Introduction
Several families of mites (Arachnida: Acariformes, Parasitiformes) parasitize the nasal cavity of vertebrates, with more than 500 species recorded in birds (Fain, Reference Fain1994). Rhinonyssidae (Mesostigmata) is the most diverse family of nasal mites found in birds, comprising eight genera that occur in a wide range of non-ratite birds worldwide (Krantz, Reference Krantz1978). Rhinonyssids are obligate haematophagous parasites that have no off-host stage and are transferred by direct contact between birds. They are slow-moving mites that live on the highly vascularized nasal epithelium of the nasal turbinates, but some species also invade the tracheal tissues, lungs and body cavity (Porter and Strandtmann, Reference Porter and Strandtmann1952; Krantz, Reference Krantz1978). Despite causing some trauma to the nasal epithelium, only a few rhinonyssid infections are considered significantly pathogenic [e.g. Sternosoma tracheacolum (Lawrence)] (Stephan et al., Reference Stephan, Kaschula and Canham1950; De Rojas et al., Reference De Rojas, Mora, Ubeda, Cutillas, Navajas and Guevara2002).
Two named species of nasal mites have been collected from the nasal cavities of penguins (Aves: Spheniscidae). Rhinonyssus sphenisci Fain and Mortelmans was described from a Humboldt penguin [Spheniscus humboldti (Meyen)] that was originally caught in Peru but died in captivity in Belgium (Fain and Mortelmans, Reference Fain and Mortelmans1959). This species has since been recorded in Magellanic penguins [Spheniscus magellanicus (J.R. Forster)] in São Paulo (Amaral and Rebouças, Reference do Amaral and Rebouças1974) and Rio Grande do Sul, Brazil (Gastal et al., Reference Gastal, Mascarenhas, Vanstreels and Ruas2017). The second species, Rhinonyssus schelli (Fain and Hyland), was initially described from Adélie penguins [Pygoscelis adeliae (Hombron & Jacquinot)] from Queen Maud Land, Antarctica (Fain and Hyland, Reference Fain and Hyland1963), and it was subsequently recorded in Adélie penguins from Victoria Land, Antarctica (Wilson, Reference Wilson and Gressitt1967) and in Gentoo penguins [Pygoscelis papua (J.R. Forster)] from South Georgia (Wilson, Reference Wilson1970) and Anvers Island in the Antarctic Peninsula (Wilson, Reference Wilson1971). Rhinonyssus schelli was originally described as a subspecies of R. sphenisci by Fain and Hyland (Reference Fain and Hyland1963) but was subsequently raised to species level by Wilson (Reference Wilson and Gressitt1967).
In this study, for the first time, we report the infection of African penguins [Spheniscus demersus (Linnaeus)] by a nasal mite, R. sphenisci sensu lato. We furthermore provide data on the epidemiology and morphology of this parasite.
Materials and methods
Seabirds are regularly admitted for rehabilitation to the Cape Town facility (33°50′02″S 18°29′29″E) of the Southern African Foundation for the Conservation of Coastal Birds (SANCCOB). For January to October 2017, in addition to routine examinations, particular attention was paid to the occurrence of nasal mites. Penguins examined included those admitted as live rescues from breeding colonies or along the coast of the Western Cape province but which died while under care (N = 40). Live penguins underwent rehabilitation following standardized protocols (Parsons and Underhill, Reference Parsons and Underhill2005); depending on their age and health status, penguins receive antiparasitic treatment upon admission (ivermectin 0.2 mg kg−1 PO) and treatment was repeated after 14 days. In addition to birds deceased while under care, recently deceased penguins found at breeding colonies also underwent post-mortem examination (N = 67).
