INTRODUCTION
Many of the compounds used to control nematode parasites of medical and veterinary importance have molecular targets within the neuromuscular system. It is reasonable to conject, therefore, that targets for new generations of nematicidal agents may also be found within the nematode neuromuscular system. From a parasitology perspective, this hypothesis has driven the study of nematode neurobiology and muscle control in human and animal parasitic species, as well as those free-living nematodes which are deemed good models for parasitic nematodes. This has been a profitable endeavour as it has greatly advanced our understanding of nematode neuromuscular function and in the process has thrown up several promising leads for parasiticide targets. Very little research, however, has been devoted to the study of plant parasitic nematode neuromuscular function. This is surprising given the impact that plant parasitic nematodes have on crop production and the lack of effective control strategies to combat nematode attack. Phytoparasitic nematodes damage crops by direct feeding, transmission of viruses and by facilitating fungal and bacterial infection. This damage is often non-specific and symptoms of infection may be difficult to detect such that the detrimental economic effects of plant parasitism tend to be underestimated; reported annual losses of US$100 billion to world agriculture (Sasser & Freckman, 1987) testify to the significance of the problem caused by plant parasitic nematodes.
Over 50 genera of plant parasitic nematodes are found parasitizing all parts of plants but the most frequent target is the root system. The Order Dorylaimida (Class Adenophora) includes the families Longidoridae and Trichodoridae, both of which comprise migratory species that are ectoparasitic on plant roots. Within these families are several important nematode vectors of soil-borne viruses. The Order Tylenchida (Class Secernentea) contains genera exhibiting a much wider range of host-parasite relationships in which an increased complexity in the host-parasite interaction is reflected in an enhanced ability of the parasite to modify and regulate host plant gene expression. Thus, appearing within these genera are migratory ectoparasites, sedentary ectoparasites, migratory ecto-endoparasites, migratory endoparasites and sedentary endoparasites. The sedentary endoparasites represent the apex of intricacy with regard to the plant-nematode interaction and their ability to regulate host plant genes is most pronounced. These nematodes can reorganise plant root morphology and physiology at the site of interaction and establish complex cellular structures that provide nutrients for nematode development and reproduction. Most plant parasitic nematode life cycles comprise six stages: the undifferentiated egg, four juvenile stages (J1 to J4) and the adult. Invasion of the host plant by these sedentary endoparasites is by the J2 stage and the modification of plant tissues allows for progression of the life cycle. These sedentary endoparasites, the most highly adapted parasites, cause severe economic damage to forestry, horticultural and agricultural crops.
Plant parasitic nematode control strategies traditionally involve the use of plant health legislation, crop rotation, resistant plant varieties and treatment with nematicidal chemicals. Intensive agricultural production relies heavily on chemical control and two major classes of product are used to reduce the impact of nematode feeding and damage. Fumigant nematicides are either general biocides (e.g. methyl bromide) or act more specifically against nematodes and other soil arthropods (e.g. 1,3-dichloropropene). Use of fumigant nematicides can be technically demanding and many of these products have been withdrawn for environmental reasons. Non-fumigant nematicides are represented by organophosphates and carbamates, both of which are acetylcholinesterase inhibitors. This mode of action means that non-fumigants act as nemastats rather than nematicides and, should levels of the compound fall in the soil, nematodes can recover leading to subsequent plant infection (Rich, Dunn & Noling, 2004). Product withdrawals due to toxicity and loss of efficacy primarily due to enhanced biodegradation by microbial adaptation (Racke & Coats, 1990) continue to restrict the list of usable chemicals for plant parasitic nematode control and, increasingly, nematode problems are being left with no effective management strategy. The development of novel nematode control compounds and techniques remains a high priority for world agriculture.
If the nematode neuromuscular system is attractive in the search for new drug targets to control human and animal parasites, the same is true for phytoparasitic nematodes. In the case of sedentary endoparasites, the establishment of parasitism within the host plant requires nematodes to migrate through the soil, invade host tissue and migrate through the host tissue to set up a feeding site. These behaviours require elaborate neuromuscular regulation and disruption of this neuromuscular regulation may be effective in controlling the parasite. Indeed, carbamate and organophosphate nemastats have just this mode of action, disrupting acetylcholine neurotransmission and paralysing the worm. Nematode nervous systems display a high degree of conservation across the phylum such that it is taken for granted that plant parasitic nematodes have similar neuromuscular biology to their free-living and animal parasitic cousins. The main nematode excitatory and inhibitory neurotransmitters are acetylcholine (ACh) and γ-aminobutyric acid (GABA); the potent acitivity of acetylcholinesterase inhibitors suggests ACh is critical to phytoparasitic nematode biology and immunocytochemical studies confirm the presence of GABA (Stewart, Perry & Wright, 1994) in the phytoparasitic nematode nervous system. Our review is concerned, however, not with these classical neurotransmitters for which there has been a paucity of research with regard to plant parasitic nematodes, but with the neuropeptidergic component of phytoparasitic nematode nervous systems, particularly in the cyst-forming genera. The aim of this review is to demonstrate not only the abundance and diversity of these peptide neurotransmitters in plant parasitic nematodes but also to delineate the roles of these molecules in plant parasitic nematode biology and perhaps validate the targeting of this system as a novel approach to plant parasitic nematode control.
