INTRODUCTION
Toxocara canis, the parasitic roundworm of dogs, can infect a number of abnormal (or paratenic) hosts such as mice and humans, due to the widespread dissemination of its ova in the environment. Following ingestion of infective eggs, second-stage larvae hatch in the intestine, and undergo a somatic migration through various organs of the body, though they do not develop to adulthood in the intestine. Toxocariasis may manifest as several clinical syndromes, involving visceral organ invasion and ocular disease (Taylor et al. 1987; Wilder, 1950; Beaver et al. 1952). A phenomenon of ecological significance in mice, and of potential public health significance in man, is that larvae exhibit a predilection for the central nervous system, resulting in an increasing number of parasites migrating to the brain as infection progresses (Sprent, 1955; Lee, 1960; Dunsmore, Thompson and Bates, 1983; Skerret and Holland, 1997). Smith (1991) reported that the migration pathway of larvae in humans and mice is very similar, and that lesions elicited in experimental mouse models and humans are also comparable. Due to these parallels, the mouse model has been widely used to study toxocariasis. Furthermore, rodents caught in the wild have been shown to harbour Toxocara larvae in their tissues and so have been hypothesized to contribute to parasite transmission if consumed by an appropriate definitive host (Dubinsky et al. 1995).
The mouse model has been used to study the impact of Toxocara infection on different aspects of murine behaviour, including baseline activity, social behaviour, and learning and memory. In general, infected mice appear less active and explorative, less responsive to novelty, and less aversive to open areas and predator stimuli than uninfected animals (Burright et al. 1982; Dolinsky, Burright and Donovick, 1981; Cox and Holland, 1998, 2001a,b). The extent of some behavioural changes could be correlated with larval burdens in the brain (Cox and Holland, 1998, 2001a,b) – such changes being more likely due to non-specific side-effects of induced pathology rather than any adaptive manipulation of host behaviour (Holland and Cox, 2001). In previous studies, larval burdens have been shown to vary between individual outbred mice receiving the same inocula (Cox and Holland, 2001a; Skerrett and Holland, 1997), suggesting a possible role of immunity and host genetics in the establishment of cerebral infection.
A large proportion of the above studies assessed murine behavioural alterations using outbred mice as models, with inbred strains receiving less attention. Individual inbred mouse strains, produced by brother-sister mating for at least 20 generations, result in animals genetically identical at >99% of loci. Utilized for immunological and genetic studies, due to the isogenicity within strains and the genetic heterogeneity between strains, they have transpired to be extremely useful tools for studying the interplay between genes and environment (Festing and Fisher, 2000; Beck et al. 2000). Undertaking behavioural studies with inbred mice is attractive since any infection-induced behavioural alterations that could be potentially masked in heterogeneous outbred mice, may appear more pronounced. We acknowledge that this may reduce applicability to wild populations but does increase experimental control. Since there can be great heterogeneity between inbred strains, their use also allows for the comparison and analysis of behavioural alterations in completely divergent strains.
We investigated the differential brain involvement of T. canis in 7 different strains of inbred mice in order to choose a strain susceptible and resistant to larval establishment in the brain. We then investigated the 2 divergent strains in terms of larval accumulation, and behavioural and immune response. This model system will provide a novel opportunity to investigate the genetic factors contributing to brain involvement, and the interaction between host behaviour and infection.
MATERIALS AND METHODS
Mouse maintenance
Male inbred mice were purchased from Harlan UK Limited, at 6–8 weeks old. A total of 140 inbred mice were purchased for Experiment 1 (20 each of the strains A/J, BALB/c, CBA/Ca, C3H/HeN, C57BL/6J, SWR and NIH – chosen to represent a range of haplotypes), and a further 190 mice for Experiment 2 (95 each of BALB/c and NIH). Mice were housed in an animal maintenance room for the duration of the experiments, in standard plastic cages with sawdust bedding. The room was maintained at approximately 22 °C, and operated on a 12 h light/dark photoperiod. Water and pelleted commercial food were supplied ad libitum, and cages were cleaned on a regular basis. The mice were individually weighed on arrival, and randomly assigned, within each strain, to groups of 5 per cage.