Carcasses were necropsied following standardized protocols (Hocken, Reference Hocken2002) and their heads were removed, stored in individual plastic bags and frozen for later analysis. Cause of death was classified into the following categories: infection (e.g. air sacculitis, pneumonia, septicaemia), debilitation (e.g. starvation, dehydration), trauma (e.g. predation, blunt force trauma, drowning) or unknown. Sex was determined through the dissection of gonads. Age class was classified according to plumage and other external characteristics (P1 = small chicks with open eyes and primary downy plumage, P2 = medium-to-large chicks with secondary downy plumage, P3 = large chicks with <50% fledging plumage, P4 = large chicks with more than 50% fledging plumage, juvenile = blue/grey plumage, adult = black-and-white plumage) (García-Borboroglu and Boersma, Reference García-Borboroglu and Boersma2013; Sherley et al., Reference Sherley, Waller, Strauss, Geldenhuys, Underhill and Parsons2014). Body condition was classified into five categories based on the quantity of chest muscle and fat deposits (Clements and Sanchez, Reference Clements and Sanchez2015).
The penguin heads were later thawed and the nasal cavity and paranasal sinuses were dissected and thoroughly examined, with all mites collected in 70% ethanol. The number of mites found in the following anatomical sites was recorded (see Supplementary File S1 for illustrations): nasal cavity, ophthalmic sinus (combination of the ophthalmicus, mesenthmoidale and frontale components), antorbital sinus, lacrimal sinus and suborbital sinus (Witmer, Reference Witmer1995; Witmer and Ridgely, Reference Witmer and Ridgely2008). All mites were examined under the light microscope to evaluate general morphological characteristics. A subset of 42 mites from four individuals were further examined for detailed morphological analysis and species identification. Mites were removed from ethanol, cleared in 85% lactic acid for 1–24 h depending on the degree of original opacity and mounted in a polyvinyl alcohol medium (6371A, BioQuip Products, Rancho Dominguez, CA, USA). Slides were cured on a slide warmer at about 40 °C for 3–4 days. Slide-mounted specimens were examined on a Leica DMLB compound microscope with differential interference contrast at 400× magnification. Species-level identifications were made using an identification key (Pence, Reference Pence1975) and descriptions from the primary literature. A subset of 14 mites were photographed and the maximum length and maximum width of the dorsal, sternal and genital shields were measured with the aid of ImageJ 1.50 (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). These measurements were also obtained from the photographs of R. spheniscus from Magellanic penguins in Gastal et al. (Reference Gastal, Mascarenhas, Vanstreels and Ruas2017). Scatter plots and principal component analysis were used to evaluate the morphometric differences of nasal mites from penguin species based on the measurements provided in the literature and those estimated in this study. To better visualize the palptarsus chaetotaxy, an additional six females were mounted and pressed firmly under the cover slip to flatten the palp.
A high-resolution composite image was prepared by merging a series of photographs at 400× magnification with the Photomerge tool of Photoshop CS6 (Adobe Systems Inc., San Jose, CA, USA). To represent the three-dimensional characteristics of the gnathosoma, a series of photographs taken at different focal distances was consolidated into a single image using the Z Project tool (minimum intensity) of ImageJ 1.50. Photoshop CS6 was used to create a series of photographs of the palptarsus chaetotaxy.
Prevalence, mean abundance and mean intensity were estimated (Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997). The χ 2 tests were used to evaluate whether the presence of nasal mites was associated with the following variables: type of carcass (carcass collected in the field, bird deceased during rehabilitation), location (Boulders Beach, Stony Point, other locations), cause of death (infection, debilitation, trauma, unknown), sex (male, female), age group (chick, juvenile, adult), chick stage (P1, P2, P3, P4), body condition (emaciated, thin, moderate, good, fat) and anti-parasitic treatment (not received, single dose, two doses). Significance level was P ⩽ 0.05 for all tests.