FMRFAMIDE-LIKE PEPTIDES
FMRFamide-like peptides or FLPs (also known as FMRFamide-related peptides, or FaRPs) are a large family of peptidergic neurotransmitter or neuromodulator molecules that appear to be ubiquitous amongst invertebrates. Their defining features are their small size, usually between four and 20 amino acids in length, and a C-terminal Phe/Tyr-X-Arg-Phe-amide (F/Y-X-R-F-NH2) motif where X is typically a hydrophobic residue. As more structural information is acquired on the composition of the nematode neurotransmitter complement, it is becoming evident that there are many short-chain peptidergic neurotransmitters present that, whilst not adhering to the constrained structural definition of the term ‘FLP’, do have an a conserved R-F-amide motif. They have similar biological activity to so-called ‘true’ FLPs and are frequently genetically co-encoded and consequently these peptides are also considered FLPs. The free-living nematode, Caenorhabditis elegans, serves as the best example to underline the remarkable diversity and sheer abundance of FLPs in the nematode nervous system. Twenty-eight flp genes have been identified from this worm, encoding 67 distinct FLPs (Li, Kim & Nelson, 1999; McVeigh et al. 2005). Indeed, analysis of Expressed Sequence Tag and genomic databases for this species indicate that this may be an underestimation of the C. elegans FLP complement. The presence of such a complex neuropeptide signaling family in an organism where the adult hermaphrodite has only 302 neurons indicates that FLP mediated neurotransmission is central to normal nematode neuronal function.
FLP DIVERSITY IN PLANT PARASITIC NEMATODES
An immunocytochemical study (Atkinson et al. 1988) was the first to demonstrate the presence of FLPs in plant parasitic nematodes, with elements of the nervous system of the soybean cyst nematode (SCN), Heterodera glycines, shown to be immunoreactive to anti-FMRFamide antisera. Although many studies were undertaken to characterize both biochemically and genetically free-living and animal parasitic nematode FLPs throughout the late 1980s and 1990s, no further work helped to elucidate the plant parasitic nematode FLP complement until Masler and colleagues in 1999. In that study, ELISA and HPLC techniques were used to compare H. glycines FLP immunoreactivity to that of the free-living nematodes, C. elegans and Panagrellus redivivus. There was found to be no significant differences in the total FLP immunoreactivity between the species, suggesting that plant parasitic nematodes have a quantitatively similar FLP complement to that of C. elegans and other free-living nematodes. The elution profile of FLP immunoreactive fractions from H. glycines extracts was, however, different from those of the free-living nematode species. Although not conclusive, this suggested that although plant parasitic nematodes may have a quantitatively similar FLP complement to free-living nematodes, the distinct peptides present in plant nematodes are structurally different.
We have also used polyclonal antisera raised against FMRFamide to examine FLP distribution in the potato cyst nematodes (PCN), Globodera pallida and G. rostochiensis (Kimber et al. 2001). This study was the first to demonstrate the extent of FLP immunoreactivity, and hence FLP distribution, in the plant parasitic nematode nervous system. Intense immunoreactivity was observed throughout the worm central nervous system and peripherally, in neurons innervating the pharynx, stylet protractor muscles and the anterior sensory organs. Previous immunocytochemical studies on C. elegans and the pig gastrointestinal nematode, Ascaris suum, have indicated that the percentage of neurons that are FLPergic is remarkably high, with between 50% of C. elegans and 60–75% of A. suum neurons being immunoreactive to anti-FMRFamide antisera (Cowden et al. 1993; Brownlee et al. 1994; Li et al. 1999). The extent of the immunoreactivity observed in PCN is on a par with these estimations, reinforcing the concept that the FLP complement of plant parasitic nematodes is comparable to free-living and animal parasitic species.
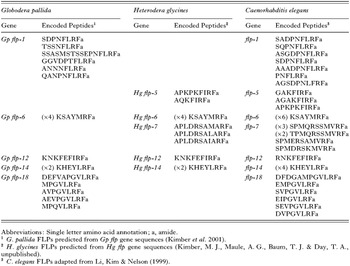
Although these chromatographic and immunocytochemical studies revealed the general distribution of FLPs in plant parasitic nematodes, they did not provide structural information regarding the individual peptides that contribute to the observed immunoreactivity. This information has begun to be accrued through the molecular characterization of the flp genes that encode FLPs in plant parasitic nematodes. We have previously used a degenerate PCR approach, in combination with RACE PCR (Rapid Amplification of cDNA Ends) to identify five FLP-encoding flp genes from G. pallida (Kimber et al. 2001). This approach used primers designed from the sequences of FLPs present in both free-living and animal parasitic nematode species and which we expected to be present in PCN. A total of 14 distinct peptides are encoded on these five genes (Table 1). This degenerate PCR methodology has proved successful in identifying the primary sequence of a number of PCN neuropeptides but, in the longer term, a more fruitful approach to peptide identification may be the mining of the burgeoning number of publicly available Expressed Sequence Tag (EST) datasets. EST datasets are collections of single pass sequencing runs of reverse transcribed mRNA and in recent years EST projects have been initiated on many of the most significant plant nematode species including H. glycines, G. pallida, G. rostochiensis and a host of root knot nematodes (Meloidogyne spp.). Although the sequences held in these datasets are not definitive, they do provide preliminary sequence information which can be used as the basis for further gene characterization studies. To illustrate this, we have searched the H. glycines EST database for potential flp gene fragments using predicted C. elegans FLPs as search strings. RACE PCR based on likely ‘hits’ has lead to the identification of five SCN flp genes, encoding eight distinct FLPs (Kimber, M. J., Maule, A. G., Baum, T. J. & Day, T. A., unpublished data; see Table 1). Homology searches of the other plant nematode datasets reveal a number of potential flp genes awaiting full characterization but there is sufficient data already present to draw some conclusions as to the nature of the plant parasitic nematode FLP complement.