Infection of mice
Mice were allowed to acclimatize for 1 week prior to commencing experiments. In Exp. 1, 140 mice were each orally inoculated by stomach intubation with 2000 T. canis ova suspended in 0·2 ml of distilled water. In Exp. 2, 120 mice were intubated with T. canis ova and 70 control mice intubated with distilled water. The inocula were supplied by the National Institute for Public Health and Environment (RIVM), The Netherlands. A dose of 2000 ova was chosen as it has previously been shown to be sufficient to cause differences in behaviour, without causing debilitation (Cox and Holland, 1998). All mice were monitored hourly after infection for 12 h, for signs of post-inocula trauma or ill effects, although none were shown. In Exp. 2 infection was allowed to establish over a 35-day period before behavioural testing commenced. Previous research has shown that T. canis larvae stabilize in the brain of infected mice between days 35 and 42 post-infection (Burren, 1971), and Good (1998) demonstrated that they peaked by day 35 post-infection in outbred CD1-ICR mice.
Larval counts
(i) Experiment 1
Five mice per strain were sacrificed by cervical dislocation on days 7, 14, 35 and 42 p.i. Larvae were recovered from brain, lungs, liver and right hind-leg muscle using the Baermann technique (Pritchard and Kruse, 1982), modified to reduce evaporation. Larvae were enumerated in the brains of all strains of mice in order to identify a susceptible and resistant strain. Once these strains had been chosen (BALB/c and NIH), larvae were enumerated in their liver, lungs and musculature. Enumeration was carried out following the same examination procedure as that described by Cox and Holland (1998).
(ii) Experiment 2
Five mice per strain were sacrificed on days 3, 7, 14, 35 and 97 p.i., and 20 mice per strain were killed on day 42 p.i., following behavioural experiments (10 infected and 10 control – two groups of 5 mice in each case). Larvae were recovered from the brain, liver, lungs and musculature, and enumerated as before. Exp. 2 was originally proposed to incorporate day 120 p.i., however, by day 97 p.i. some of the mice were exhibiting central nervous symptoms (i.e. loss of co-ordination and balance), so for ethical reasons, the mice were sacrificed on this day.
Behavioural testing – Exp. 2
(i) Activity
Activity was determined prior to infection, and again on day 35 p.i. The behaviour of individual mice was measured by observing their activity in the homecage. Six different categories of murine behaviour were measured, based on previous research (Hutchison et al. 1980). (1) Ambulation – walking, running, jumping, rolling, circling, stretching. (2) Grooming – biting coat, licking coat, washing face, scratching, washing genitals, chewing tail. (3) Rearing – standing on cage, straight rearing. (4) Digging – digging bedding backwards, pushing bedding forwards. (5) Immobility – lying on stomach, standing still. (6) Climbing – on bars (upside down).
Testing was performed in silence, with food and water removed, on 2 consecutive days (testing 1 strain per day) between 09.00 h and 19.00 h. Before testing each mouse, the other 4 mice were removed from the homecage, and the cage was placed in the testing location – i.e. in such a position that the mouse could not see the observer. A 5-min habituation period preceded testing to allow the mouse to acclimatize to being alone in the homecage. The behaviour of the mouse was then recorded over a 20-min period on videotape using a Sony Camcorder, suspended above the cage, and linked to a video-monitor, allowing the animal's behaviour to be observed from a discrete position. Analysis of the videos was performed blind, using a True Basic© computer programme (Cox, 1996), which produced an individual summary of behaviour for each mouse over the 20-min period.
(ii) Learning and memory
The water-finding apparatus used in this test was similar to that described by Cox and Holland (2001b), consisting of an open-field enclosure with a Perspex-covered alcove situated on the back wall. The floor of the open field was divided into 15 identical squares to allow measurement of the subject's movement. A water-bottle was suspended from the centre of the alcove ceiling, with its tip 6 cm above the floor. Habituation involved placing each mouse in the left-hand corner of the apparatus and monitoring its behaviour over a 3-min period, making the following measurements: the number of squares crossed in the open field, the number of times the subject touched or sniffed the water-bottle in the alcove, and the number of rears performed. After the habituation period, mice were returned to their homecage, and tested 48 h later, having been deprived of water 24 h pre-test. Testing followed the same pattern as habituation, and the following measurements were taken: the time taken to enter the alcove after beginning exploration, the time taken to touch or sniff the water-bottle after beginning exploration, and the time taken to drink from the water-bottle after beginning exploration. In cases where the mouse did not find the water-bottle, or drink, the animal was given the maximum time latency (180 s). All behaviours were recorded on a Sony camcorder as before.