Results
Nasal mites were recorded in penguin carcasses collected at Boulders Beach (34°12′S 18°27′E) and Stony Point (34°23′S 18°54′E), as well as in live penguins admitted for rehabilitation from Boulders Beach, Stony Point, Langebaan (33°03′S 18°02′E), Clifton (33°56′S 18°22′E) and Silwerstroomstrand (33°35′S 18°21′E). Infection by nasal mites was recorded in all age groups; the youngest infected bird was a P1 chick (<15 days old; see Sherley et al., Reference Sherley, Waller, Strauss, Geldenhuys, Underhill and Parsons2014) weighing 240 g found dead at Stony Point.
Nasal mite prevalence was 19.6% (21 infected penguins out of 107 examined), mean abundance was 1.16 ± 6.06 mites/examined host (mean ± s.d.) and mean intensity was 5.90 ± 12.86 mites/infected host (ranging from 1 to 60). The mites were occasionally visible by forcefully opening the choanal slit (Fig. 1), but more frequently, they could only be detected by thoroughly dissecting and examining the nasal cavity and paranasal sinuses. Mites were present in the nasal cavity of all the 21 infected birds (mean intensity 3.95 ± 6.34 mites, range 1–28). In some of these birds, mites were also present in the ophthalmic (four birds; mean intensity 9.00 ± 15.34 mites, range 1–32), antorbital (one bird; three mites) and lacrimal sinuses (one bird; two mites).
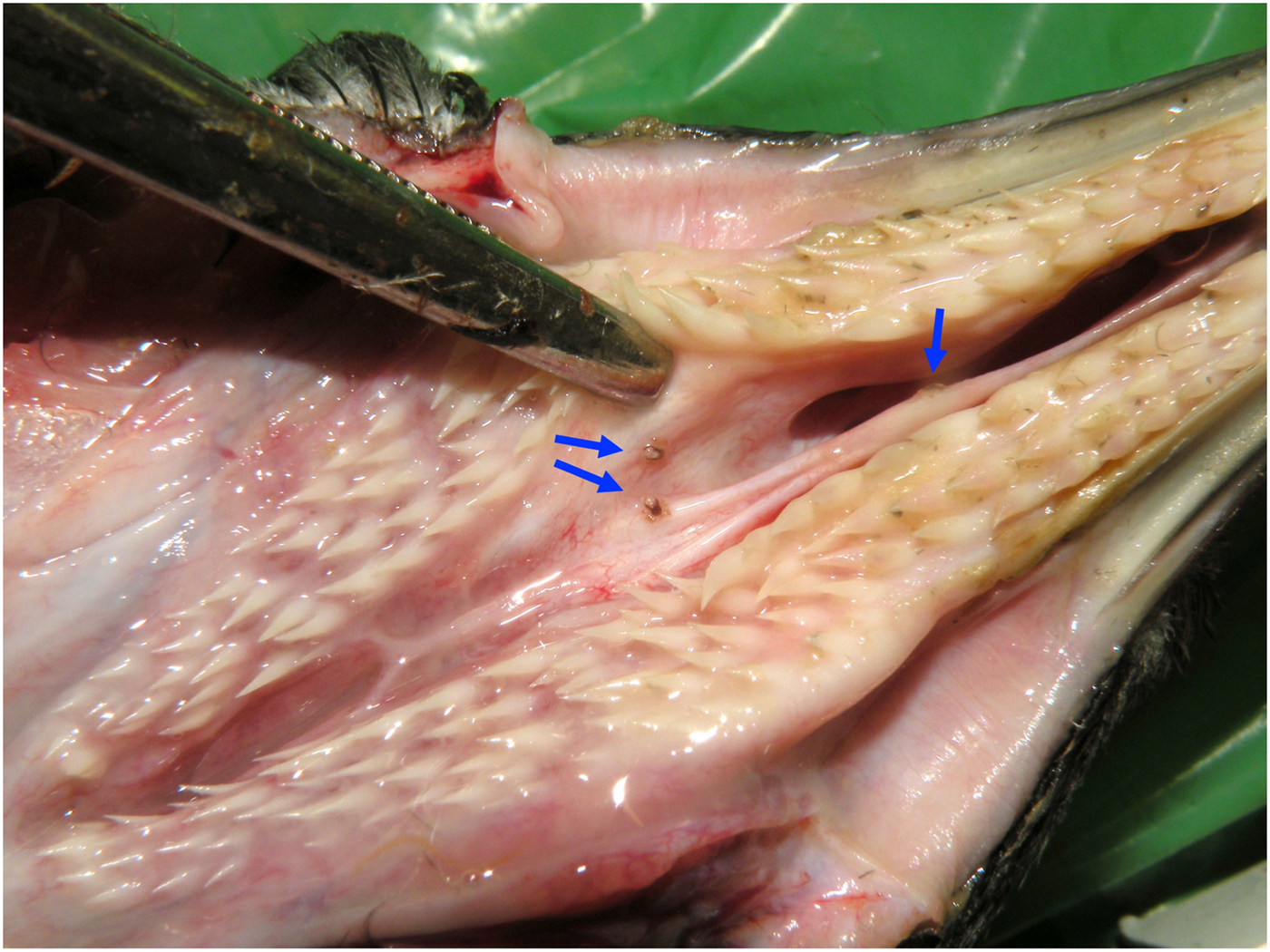
Fig. 1. Nasal mites (blue arrows) in the choanal slit of an African penguin (Spheniscus demersus). Photo: R.E.T. Vanstreels.
The only variable that was significantly associated with the presence of nasal mites was age group (χ 2 = 6.182, df = 2, P = 0.045), with chicks having a lower prevalence (9.8%; 4/41) than juveniles (29.4%; 5/17) and adults (26.7%; 12/45). Mean abundance was 0.09 ± 0.29 in chicks, 4.82 ± 14.74 in juveniles and 0.84 ± 1.73 in adults. All infected chicks had one mite each, and mean intensity was 16.40 ± 25.13 in juveniles (range 1–60) and 3.17 ± 1.99 in adults (range 1–6). The remaining variables tested were not significantly