The cadre of FLPs identified in plant nematodes so far can be broadly categorized into two main groups. Firstly, there are those FLPs that have been repeatedly identified in a wide range of nematode species such that they appear to be ubiquitous throughout the phylum. Examples of such FLPs include KSAYMRFamide and KHEYLRFamide, which we have identified in both G. pallida and H. glycines and which have previously been found in C. elegans (Marks et al. 1995, 1998), P. redivivus (Maule et al. 1994a,b), A. suum (Davis & Stretton, 1996), and the sheep roundworm, Haemonchus contortus (Keating et al. 1995; Marks et al. 1999). Others have speculated that the highly conserved nature of these FLPs suggests some common function critical to nematode neurobiology and also draw attention to the fact that these FLPs are multiply encoded on their own flp genes and are usually the most abundant FLPs in nematode extracts (Maule et al. 2002).
The remainder of the plant nematode FLP complement is composed of peptides which have C-terminal signature motifs that consistently appear throughout the phylum. The N-terminal extensions of these conserved motifs, however, have generally not been identified in any free-living or animal parasitic nematodes. Thus, these FLPs appear to be specific to plant parasitic nematodes and indeed, some appear specific to certain genera of plant parasitic nematodes. Having noted this, it must be reiterated that the C-terminal motifs of these FLPs do appear conserved in other nematodes such that although the mature peptide may be structurally different at the N-terminus, the disparate FLPs may be considered homologous. To illustrate this distinction, we have identified a gene in G. pallida that encodes at least five distinct FLPs, all possessing the C-terminal motif PGVLRFamide or PQVLRFamide. Although the N-terminal extensions of four of these PGVLRFamides appear unique to plant parasitic nematodes, PGVLRFamides have also been identified in C. elegans (Li et al. 1999), encoded on the flp-18 gene and in A. suum (Edison, Messinger & Stretton, 1997). Indeed, one of the G. pallida FLPs, AVPGVLRFamide has been biochemically purified from Ascaris (Cowden & Stretton, 1995). Thus, although many plant parasitic nematode FLPs appear specific to phytoparasitic nematodes, they do have homologues throughout the phylum, based on this C-terminal sequence similarity. It would seem likely that these peptides are orthologous but as their ancestral heritage has yet to be determined, this designation is inappropriate and other authors have adopted the term ‘sequelog’ in an attempt to better define this relationship between distinct but structurally similar molecules (Varshavsky, 2004). The extent of nematode FLP homology has lead to the proposition of a universal flp gene nomenclature for genes being identified in parasitic species, one based on the corresponding homologous C. elegans flp genes. It follows that as the C. elegans flp gene encoding PGVLRFamides has been designated in a chronological fashion flp-18, our G. pallida gene encoding PGVLRFamides should be termed Gp flp-18 (see Table 1) as it encodes structurally similar peptides. A comprehensive review of pan-phylum FLP distribution can be found in Maule et al. (2002).
In summary, the diversity of FLPs in plant parasitic nematodes is just beginning to be realized and, compared to model free-living and animal parasitic nematodes, we know relatively little. A general picture is emerging that plant parasitic nematodes appear to have a quantitatively similar FLP complement to that of C. elegans, that is, likely upwards of 60 FLPs. A number of these FLPs are identical to those found in all other nematodes but the majority are species or genera specific FLPs with conserved C-terminal structural motifs that repeatedly appear throughout the phylum.
FLP FUNCTION IN PLANT PARASITIC NEMATODES
If most of our structural knowledge of FLPs comes from C. elegans, the majority of our functional data on FLPs comes from the nematode physiological model, A. suum. The conserved nature of the nematode nervous system means that physiological information gained from this species can be taken as representative of the phylum as a whole. The majority of FLPs tested in A. suum preparations potently modulate nerve and muscle activity in a concentration-dependent and reversible manner and this activity has been the subject of several reviews (Day & Maule, 1999; Geary et al. 1999). For example, the FLP KHEYLRFamide produces a biphasic response resulting in increased contractility of body wall muscle at a threshold of 1 pM (Bowman et al. 1996). FLPs also modulate nematode pharyngeal muscle activity in A. suum; KSAYMRFamide inhibits serotonin-induced pharyngeal pumping at concentrations as low as 1 nM (Brownlee et al. 1995; Brownlee & Walker, 1999). As in somatic musculature, FLPs produce multiple responses in the ovijector musculature, ranging from transient excitation to persistent or transient inhibition (Fellowes et al. 1998, 2000). The fact that FLPs shown to be present in plant parasitic nematodes, and the structural homologues thereof, potently modulate nematode body wall, pharynx and ovijector supports the view that these peptides play a central role in somatic muscle control, feeding and reproduction. Disruption of these behaviours in plant parasitic nematodes represents an attractive novel control strategy as it would impinge upon the worm's ability to hatch, to migrate through the soil, to gain access to the host, to feed off host plant tissues and to mate.