Statistical analysis
All statistical analysis was carried out at the 95% confidence limit, and data were checked for normality prior to testing using the Levene test of homogeneity.
(i) Experiment 1
Overall effects of day and strain on larval recoveries from the brain were investigated across all 7 strains of mice by means of a 2-way ANOVA, and F-ratios, with degrees of freedom, and P values are given in the text. Separate strain comparisons were made using least significant difference (LSD) post-hoc tests, in order to ascertain a resistant and susceptible strain of mouse (with regard to cerebral larval establishment), and P values are reported in the text. Once the susceptible and resistant strains had been chosen, effects of day and strain on larval recoveries from the brain and visceral organs were investigated using a MANOVA, which allowed for more than one observed variable to be analysed at once – since the number of larvae in the organs may not be independent of each other. Between-factor differences by both day and strain were investigated using LSD post-hoc tests, and the appropriate statistics are quoted where necessary.
Effects of day and strain on the percentages of total larvae recovered from each organ were investigated using a MANOVA with post-hoc tests (data were arcsine square root transformed prior to analysis), and F ratios, with degrees of freedom, and P values are given where appropriate. Separate 2-way ANOVAs with post-hoc tests were then performed for each strain to further investigate the effects of organ and day on larval recoveries, and for each day post-infection to investigate the effects of strain and organ on larval recoveries. Appropriate statistics are reported in the text.
(ii) Experiment 2
A MANOVA with post-hoc tests was used to investigate effects of day and strain on larval recoveries from the brain and visceral organs of BALB/c and NIH mice, and F ratios, with degrees of freedom, and P values are given in the text. As for Exp.1, effects of day and strain on the percentages of total larvae recovered from each organ were investigated using a MANOVA (on transformed data), and statistics are given in text. Separate 2-way ANOVAs with post-hoc tests were then performed for each strain to further investigate the effects of organ and day on larval recoveries, and appropriate statistics are reported.
Effects of strain and infection status on each of the observed behavioural activities were assessed using a MANOVA with post-hoc tests. F ratios, with degrees of freedom, and P values are given in the text. Results from the water-finding task were analysed using a 2-way ANOVA, to determine effects of strain, infection status, and the interaction between these factors, on observed activities, and P values from LSD post-hoc tests are reported. Chi-square analysis was used to assess the numbers of mice that drank compared to those that did not, for both strains, and the appropriate statistics are given.
In order to assess any effect of cage on behavioural observations, nested 2-way ANOVAs were carried out for each set of behavioural data, where cage was a random factor nested within the interaction between strain and infection status. In the case of activity, where multiple comparisons were carried out on essentially the same data set (since each activity is not independent of the others), the Bonferroni method was applied, reducing the critical P value from 0·05 to 0·008.
RESULTS
Larval counts
(i) Experiment 1
Toxocara canis larvae were recovered from the brains of all strains of mice, on each post-mortem date in Exp. 1, and recoveries, as a percentage of inoculum, ranged from 3 to 19% (Table 1). In all strains, larvae had reached the brain in appreciable numbers by as early as day 7 p.i. – the highest numbers being observed in the brains of A/J mice. The general pattern of accumulation in the brain mirrored that of previous studies, i.e. numbers increased over the course of infection, and peaked around day 35 p.i. The number of larvae recovered from NIH mice was generally lower than all other strains, with the highest burden being recovered on day 35 p.i.