associated with the presence of nasal mites: type of carcass (P = 0.940), location (P = 0.468), cause of death (P = 0.140), sex (P = 0.833), chick stage (P = 0.458), body condition (P = 0.230), anti-parasitic treatment (P = 0.466). There were four cases where penguins had nasal mites in spite of having received anti-parasitic treatment (ivermectin single dose upon admission), however in these cases the birds died <3 days post-admission.
Mites were identified as R. sphenisci sensu lato based on the following characteristics and comparison to illustrations and measurements in Fain and Mortelman (Reference Fain and Mortelmans1959) and Fain and Hyland (Reference Fain and Hyland1963): stigmatal openings dorsal, peritreme absent but stigmatal opening surrounded by a small chitinized ring, genital shield present in the female, chelicerae approximately uniform in diameter with two robust digits, posterior adhesive disk absent, tritosternum absent, sternal shield well developed and approximately rectangle-shaped, dorsal shield diamond-shaped with irregular contours, ventral opisthosomal setae with distinctively thickened bases, anus terminal with two dorsally positioned setae. Ventral opisthosomal setae were counted in 11 individuals and ranged from 31 to 45. Individual measurements of dorsal, sternal and genital shields are provided in Supplementary File S3 and high-resolution photographs are provided in Supplementary File S4. There was a considerable variation in the morphology of the dorsal, sternal and genital shields, as illustrated in Fig. 2, and the measurement of the shields is summarized in Table 1 and compared in Fig. 3. Figure 4 illustrates the palptarsus chaetotaxy; because the three-dimensional nature of this structure renders it difficult to faithfully represent it in a single photograph; a series of photographs is provided in Supplementary File S5.

Fig. 2. Comparison of the morphology of the dorsal, sternal and genital shields of Rhinonyssus mites recovered from penguins.