Elucidating FLP function: immunocytochemistry
Investigation of the physiological role of FLPs in plant parasitic nematodes is made difficult due to their small size which precludes the use of standard physiological techniques. FLP function has previously been inferred through the visualization of the distribution of these peptides in the plant nematode nervous system. As mentioned, we have used indirect immunocytochemical (ICC) techniques to examine FLP distribution in PCN (Kimber et al. 2001). Those studies on Ascaris demonstrated that FLPs are potently myoactive on nematode body wall muscle and pharynx preparations. In PCN, the dorsal, ventral and lateral nerve cords containing the motor- and interneurons that govern body wall muscle function all reacted strongly to the anti-FMRFamide antisera, as did the circumpharyngeal and perianal nerve rings and their associated ganglia. This innervation pattern is consistent with the hypothesis that FLPs modulate plant nematode body wall muscle and hence locomotion. Plant nematodes have highly muscular triradiate pharynges used to draw food through the hollow stylet. The complex muscular organization of this organ is mirrored by an equally complex pattern of FLPergic pharyngeal innervation in PCN, composed of an extensive FLPergic neuronal plexus and nerve ring. This intricate innervation points to a fine level of coordination and control of this important feeding organ by FLPs. These preliminary findings are sufficient to demonstrate a continuity of FLP function between the physiological models and plant parasitic nematodes.
These ICC studies also suggested further roles for FLPs which would be critical to the biology of plant parasitic nematodes. Several large FLPergic neurons were observed running from the circumpharyngeal nerve ring and pharyngeal innervation towards the anterior of the parasites. Several of these nerves were seen to associate closely with the stylet protractor muscles. These are the muscles that produce the motive force for the thrusting of the needle-like stylet, the stomal tool used by plant nematodes for emergence from the egg, gaining access to the host, and in the case of sedentary endoparasites, for migration through the host to the vascular tissue and the establishment and utilization of the feeding site. These anterior-running FLPergic neurons also appear to innervate sensory apparatus. The main concentration of nematode sensory structures, including the sensilla and amphids, is located at the anterior end of the worm. Of these sensory structures, the function of the amphids in plant parasitic nematodes is best detailed (Perry, 1996, 2001) and they appear to act mainly as chemosensors critical for both host and mate location. Immunoreactivity to FMRFamide has been identified in sensory neurons of other nematode species (Schinkman & Li, 1992, 1994; Brownlee et al. 1993; Jagdale & Gordon, 1994) suggesting that FLPs may be involved in the transduction of nematode sensory stimuli. Disruption of plant nematode chemosensation has long been suggested as a possible control approach (Zuckermann, 1983) and may indeed be the mode of action of some current nematicides (Winter, McPherson & Atkinson, 2002). The demonstration that FLPs are involved in plant nematode chemosensation as well as being potent myomodulators would enhance the appeal of FLP neurotransmission as a novel target for plant nematode control. A final observation based on the ICC studies was a large FLPergic neuron terminating at the base of the PCN odontophore (posterior part of stylet). It is unclear what structure this neuron is innervating, however, the oesophageal gland ampulla is located in this region. A valve structure in this ampulla controls the secretion from the dorsal oesophageal gland which is delivered through the stylet. Much research into the nature of these glandular secretions and their role in defining the plant-nematode interaction has been carried out and readers are directed to excellent reviews on the subject (Davis et al. 2000; Williamson & Gleason, 2003; Davis, Hussey & Baum, 2004). Glandular secretions from phytoparasitic nematodes include lytic and degradative enzymes which aid penetration of host tissues, factors that establish and regulate the worm's feeding site and factors that alter the host plant's metabolism and defence mechanisms. In short, these secretions are essential to the establishment of parasitism in the plant. Some of these secretions also emanate from subventral glands which empty into the oesophagus through the pharynx, a process believed to be under nervous control (Anderson & Byers, 1975). The region of the pharynx where this occurs is rich in FLP innervation. Work is currently being undertaken to assess the relationship between FLP containing neurons and these glandular structures with a view to determining if there is FLPergic control of secretion. It is interesting to hypothesise that these peptidergic transmitter molecules can modulate not only the neuromuscular function of plant parasitic nematodes but also regulate the release of glandular secretions and hence the modification of host plant tissues and the establishment of an endoparasitic existence.