A 2-way ANOVA revealed overall significant effects of day and strain on the numbers of larvae in the brain (day: F3,107=17·0, P≤0·0001; strain: F6,107=7·3, P≤0·0001). Post-hoc tests demonstrated that larval recoveries from BALB/c mice differed significantly from NIH mice on days 7, 35 and 42 p.i. (P=0·05, 0·02, and ≤0·0001, respectively) (Fig. 1), and larval recoveries from CBA/Ca mice also differed significantly from NIH mice on days 14, 35 and 42 (P=0·03, 0·01, and ≤0·0001, respectively). Since NIH mice carried the lowest larval burden overall, these mice were chosen as the resistant strain to T. canis infection. BALB/c mice were chosen over CBA/Ca mice as the susceptible strain, since these mice had a statistically higher larval burden in the brain than NIH mice on days 7, 35 and 42 p.i., and they had previously been reported to be more susceptible to toxocariasis.

Fig. 1. Mean number (±S.E.M.) of larvae recovered from the brains of Toxocara canis-infected BALB/c and NIH mice in Exp. 1.
Results from a MANOVA demonstrated significant effects of day and strain on larval recovery from all organs of BALB/c and NIH mice (day: F3,93=2·6, P=0·004; strain: F1,29=4·5, P=0·006). Highest larval recovery from the lungs of both strains of mice occurred on day 7 p.i. (mean±S.D.: 44±17·10 and 24±11·40, BALB/c and NIH mice respectively) and then decreased as infection progressed, although overall numbers were higher in BALB/c mice. LSD post-hoc tests demonstrated a significantly higher lung burden in BALB/c mice on day 7 p.i. compared with NIH mice (P=0·005). Recoveries on all other days were similar between strains. The number of larvae recovered from the liver increased over infection, with both strains carrying similar burdens on days 7, 35 and 42 p.i. The number of larvae recovered from the musculature was generally lower than the other organs, and varied throughout infection. Post-hoc tests demonstrated that BALB/c mice had a significantly higher larval burden in their muscle than NIH mice on day 42 p.i. (P=0·006).
When total numbers of larvae recovered from each mouse were compared between strains, it was noted that more larvae were recovered from BALB/c than NIH mice. Larval recoveries from each organ were then investigated as a percentage of the total recovery. On all days post-infection, the vast majority of larvae were recovered from the brains of BALB/c and NIH mice compared to all other organs. Results of a MANOVA revealed significant effects of day and strain on larval recoveries (day: F3,93=2·99, P=0·001; strain: F1,29=3·25, P=0·026). Separate 2-way ANOVAs for each strain revealed significant effects of organ and the interaction between organ and day, on the percentage of larvae recovered (BALB/c: organ: F3,64=502·25, P≤0·0001; organ×day: F9,64=5·43, P≤0·0001; NIH: organ: F3,64=145·2, P≤0·0001; organ×day: F9,64=3·00, P=0·005). Post-hoc tests revealed that there were significantly more larvae recovered from the brain than in the visceral organs, on all days post-infection, for both strains (P≤0·0001, for all comparison). Post-hoc tests from separate 2-way ANOVAs for each day post-infection revealed that a significantly lower percentage of larvae were recovered from the brains of NIH mice on days 35 and 42 post-infection, compared with BALB/c (day 35 p.i.: P=0·04; day 42 p.i.=0·007). There were fewer larvae recovered on days 7 and 14 p.i. also, although the differences did not reach statistical significance.
(ii) Experiment 2
Larvae were recovered from the brains of BALB/c and NIH mice on all days post-infection in Exp. 2. Although the recoveries, as a percentage of inoculum, were similar to Exp. 1 (ranging from 3 to 18%), the individual counts for each day p.i. were very different to the first experiment, and, in fact, the strains no longer appeared to be divergent. The counts for both strains were very similar, with larval numbers increasing in both strains between days 3 and 7 p.i., then decreasing slightly by day 14 p.i., before increasing again. On day 42 p.i. there were more larvae in the brains of NIH mice compared with BALB/c mice (mean±S.D.: 350·8±35·5 and 277±64·5, respectively). By day 97 p.i., however, more larvae were in the brains of BALB/c mice, and larval numbers had decreased in the brains of NIH mice (mean±S.D.: 304·4±113·1 and 250·7±42·9, respectively).