Fig. 3. Scatter plots of the maximum length and width of the (A) dorsal, (B) sternal and (C) genital shields of Rhinonyssus mites recovered from Pygoscelis and Spheniscus penguins, and (D) plot of the two first principal components of these measurements.

Fig. 4. Comparison of the palp tarsal chaetotaxy of Rhinonyssus mites recovered from penguins. The top two illustrations are taken from the original descriptions of Rhinonyssus sphenisci and R. sphenisci schelli, and the bottom three are from the specimens observed in the present study.
Table 1. Summary of the maximum length and width of the shields (in micrometres) of Rhinonyssus sphenisci sensu lato recovered from African penguins (Spheniscus demersus) (n = 14)

Discussion
We documented, for the first time, the occurrence of nasal mites in African penguins. These mites were relatively frequent in all age classes, albeit at varying prevalence and intensity, and across breeding locations on the Western Cape province of South Africa.
Morphology and taxonomy
Thus far, R. sphenisci has only been reported from Spheniscus penguins (S. humboldti and S. magellanicus), whereas R. schelli has only been reported from Pygoscelis penguins (P. adeliae and P. papua). The two mite species share a range of morphological characteristics, but according to Fain and Hyland (Reference Fain and Hyland1963), seven criteria can be used to differentiate them (bearing in mind that these authors described schelli as a subspecies of R. sphenisci): (1) R. schelli has a smaller body; (2) R. schelli has a shorter and wider sternal shield, with the width being greater than the length (whereas the opposite is true for R. sphenisci); (3) R. schelli has a dorsal shield that is longer and narrower on the posterior part; (4) R. schelli has a shorter genital shield, the posterior edges are wider and the lateral edges are rounder; (5) R. schelli has fewer ventral opisthosomal setae; (6) R. schelli has thicker and more closely spaced palps, a shorter and thicker palp tarsus, and a shorter gnathosomal base; and (7) R. schelli has a distinct chaetotaxy of the palp tarsus, with the outer seta being replaced by a soft spine.
The characteristics mentioned in criteria (1) and (6) can vary substantially depending on whether the mite was oviparous and the pressure exerted by the cover slip when the slide is mounted, and therefore may not be reliable for species identification. The sternal shield (criterion 2) of the specimens in this study had a width that was similar to, or marginally greater, than its length, with extensive individual variability that resulted in morphometric overlap with both R. sphenisci and R. schelli (Fig. 3B). The dorsal shield (criterion 3) of the specimens in this study was generally longer (and wider) than the measurements available for R. schelli, but as for the sternal shield, there was a significant individual variation that resulted in some morphometric overlap (Fig. 3A). It is worth noting that the dorsal shield of some of the specimens in this study presented relatively narrow posterior parts, forming a shape that resembles the illustrations of R. schelli (Fig. 2). The length of the genital shield (criterion 4) was the only morphometric parameter that was unambiguously different between rhinonyssids from Spheniscus penguins (including the specimens in this study) and those from Pygoscelis penguins (Fig. 3C). However, in some of the specimens examined in this study, the posterior and lateral edges of the genital shield were wide and rounded, with an overall shape that resembles the illustrations of R. schelli (Fig. 2). The number of ventral opisthosomal setae (criterion 5) is reportedly 53 in the holotype of R. sphenisci (Fain and Mortelmans, Reference Fain and Mortelmans1959), 45 in R. sphenisci recovered from Magellanic penguins (Gastal et al., Reference Gastal, Mascarenhas, Vanstreels and Ruas2017) and 32–45 in R. schelli (Fain and Hyland, Reference Fain and Hyland1963), whereas the specimens in this study had 31–45 setae.