Elucidating FLP function: in situ hybridization
A major drawback of ICC is that the antibodies used are indiscriminate in their recognition of members of the closely related FLP family and therefore it is impossible to attribute observed immunoreactivity to individual peptides. Li and colleagues (1999) overcame this problem in the genetically tractable C. elegans by transforming worms with green fluorescent protein reporter constructs driven by select flp gene promoters. This demonstrated distinct but often overlapping expression patterns for a number of flp genes. Understanding flp gene expression would be an important step in understanding the function of individual FLPs in plant parasitic nematodes. Adopting similar transgenic approaches in phytonematodes is currently unfeasible; we have therefore used an in situ hybridization methodology to visualize the expression patterns of flp genes in the infective J2 stage of our model plant parasitic nematode, G. pallida (Kimber et al. 2002). Highly localized expression patterns were generated for four out of five of our flp genes and comparison of positively stained cell bodies with the C. elegans neuronal map (White et al. 1986) allowed us to identify the neurons we observed and consequently speculate as to the function of the peptides encoded on those genes. The FLP, KSAYMRFamide, is one that appears throughout the phylum and in G. pallida, Gp flp-6, the gene which encodes four copies of the peptide has a diffuse expression pattern in the nematode circumpharyngeal and perianal nerve rings. Those neurons which could be positively identified unequivocally were interneurons upstream and downstream of a host of processes such that it was difficult to designate exact functions to this peptide. We were, however, able to be more explicit with regard to the other genes. The predicted G. pallida FLP, KNKFEFIRFamide, may be considered a homologue of the C. elegans flp-12 peptide, RNKFEFIRFamide and, based on its expression pattern in G. pallida, is a critical peptide involved in a number of worm behaviours. It is expressed in neurons innervating body wall muscle suggesting a role for this putative peptide in worm locomotion. Further, it is expressed in neurons which are associated with vulval musculature in C. elegans, as well as nerves leading from sensory structures. Thus in addition to an involvement in parasite movement, this peptide may also contribute to the coordination of worm reproduction and how the nematode senses its environment. KHEYLRFamide is another FLP present throughout the phylum and in G. pallida is encoded on Gp flp-14. This gene is expressed in interneurons and motorneurons associated with body wall muscle in the head region of the worm. Interestingly, all the nerves expressing this peptide appear to have input from sensory structures suggesting KHEYLRFamide may form an integral part of a neuromuscular network controlling worm head movement in direct response to external stimuli. The final gene which produced positive results encodes multiple FLPs terminating FLRFamide, homologues of the C. elegans flp-1 gene. Based on their expression pattern in motorneurons of the retrovesicular ganglion in PCN, these peptides appear to be involved in the coordination of plant nematode body wall muscle. We can draw several apparently contradictory conclusions from these studies. First, based on the conserved nature of the nematode nervous system, the bioactivity of FLPs on muscle preparations of Ascaris is frequently taken to be representative of the phylum in general. Here we see the in vitro actions of certain FLPs mirrored in their in vivo distribution within plant parasitic nematodes, thereby validating the physiological assays. For example, KHEYLRFamide displays a biphasic excitatory effect in Ascaris body wall muscle (Cowden & Stretton, 1993; Maule et al. 1995) and in G. pallida, appears to be involved in the control of body wall muscle in the head region of the worms. A second, seemingly paradoxical conclusion is that the expression patterns of the G. pallida flp genes and those C. elegans flp genes encoding the homologous peptides (where this information is known) do not overlap. In G. pallida, Gp flp-12, encoding KNKFEFIRFamide, is expressed in a set of cells in the retrovesicular ganglion (most likely motorneurons), in the BDU interneurons and in the pre-anal and lumbar ganglia. In C. elegans however, flp-12 is expressed in the ASE and PVM sensory neurons and the URX interneurons (Li et al. 1999). This disparity demonstrates that although some peptides or their characteristic motifs may be conserved in divergent species, the function of these peptides in vivo may not be conserved. A comparative summary of flp gene expression patterns in PCN and C. elegans, where available data permit, can be found in Table 2. A cautionary consideration is that expression patterns were localized using different methodologies in the two species; however, these findings do highlight the danger of drawing conclusions as to the role of FLPs in parasitic species based on data from model free-living nematodes. These localization studies certainly provide evidence supporting the central role FLPs play in phytoparasitic nematode neuromuscular function and consequently contribute to the validation of FLP-mediated neurotransmission as a potential source of plant nematode control targets.

Elucidating FLP function: gene silencing studies
Determining the contribution of FLPs to parasitic nematode neuromuscular biology is an excellent example of the task facing investigators in the so-called ‘post-genomic’ era. A multitude of genomic and related projects (e.g. ESTs) are producing a surfeit of sequence data regarding the genetic make up of a host of organisms, including parasitic nematodes. The challenge now is to attribute functional significance to these sequences. The identification of RNA interference (RNAi) has been invaluable in this respect. It is beyond the scope of this review to summarize the cellular mechanism of RNAi but readers unfamiliar with the process are directed to an excellent recent comprehensive review by Tomari & Zamore (2005). What is clear is that RNAi is an apparently ubiquitous mechanism for gene regulation or antiviral defence through the highly sequence-specific degradation of mRNA, the suppression of mRNA transcription or the formation of suppressive heterochromatin. The trigger for this ‘gene silencing’ is the cleavage of long, double-stranded RNA (dsRNA) molecules into 21–27 nucleotide small RNAs which, when incorporated into enzymatic complexes, serve to prime the specific silencing of genes with an homologous sequence to that of the dsRNA. This potent, and importantly systemic, gene-specific silencing was first illustrated by Fire and colleagues (1998) who injected long dsRNA molecules into C. elegans and observed inhibition of gene expression not only in those injected worms but also in their progeny. The utility of RNAi as a reverse genetics tool to investigate gene function by silencing particular genes of interest was immediately apparent. However, although a myriad of studies have now established the technique as routine, progress in applying RNAi to the study of parasitic nematode genes has been slow. Initial papers in the field have focused on demonstrating that the technique is indeed applicable to parasitic nematodes using simple protocols adapted from C. elegans RNAi studies. Selkirk and colleagues (Hussein, Kichenin & Selkirk, 2002) were the first to show this, inhibiting acetylcholinesterase activity in Nippostrongylus braziliensis by soaking the adult nematodes in dsRNA, an approach that has proved amenable with C. elegans (Tabara, Grishok & Mello, 1998). A major concern regarding the use of RNAi to study gene function in parasitic nematodes is whether the dsRNA trigger could be taken up by the nematode given that many species do not actively feed either outside the host or during those life cycle stages which are used for in vitro studies. Aboobaker & Blaxter (2003) and Lustigman and colleagues (2004) have shown that these concerns are unfounded by knocking down gene expression in Brugia malayi and Onchocerca volvulus respectively, again using a soaking protocol. Two studies have shown that RNAi can be used to study gene function in plant parasitic nematodes. Urwin, Lilley & Atkinson (2002) successfully reduced expression of cysteine proteinase and major sperm protein in infective stage (J2) H. glycines and G. pallida. Their laboratory also used a soaking protocol, one which included 50 mM octopamine to stimulate pharyngeal pumping in this non-feeding larval stage and thereby ensure dsRNA uptake via an oral route. More recently, Fanelli et al. (2005) have shown that RNAi can also be effective in eggs of phytonematodes by knocking down a chitin synthase gene in the root knot nematode, Meloidogyne artiellia.