Results of a MANOVA showed a significant effect of day, but not strain, on larval recoveries from all organs of BALB/c and NIH mice (day: F5,168=4·45, P≤0·0001). LSD post-hoc tests demonstrated no significant differences in larval burdens in the brains of BALB/c and NIH mice. Larval recoveries from the liver and lungs were very similar between strains on all days post-infection. Highest recoveries from the liver occurred on day 3 p.i. (mean±S.D.: BALB/c: 228±85·56; NIH: 199±164·22), and then decreased dramatically by day 7 p.i. (mean±S.D.: BALB/c: 35±14·14; NIH: 47·5±11·90), and remained low for the duration of infection. A similar pattern was seen in the lungs – with highest recoveries from both strains being made on day 3 p.i., and numbers decreasing by day 7 p.i., and remaining low throughout infection. Larval recoveries from the musculature varied throughout infection, and numbers peaked in BALB/c mice on day 7 p.i., and in NIH mice on day 14 p.i. Numbers then decreased over infection. Post-hoc tests revealed that BALB/c mice carried a significantly higher larval burden in the muscle than NIH mice on day 7 p.i. (P=0·03).
On the whole, the total number of larvae recovered from mice over the course of infection was similar in both strains, but generally lower than Exp. 1. As for Exp. 2, larval recoveries from each organ were investigated for each strain as a percentage of total larvae recovered, and are shown in Figs 2 and 3. It is clear to see that in early infection, the majority of larvae were recovered from liver and lungs and, as infection progressed, the percentage of larvae recovered from these organs decreased and the majority of larvae were recovered from the brain. Results of a MANOVA revealed significant effects of day, strain and the interaction between these factors, on the percentage of larvae recovered (day: F5,168=6·17, P≤0·0001; strain: F1,39=12·18, P≤0·0001; day×strain: F5,168=1·87, P=0·017). Post-hoc tests demonstrated that BALB/c mice carried a significantly higher percentage of larvae in the liver on days 35 and 42 p.i. compared with NIH mice (P=0·02, for both comparisons). More notably, post-hoc tests demonstrated that NIH mice carried a significantly lower percentage of larvae in the brain on days 14, 35 and 97 p.i., compared with BALB/c mice (day 14 p.i.: P=0·02; day 35 p.i.: P=0·003; day 97 p.i.: P≤0·0001) (Fig. 4).

Fig. 2. Mean percentage (±S.E.M.) of total larvae recovered from each organ of Toxocara canis-infected BALB/c mice in Exp. 2.

Fig. 3. Mean percentage (±S.E.M.) of total larvae recovered from each organ of Toxocara canis-infected NIH mice in Exp. 2.

Fig. 4. Mean percentage (±S.E.M.) of total larvae recovered from the brains of Toxocara canis-infected BALB/c and NIH mice in Exp. 2.
Separate 2-way ANOVAs for each strain revealed significant effects of day (in the case of NIH mice only), organ, and the interaction between organ and day, on the percentage of larvae recovered (BALB/c: organ: F3,92=435·13, P≤0·0001; organ×day: F15,92=34·14, P≤0·0001; NIH: day: F5,76=4·37, P=0·0015; organ: F3,76=226·35, P≤0·0001; organ×day: F15,76=24·98, P≤0·0001). Post-hoc tests revealed that a significantly higher percentage of larvae were recovered from the liver and lungs of both strains on day 3 p.i., compared with the muscle and brain (P≤0·0001, for all comparisons). The tests also revealed that from day 7 p.i. onwards, a significantly higher percentage of larvae were recovered from the brains of both strains of mice compared with the other organs (P≤0·0001, for all comparisons).
Behavioural testing
(i) Activity
The activity of BALB/c and NIH mice differed prior to infection (data not shown). BALB/c mice spent most of their time climbing, digging, and rearing, followed by ambulating and grooming. They spent very little time immobile. NIH mice spent most of their time rearing, and rest of the time was split similarly between ambulating, climbing and digging. Like BALB/c mice, NIH mice spent very little time immobile. Post-infection, BALB/c mice spent similar amounts of time ambulating, digging and rearing, much less time climbing and grooming, and were more immobile than pre-infection. NIH mice spent similar amounts of time ambulating, rearing, grooming and climbing, although in the latter activity, this was much reduced in infected mice. They spent much less time digging, and were more immobile, although this was more pronounced in the infected mice. Due to these differences between and within strains, statistical analysis for activity was performed on post- minus pre-infection data, and differences in activity are shown in Figs 5 and 6.