The original illustrations of the palp tarsus chaetotaxy of R. sphenisci and R. schelli (criterion 7) show that both species have two long setae and a clearly defined round pad with four short setae (Fig. 4). In R. sphenisci, the illustration shows one additional medium-sized lateral seta, and the four setae within the round pad have a similar length and width. On the other hand, in R. schelli, there are an additional two medium-sized lateral setae, and one of the setae within the round pad is larger than the other three. In the specimens examined in this study, the presence of two long setae and two medium-sized lateral setae was consistently observed in most specimens; however, the presence of a round pad with short setae was not clearly discernible (e.g. Fig. 4A and B). Only in one of the 44 examined palp tarsi (from N = 22 female mites), there was a discernible round pad (Fig. 4C), but the setae within it varied considerably in size. It is therefore clear that there is a variation in the chaetotaxy of palp tarsi of the specimens in this study, and that the presence of a round pad is either inconsistent or its discernibility is highly dependent on the orientation of the palp when the specimen is mounted.
The specimens examined in this study therefore present an ambiguous morphology, with some characteristics being more consistent with R. sphenisci, whereas others are more consistent with R. schelli. With the exception of the length of the genital shield, the shield measurements of the specimens in this study overlapped with those of both species. We therefore conservatively classify our specimens as ‘R. sphenisci sensu lato’ (encompassing R. sphenisci sensu stricto and R. schelli). Our observations raise the question of whether Wilson's (Reference Wilson and Gressitt1967) raising of R. sphenisci schelli to specific status was justified. Unfortunately, at present, there is no sufficient information on morphological variability of R. sphenisci and R. schelli from their type hosts to allow for a conclusion on this matter. Future studies evaluating variation in the morphology of rhinonyssid mites from other penguin species and characterizing them genetically would therefore be valuable in order to determine the appropriate taxonomy for these parasites.
While it is unclear whether R. schelli and R. sphenisci are distinct species or represent regional or host-specific variations within a single species, it is worth noting that the existence of morphological differences between the rhinonyssid mites of Pygoscelis and Spheniscus penguins is consistent with the evolutionary history and geographic distribution of these hosts. The penguin lineages that evolved into the Pygoscelis and Spheniscus genera diverged c. 12 million years ago, whereas the species of Pygoscelis diverged c. 6.1 million years ago and those of Spheniscus diverged only c. 1.6 million years ago (Gavryushkina et al., Reference Gavryushkina, Heath, Ksepka, Stadler, Welch and Drummond2017). At present, these genera occupy different geographic regions (Pygoscelis spp. are Subantarctic/Antarctic, whereas Spheniscus spp. are tropical/temperate) and are sympatric in only a few areas (the breeding distribution of S. magellanicus and P. papua overlap in some areas of Southern Chile and the Falkland/Malvinas Islands) (García-Borboroglu and Boersma, Reference García-Borboroglu and Boersma2013). It is therefore reasonable to expect that there is little current opportunity for gene flow among the rhinonyssid mites of penguins from these two genera.
Epidemiology and pathology
We found nasal mites to be remarkably common in African penguins (c. 27% in juveniles and adults) when the nasal cavity and paranasal sinuses were thoroughly dissected and examined. Because the nasal mites were recorded in chick carcasses recovered from breeding colonies, it is clear that the infection naturally occurs at a relatively young age, presumably with mites being transmitted to the chicks when they introduce their beak into their parents’ beaks to obtain regurgitated food (Fig. 5). However, the prevalence of nasal mites in African penguins was significantly lower in chicks (9%) than in juveniles and adults (c. 27%), suggesting that there must be additional opportunities for the transmission of nasal mites in later stages of life, perhaps through courtship behaviour, allopreening, or when penguins sleep in close proximity to one another.

Fig. 5. Parental feeding likely provides opportunities for the transmission of nasal mites to penguin chicks. Photo: R. Hurtado.