Application of these RNAi techniques will be an important pathway to furthering our understanding of FLPs and flp gene function in plant parasitic nematodes. To demonstrate this, we have amassed a significant amount of data which not only shows that the technique can easily be applied to the study of parasitic nematode neuromuscular function, but also reveals the central role FLPs play in plant parasitic nematode biology (Kimber, M. J., McKinney, S., McMaster, S., Fleming, C. C. & Maule, A. G., unpublished).
Our initial experiments were directed at investigating the susceptibility of flp genes to RNAi in our model phytoparasitic nematode, G. pallida. We focused on Gp flp-6 as previous in situ hybridization studies revealed a more extensive distribution within the G. pallida nervous system compared to the other flp genes (Kimber et al. 2002) thus perhaps offering a higher likelihood for success. Infective stage juveniles (J2) were treated with dsRNA homologous to Gp flp-6 then assayed for any detectable null, or ‘lack-of’, phenotype induced by flp gene silencing. A basis of dsRNA induced gene silencing is that it operates, at least in the most part, at the post-transcriptional level, mediating the sequence specific degradation of mRNA (Montgomery, Xu & Fire, 1998). To confirm Gp flp-6 silencing, an RT-PCR was performed on poly A+ RNA extracted from worms incubated in the dsRNA. Using this approach, our laboratories found not only that RNAi can be used to potently inhibit FLP-encoding flp genes in plant parasitic nematodes but that this effect was dependent on the size of the dsRNA used as a trigger. It is significant that neuronally expressed flp genes can be silenced using RNAi. Neuronal targets have traditionally been considered refractory to the process, dogma based mostly on an absence of detectable phenotype in high throughput C. elegans screens, but also reflecting highly variable results using smaller scale microinjection studies (Fraser et al. 2000; Kamath et al. 2000; Tavernarakis et al. 2000; Maeda et al. 2001). The cellular machinery by which RNAi operates is clearly present in nematode neuronal tissue; this has been made evident through the generation of transgenic C. elegans capable of in vivo RNAi (Tavernarakis et al. 2000) and so it seems likely that access to neuronal tissue by exogenous dsRNA is the major stumbling block. Why RNAi silences the neuronally expressed flp genes in G. pallida but not as readily for neuronal targets in other nematode species is unclear. For whatever reason, what is apparent is that RNAi can be used to silence neuronal targets in G. pallida. This affords the opportunity, not just to investigate FLP and flp gene function in these worms, but to use this species as a model to study neuromuscular biology in nematodes in general.
Many of those FLPs tested in physiological assays are potently myoactive. Our hypothesis was that the Gp flp-6-silenced nematodes would display altered myoactivity, primarily an inhibition of motility. Therefore, a motility or migration assay was chosen to detect aberrant motility in the J2. Worms incubated in dsRNA for varying lengths of time were assessed for impaired motility using a sand column assay whereby nematodes under investigation migrate the length of a 5 cm wet sand column. Under normal conditions, nematodes migrate down the moist sand column; worms with disrupted motor function, however, have impaired ability to complete this migration. Worms incubated in the effective Gp flp-6 dsRNA constructs showed significant inhibition of motility. Gp flp-6 encodes four copies of the FLP, KSAYMRFamide, arguably the most well studied nematode FLP. It has been identified in representative species throughout the phylum including a number of free-living (Maule et al. 1994b; Marks et al. 1998) and animal parasitic nematodes (Cowden & Stretton, 1995; Marks et al. 1999) and been characterized at the gene level from several plant parasitic species (Kimber et al. 2001 and Kimber, M. J., Maule, A. G., Baum, T. J. & Day, T. A., unpublished). It is potently myoactive, eliciting reversible, nerve cord dependent effects on Ascaris suum somatic body wall muscle strips (Maule et al. 1995), it inhibits the inherent contractility of the Ascaris ovijector (Fellowes et al. 1998) and it inhibits serotonin-induced pumping of the Ascaris pharynx (Brownlee et al. 1995). Our in situ studies were unable to establish unequivocally a role for this peptide in PCN but on the basis of these RNAi experiments KSAYMRFamide is clearly involved in modulating phytonematode motile behaviour, consistent with the myoactive properties of the peptide on Ascaris body wall preparations.
