the branches are bootstrap support values in percentage.
Table 2. Comparison of current microsporidium and other related microsporidia based on the 16S small subunit ribosomal RNA gene (16S SSU rRNA) and the largest subunit of RNA polymerase II (RPB1) gene by query cover, by nucleotide identity, by Pairwise distance analysis and GC% content.

‘-’No significant similarity found.
The RPB1 gene sequence of the current microsporidium (969 bp) was deposited in GenBank with (MT461295) accession code. Similar parameters that were assessed for the 16S SSU rRNA like the GC content, the distances and etc. were analysed too and they were summarized in Table 2. As in the maximum parsimonic tree constructed for 16S SSU rRNA, the current microsporidium settled again in the same branch with N. fumiferanae in the maximum parsimonic tree which was prepared with RPB1 base sequences.
An 1110 bp long alignment of the current microsporidium showed an SSU rRNA gene difference of only 0.0009, corresponding to >99.91% sequence similarity with Nosema fumiferanae, while RPB1 gene sequences were 98.03% similar within an alignment of 969 bp. These two species were, therefore, very closely related to their biological and morphological features that were evidently similar. Consequently, the phylogenetic status, light and electron microscopical observations showed that the microsporidian pathogen of A. ceratoniae is the new isolate of the Nosema fumiferanae.
Discussion
The date moth [Apomyelois (Ectomyelois) ceratoniae, Zeller] is a cosmopolite pest of the many fruits, nuts and dried fruits during storage (Gothilf, Reference Gothilf1984; Warner, Reference Warner1988). Therefore, numerous studies have been conducted in different parts of the world to determine the organisms that can be used in the control of this insect (Alrubeai, Reference Alrubeai1988; Elsayed and Bazaid, Reference Elsayed and Bazaid2011; Mnif et al., Reference Mnif, Elleuch, Chaabouni and Ghribi2013). Similarly, in the study, conducted by Lange in 1991 from Argentina, they declared the microsporidiosis from A. ceratoniae which were collected from walnuts. Although it was stated that the microsporidium isolated in this study belong to the genus Nosema, no definition could be made at the species level. The determined Nosema sp. was identified via light and electron microscopy in this study. There are obvious similarities between the taxonomic characters examined in Lange's study and the current research. For instance, fresh spore shape, dimensions, disporoblastic (Nosema type) development, electron-dense wrinkled exospore, etc. characters determined as nearly the same in both of the two studies. While the fresh spore dimension of the Nosema species presented in here 3.29 ± 0.23 μm × 1.91 ± 0.23 μm, the Lange's record was 3.7 ± 0.01 μm × 1.3 ± 0.006 μm. The disporoblastic life cycle was determined in both studies. Also, microsporidia were detected in both studies in direct contact with the host cell cytoplasm. The number of polar coils provides very effective taxonomic information for discriminating microsporidia species (Cheung and Wang, Reference Cheung and Wang1995). The current microsporidium has an isofilar 10-12 polar filament coils and mature coils measure 80–102 nm in diameter. Similarly, Lange reported 9-12 polar filament coils from the isolated microsporidium. Unfortunately, there was no molecular data for identifying the Nosema species that was determined by Lange (Reference Lange1991).
In South Africa, Lloyd and friends reported the second microsporidiosis from the A. ceratoniae in Reference Lloyd, Knox, Thackeray, Hill and Moore2017. Although this study mentioned the presence of microsporidial spores with similar size and morphological characteristics as those reported previously by Lange, there was no data or figure in this study put forward to demonstrate this. On the other hand, in this study, molecular data were available in contrast to the study of Lange. In this study, researchers mentioned that approximately 1148 bp SSU sequence was amplified and according to their BLAST search the isolate showed 99% similarity with the Nosema carpocapsae (AF426104) and Nosema oulemae (U27359) sequences. Also, their phylogenetic analysis revealed that this isolate clustered with the Nosema/Vairimorpha group rather than the ‘true’ Nosema group. Despite revealing such important data and making phylogenetic determinations, there were not any SSU base sequences or GenBank accession codes related to this study. Therefore, it was not possible to phylogenetically compare the current microsporidium with this isolate. The Nosema genus has been recently suggested for a new classification by researchers and with this perspective, the RPB1 gene sequence was determined in addition to the 16S rRNA sequence in this study (Tokarev et al., Reference Tokarev, Huang, Solter, Malysh, Becnel and Vossbrinck2020).
According to the phylogenetic tree, the microsporidium presented in this study grouped in the same branch with Nosema fumiferanae (KT020736) reported from Choristoneura fumiferana Clemens (Lepidoptera: Torticidae) in the ‘true’ Nosema group (Thomson, Reference Thomson1955). Although host species and tissue specificity have historically been important taxonomic characteristics in microsporidia, last researches show that some microsporidian species easily switch hosts among different families (Sprague et al., Reference Sprague, Becnel and Hazard2008; Tokarev et al., Reference Tokarev, Huang, Solter, Malysh, Becnel and Vossbrinck2020). Given this situation, it is quite likely that the existing microsporidium isolated from A ceratoniae is N. fumiferanae. The N. fumiferanae isolate described by Thomson in 1955 from Ontario, Canada, have several similarities compared to the current microsporidium concerning in site of infection (most tissues, esp. midgut, fat body), spore dimension (fixed mature spore 3-4 μm, fresh mature spore 3–5 μm) (Table 3). The second study that was conducted for identifying Nosema fumiferanae by Hopper et al., in 2016, clarified the ultrastructural features of the N. fumiferanae. The ultrastructural characteristics of spore structure, especially polar filament structure, are important parameters for the comparison of microsporidian species (Canning and Vávra, Reference Canning, Vavra, Lee, Leedale and Bradbury2000; Becnel et al., Reference Becnel, Jeyaprakash, Hoy and Shapiro2002; Ovcharenko et al., Reference Ovcharenko, Swiatek, Ironside and Skalski2013). While the current microsporidium polar filament number is 10–12 coils (80-102 nm diameter); N. fumiferanae has 12–15 coils (80-90 nm diameter). And the spore wall thickness of the current microsporidium is (106–203 nm) thicker than N. fumiferanae (60–160 nm) (Table 3). All these diagnostic features are important taxonomic characteristics in microsporidia systematics (Larsson, Reference Larsson1986, Reference Larsson1988; Undeen and Vavra, Reference Undeen, Vávra and Lacey1997; Canning and Vavra, Reference Canning, Vavra, Lee, Leedale and Bradbury2000). And in all these comparisons between the current microsporidium and the isolate of Hopper et al., it was observed that the measurement and taxonomic characters were overlaps.
Table 3. Characteristics of the new Nosema fumiferanae isolate described in the present study and other Nosema fumiferanae isolates

In conclusion, the phylogenetic status, light and electron microscopy observations suggest that the described Nosema species from Apomyelois (Ectomyelois) ceratoniae is a new isolate of the Nosema fumiferanae. We named it as Nosema fumiferanae TY61. This work is the first study that confirmed Apomyelois (Ectomyelois) ceratoniae as a host of the Nosema fumiferanae isolate.
Acknowledgements
The TEM analysis was carried out in Eskişehir Osmangazi University (ESOGU) Central Research Laboratory, Research and Application Centre (ARUM). I am thankful to Prof. İlknur Dağ and her team for making this study possible and for her valuable comments, to Assoc. Prof. Mehmet MAMAY for insect identification.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Not applicable.