The prevalence of nasal mites in juvenile (17.6%) and adult (12.5%) Magellanic penguins in wintering waters in southern Brazil (Gastal et al., Reference Gastal, Mascarenhas, Vanstreels and Ruas2017) is considerably lower than that in juvenile (29.4%) and adult (26.7%) African penguins in this study. This could be related to the fact that the African penguin is a sedentary species, consistently returning to the colony during the non-breeding season, whereas the Magellanic penguin is a migratory species that only returns to the colony in the breeding season (García-Borboroglu and Boersma, Reference García-Borboroglu and Boersma2013). A possible hypothesis is that as the Magellanic penguins spend time away from their breeding colony, they are able to gradually eliminate some of the nasal mites (through their immune response, mucus shedding and sneezing), and by the time they arrive in Brazilian waters, the prevalence has substantially decreased. In contrast, by frequently returning to the colony and closely interacting with other individuals, the African penguin would have more opportunities for re-infection and sustained transmission of nasal mites. Comparative studies of the seasonal distribution of nasal mites in penguin species with different migratory behaviour would therefore be valuable to elucidate the ecology of these parasites. Additional studies would also be important to evaluate if nasal mites are similarly common in African penguins in other regions of the species’ distribution (Namibia and the Eastern Cape province of South Africa).
Most mites parasitized the nasal cavity and sometimes they could be seen without fully dissecting the nasal cavity (e.g. Fig. 1). Although several hundreds of African penguins are admitted for rehabilitation to SANCCOB each year, infection by nasal mites has never been noted in a live African penguin during veterinary procedures or routine handling. A possible explanation is that perhaps the nasal mites remain hidden in the upper respiratory tract in live penguins, and only wander into the choanal aperture after the host's death. It is worth noting that because nasal mites were sometimes located deeply within the paranasal sinuses (in one case as many as 32 mites were found in the ophthalmic sinus), it is possible that other sampling methods such as syringe-flushing the nasal cavity could lead to an underestimation of the infection intensity.
No mites were seen in the trachea, air sacs, lungs and body cavity of African penguins, and it is therefore unlikely that R. sphenisci sensu lato would lead to the same level of respiratory distress as is observed in lung-infecting species such as S. tracheacolum (Fain and Hyland, Reference Fain and Hyland1962; Krantz, Reference Krantz1978). Some of the penguins examined in this study presented a reddened nasal mucosa (Supplementary File S2), suggesting that the parasites can cause mild-to-moderate sinusitis. Because nasal mite infection could only be diagnosed post-mortem, we were unable to evaluate whether it was a mortality risk factor during rehabilitation. However, none of the infected birds died due to air sacculitis or pneumonia, and it would therefore seem that nasal mite infection does not predispose to opportunistic respiratory infections. Nevertheless, even if the presence of the mites in the nasal passages lacks lethal effects, it is safe to presume that the penguins experienced some level of discomfort. Medical treatment of these mites with anti-parasitic agents (e.g. ivermectin) might therefore benefit the welfare of penguins at rehabilitation centres and zoological collections (and perhaps improve the weight gain and recovery during rehabilitation).
Conclusion
The African penguin is currently classified as an endangered species with <3% of the original population remaining and populations are still declining (Crawford et al., Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Geldenhuys, Makhado, Pichegru and Ryan2011; Department of Environmental Affairs, 2013). It is striking that nasal mites may infect as many as one in four juveniles and adults in the Western Cape and yet remained unrecorded until now; this illustrates how there may be other parasites and pathogens that challenge the health of this species but are still unknown. Further studies on the pathogens that potentially impact the recovery of this species will therefore be important in order to successfully protect it.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018000999.
Acknowledgements
We wish to thank the many staff, collaborators, interns and volunteers at SANCCOB.
Financial support
SANCCOB is supported by a wide range of local and international donors including international zoos and aquaria, foundations and trusts, corporates and individuals. This research was supported by the National Research Foundation (PAP, RETV) and by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (HP).
Conflict of interest
None.
Ethical standards
SANCCOB worked under annual permits for the transport and rehabilitation of seabirds from the Department of Environmental Affairs for this study.