Having demonstrated that Gp flp-6 could be silenced using RNAi and that silencing this gene grossly altered normal worm behaviour, we investigated the effects of silencing other G. pallida flp genes. Silencing of four other Gp flps resulted in profound inhibition of migration down the sand column (Kimber, M. J., McKinney, S., McMaster, S., Fleming, C. C. & Maule, A. G., unpublished). The silencing of all of the flp genes examined was discernible as a remarkable inhibition of normal migratory behaviour in the functional sand column assay. This inhibition of motility may be attributable to a number of factors. Firstly, it may be as a result of dysfunction in body wall muscle coordination manifesting itself as impaired movement to the extent of paralysis. This seems plausible based on the myoactive properties of FLPs on nematode body wall muscle preparations. It is also consistent with the expression of the peptides in PCN motorneurons as visualized by in situ hybridization. Another alternative is that it may indicate some sensory dysfunction rendering the nematodes incapable of some inherent geotactic behaviour (the sand columns were orientated vertically, with worms moving from the top to the bottom of column). Again, in situ hybridization indicated that some of the FLPs were expressed in neurons with sensory inputs. In summary, the almost complete inhibition of worm motility upon flp gene silencing clearly demonstrates how critical FLP mediated neurotransmission is to nematode biology. Such remarkable impairment of normal plant parasitic nematode behaviour validates the suggestion that the FLPergic neuromuscular system may provide important future targets from a control perspective.
PHYTONEMATODE CONTROL USING RNAI
RNAi will clearly be instrumental in the future analysis of neuromuscular gene function in parasitic nematodes and as such is valuable in supporting more traditional genetic tools. The technique may also have more applied usage. The ability to replicate the in vitro motile dysfunction described above in parasitic nematodes in the host or in the field would constitute an exciting novel control mechanism. Given the life cycle of medically and veterinary important parasites however, it is difficult to envisage how RNAi may be adapted for such control purposes as delivery of the dsRNA would surely be too problematic. For these nematode species, RNAi will likely remain an in vitro experimental device. However, both the migratory and sedentary phases of the life cycle of plant parasitic nematodes in host tissues provide opportunities to affect RNAi in the invading worms through in planta production of dsRNA. The viability of using RNAi to knockout genes essential to plant pathogens has already been demonstrated. The proven approach is to engineer transgenes into the host plant which either have regions of self-complementarity or consist of inverted repeats of the particular genes of interest. Upon transgene transcription, the RNA adopts a hairpin formation consisting of a double stranded stem and single stranded loop structure. This conformation is sufficient to act as an RNAi trigger and induce the silencing of the homologous gene in the pathogen. This approach has been used with great effect to generate transgenic plants with either complete or almost complete resistance to viral pathogens (Waterhouse, Graham & Wang, 1998; Smith et al. 2000; Kalantidis et al. 2002). Escobar et al. (2001) have also shown that the approach can be used to generate transgenic plants with conferred resistance to bacterial pests by transforming Arabidopsis and Lycopersicon with inverted repeat transgenes homologous to Agrobacterium oncogenes. There has been no published report, however, documenting the use of this technique to generate plants with resistance to metazoan pests. There is evidence confirming that in planta-generated RNAi triggers are effective at inducing gene silencing in nematodes. Boutla et al. (2002) isolated RNA from transgenic N. benthamiana plants actively silencing GFP expression. This RNA was then microinjected into transgenic C. elegans expressing GFP. The subsequent silencing of nematode GFP indicated that the plant RNAi trigger (dsRNA) could cross kingdoms and that plant derived dsRNA could knock out nematode genes.
The generation of engineered host plants which use RNAi-mediated silencing of pathogen genes as a novel defence mechanism is the subject of ongoing research by several groups. Most of these studies are using the pHANNIBAL/pKANNIBAL vector system (Wesley et al. 2001) to introduce inverted repeat transgene precursor constructs into host plants. It is conceivable that the same approach could be used to introduce inverted repeats of phytonematode flp genes, the expression of which in the host root tissue or indeed feeding site may produce the same motile dysfunction in worms we have demonstrated in vitro. A transgenic plant which produces complete inhibition of worm motility in planta would be of great potential in controlling plant parasitic nematodes and reducing yield losses. The highly gene-specific nature of RNAi would mean any control based around its application would also have the added benefit of species specificity, the control could be targeted at a specific pest as RNAi operates at the gene, rather than the protein level. Paradoxically, this may also be regarded as a drawback to using the technique as the trend in parasite control is a desire for any therapeutic or compound to have broad-spectrum efficacy. For this to be achieved using the RNAi approach multiple transgenes would have to be introduced corresponding to multiple pest species.