Fig. 5. Mean time difference (±S.E.M.) spent at each activity between pre- and post-infection, in BALB/c mice.

Fig. 6. Mean time difference (±S.E.M.) spent at each activity between pre- and post-infection, in NIH mice.
Fig. 5 demonstrates that, overall, BALB/c mice spent more time ambulating, rearing and had longer periods of immobility after infection, and less time climbing and grooming compared with pre-infection. The time spent digging differs between control and infected mice – with infected mice spending less time at this activity. Fig. 6 demonstrates that, overall, NIH mice spent slightly more time grooming, less time digging, and much more time immobile, after infection – although the latter activity differs between control and infected mice. Time spent ambulating, climbing and rearing differs between control and infected mice, with infected mice spending less time climbing and more time rearing and ambulating. Results of a MANOVA demonstrated overall effects of strain, infection status and the interaction between these two factors on activity levels (strain: F1,30=2·75, P=0·037; infection status: F1,30=10·61, P≤0·0001; strain×infection status: F1,30=4·88, P=0·002). Post-hoc tests revealed that both infected BALB/c and NIH mice spent significantly more time ambulating compared with control mice (BALB/c: P=0·005; NIH: P=0·003). However, infected NIH mice were also significantly more immobile than their control counterparts (P=0·002), and spent significantly less time climbing (P=0·018), whereas there were no significant differences between control and infected BALB/c mice for these activities. Infected NIH mice spent less time digging and grooming, and more time rearing compared with control mice, but the differences were not significant. Infected BALB/c mice also spent less time digging and grooming than control mice, but the differences were not significant.
Results of separate nested 2-way ANOVAs carried out for each activity, with cage as a random factor nested within the interaction between strain and infection status, revealed a significant effect of cage on the level of rearing observed (P=0·002).
(ii) Learning and memory
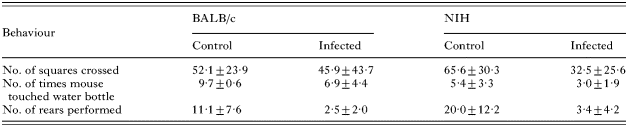
During habituation (Table 2), significant differences were noted between, and within, both strains of mice for some of the measurements recorded. Post-hoc tests, from separate 2-way ANOVAs for each observation, revealed that infected NIH mice crossed significantly fewer squares in the open field (P=0·037), and performed significantly fewer rears (P≤0·0001), compared with control mice. They also touched/sniffed the water-bottle significantly less often than infected BALB/c mice (P=0·012). Infected BALB/c mice also performed significantly fewer rears than their control counterparts (P=0·018), and touched/sniffed the water-bottle fewer times than the control mice, although this difference did not reach significance (P=0·06). Results of separate nested 2-way ANOVAs for each activity revealed a significant effect of cage on the number of times the water-bottle was touched/sniffed (P=0·04).
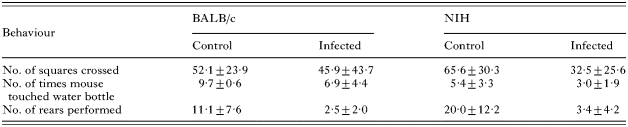
During the testing period, 9 out of 10 control BALB/c mice drank from the water-bottle, compared with only 2 out of 10 infected BALB/c mice (χ2=9·899, P=0·002, D.F.=1), and 6 out of 10 control NIH mice drank, compared with 3 out of 8 infected NIH mice (χ2=0·900, P=0·343, D.F.=1). Post-hoc tests, from 2-way ANOVAs for each activity, revealed that infected BALB/c mice took longer to enter the alcove, and find the water-bottle, after beginning exploration, compared with control mice, although these differences were not significant (Fig. 7; time taken to enter alcove: P=0·121; time taken to find water-bottle: P=0·06). There were significant effects of infection status, and the interaction between strain and infection, on the time it took for mice to start drinking after beginning exploration (infection status: F1,34=16·73, P=0·0002; strain×infection status: F1,34=6·17, P=0·018). Post-hoc tests revealed that infected BALB/c mice took a significantly longer amount of time to drink from the water-bottle, compared with control mice (P≤0·0001), suggesting some degree of memory impairment (Fig. 7). Although there were no significant differences between control and infected NIH mice, infected mice generally took longer to locate the water-bottle and drink from it compared with control mice.