OTHER NEUROMUSCULAR TARGET MOLECULES
Receptors
Up to this point we have demonstrated that FLP-mediated neurotransmission is central to plant nematode biology and as such is an attractive novel control target. We have also outlined how RNAi can be used to investigate FLP function in these parasites and how it might also be adapted as a future control mechanism itself. Other more conventional approaches, such as the application of compounds which disrupt FLP neurotransmission, may also be exploited as novel means of control. Many chemotherapeutics directed towards controlling animal parasitic nematodes have molecular targets within the nematode neuromuscular system and all these targets are neurotransmitter receptors, primarily ionotropic receptors. Consequently, if we are searching for the molecules that will ultimately serve as the substrate for intervention strategies which exploit plant nematode FLP neurotransmission, the identification of plant parasitic nematode FLP receptors is paramount. Unfortunately, we know very little regarding the structure and function of FLP receptors in nematodes; our understanding is reviewed elsewhere in this supplement. Although there is evidence some FLPs may directly gate ion channels in nematodes (Purcell et al. 2002), the weight of physiological data supports the hypothesis that FLP receptors are primarily G-protein-coupled receptors (GPCRs). A number of neuropeptide GPCRs have been identified from C. elegans, initially based on homology with vertebrate peptide receptors, and have been shown to be stimulated by FLPs when heterologously expressed in mammalian cell lines (Rogers et al. 2003; Kubiak et al. 2003a,b; Mertens et al. 2005a,b, 2004). A cursory search of plant parasitic nematode EST datasets using these receptors as search strings yields several encouraging ‘hits’ suggesting homologous receptors are expressed in plant parasitic nematodes. Comprehensive studies directed at the structural and functional characterization of these receptors will be essential in helping to understand FLP neurobiology in phytoparasitic nematodes. Additionally, they may validate FLP receptors as potential targets and indicate which of the receptors should be given more detailed consideration as templates in drug discovery efforts. Those plant nematode FLP receptors which serve as mediators in FLP pathways which modulate critical worm behaviour would be excellent candidates towards which non-peptide agonists or antagonists could be developed. These compounds would have great potential as novel control agents.
Processing enzymes
Current plant nematode control compounds whose targets are within the neuromuscular system act on neurotransmitter processing enzymes; both the carbamate and organophosphate classes of nematicide act as cholinesterase inhibitors and retard the degradation of the excitatory neurotransmitter acetylcholine. It seems likely that molecules associated with the biosynthesis and/or degradation of neuropeptides may prove to be targets for future control compounds. Neuropeptides and neurohormones undergo a number of post-translational cleavage and modification steps before they become biologically active. Essential to this process is the endoproteolytic cleavage of the neuropeptide precursor from the prepropeptide by members of the prohormone or precursor convertase (PC) family of enzymes. Conceptually, inhibition of the prohormone convertases responsible for processing neuropeptides, including FLPs, in plant parasitic nematodes would prohibit their biosynthesis and thereby disrupt normal worm behaviour. Kovaleva et al. (2002) have identified a cDNA from H. glycines, dubbed HglPC2, with high homology to all known PC2 convertases. Further study will be required to appreciate the significance of this putative enzyme to plant nematode neurobiology and understand its contribution to neuropeptide processing however, its functional characterization may lead to the identification of agents which interfere with its action and perhaps be useful from a control perspective.
Endoproteolytic cleavage from the prepropeptide through convertases yields a C-terminal glycine-extended propeptide precursor. This glycine moiety serves as the donor of an α-amide group in the generation of the C-terminally amidated mature neuropeptide; C-terminal amidation is essential for neuropeptide activity. In invertebrates, this conversion of a glycine-extended precursor into the mature peptide is mediated by two enzymes, peptidylglycine α-hydroxylating monoxygenase (PHM) and peptidyl-α-hydroxyglycine α-amidating lyase (PAL). Compounds which disrupt either of these two enzymes could prevent the formation of biologically active neuropeptides such as FLPs in plant parasitic nematodes and may have potential as effective nematicides. We have cloned a cDNA from H. glycines J2 encoding a putative PHM enzyme (GenBank Locus: AY242521) which has high homology to known invertebrate PHM molecules. Again, as with HglPC2, functional characterization of this molecule will be required, not only to confirm its designation as a PHM, but also to identify agents which may selectively inhibit the enzyme and thereby act as possible novel control compounds.
CONCLUDING REMARKS
The focus of much research into parasitism of plants by nematodes is the interaction between the parasite and host. Hopefully, a better understanding of the mechanisms by which the worm invades the host, establishes and maintains its feeding site and evades host defence mechanisms, that is, how the worm interacts with the plant, will lead to a more effective means of plant nematode control than currently exists. Here, we have provided evidence which validates an alternative set of possible intervention targets which may be useful for plant parasitic nematode control, one which does not directly focus on the plant-nematode interaction. FLP neurotransmission is central to plant nematode biology. FLPs appear integral to how these parasites move, feed, sense their environment and perhaps establish parasitism within the plant. Successful strategies to plant nematode control involve the disruption of processes including these which are critical to the parasite. We have demonstrated that the disruption of FLP neurotransmission grossly affects worm behaviour. Consequently, it is evident that FLP neurotransmission appears to have great potential as a source of targets for a novel approach to phytonematode control; be it through the application of chemicals or nematicides which adversely affect FLP neurotransmission or through the use of transgenic crop plants which may utilize fledgling techniques such as RNAi to allow the host to silence FLP neurotransmission in invading nematodes. FLPs themselves are unlikely to constitute the molecular targets upon which any control strategy which disrupts FLP neurotransmission will be based. Rather, specific molecules which orchestrate FLP neurotransmission will better serve this function, such as FLP receptors or the enzymes associated with FLP processing. In this respect, advancing our knowledge of the structure and function of these molecules and developing RNAi both as a reverse genetic tool to aid in this, and also as a mechanism of engineering host plant resistance, will be the next step in moving forward this novel approach to the control of plant parasitic nematodes.
ACKNOWLEDGEMENTS
This work was supported in part by the Department of Agriculture and Rural Development for Northern Ireland.