Results of separate nested 2-way ANOVAs for each activity revealed no significant effect of cage on behavioural observations (P>0·05, for all activities).

Fig. 7. Mean time (±S.E.M.) taken by control and infected BALB/c mice to carry out the various observations recorded during the water-finding, task-testing period (* comparison of infected and control mice, P≤0·0001).
DISCUSSION
The most striking result from this study was that Toxocara canis infection led to some degree of memory impairment in infected inbred mice. Spatial awareness, and the ability to use visual cues from the surrounding environment to remember locations of specific resources, is vital to the survival of small rodents. In this study, learning and memory was assessed in T. canis-infected mice using a water-finding task. In general, infected animals showed a reduction in exploratory behaviour and spatial awareness, taking longer to enter the alcove (after a period of water-deprivation), and locate and drink from the water-bottle, compared with control animals. These findings were most pronounced in infected BALB/c mice, with animals taking significantly longer to drink from the water-bottle, after beginning exploration, compared with control mice. Infected BALB/c mice also took longer to enter the alcove and locate the water-bottle in comparison to control mice, but these differences did not reach statistical significance. Although the time taken to enter the alcove did not differ between infected and control NIH mice, infected NIH mice did take a longer amount of time to locate the water-bottle and drink from it, compared with controls, although these differences did not reach statistical significance. A possible explanation for this may be that the drive to enter the alcove was not influenced by the water source specifically but rather the need for mice to seek narrow places of shelter/safety.
An alternative explanation for infected BALB/c mice taking longer to enter the alcove, and drink from the water bottle, is general lethargy – since parasitic infection can render a subject less energetic than uninfected individuals. Infected BALB/c mice could have been more sluggish than uninfected mice and therefore took longer to complete any of the tasks set in the experiment. However, considering the reported activity of these mice, it is evident that although infected BALB/c mice were less active for some recorded behaviours, they were more ambulatory than their control counterparts, and spent less time immobile – suggesting that they were not lethargic. It was also noted that once each subject was returned to the homecage after testing, they displayed an eagerness to drink – indicating that the length of time taken to locate the water source was unlikely due to thirst suppression. In a related experiment, learning and memory was assessed in control and infected BALB/c and NIH mice under the same experimental conditions, although using modified apparatus (Flanagan, 2005). Instead of the open-field apparatus having only one alcove containing the water-bottle, the modified apparatus utilized a further 2 separate alcoves – making it possible to test whether or not the infected subject could correctly relocate the water source after a period of habituation. The results of the related study were similar to the results obtained in the present study, and the most notable finding was that infected BALB/c mice took significantly longer to drink from the water-bottle than uninfected control mice. They also took longer to enter the correct alcove, and locate the water-bottle within, compared with controls, although these differences were not significant. The idea that T. canis-infected mice have significantly reduced spatial awareness and exploratory skills during infection carries implications for their survival in the wild, and also implications for cognitive development in children.
Previous studies have reported the possibility of learning and memory retardation due to T. canis infection in outbred mice, but the levels of impairment have rarely reached statistically significant levels (Dolinksy, Burright and Donovick, 1981). In a study using similar apparatus to that used in this study, Cox and Holland (2001b) demonstrated that outbred LACA mice in moderate and high larval dose groups showed a greater latency to enter the alcove, locate the water-bottle and drink from it, compared with control mice, suggesting some degree of memory impairment, although the differences were not statistically significant. Olson and Rose (1966) reported that the ability of rats to solve maze problems was impaired as a result of T. canis infection, and this effect was dose dependent, with rats infected with 20000 ova making significantly more errors than control rats. Effects on spatial learning have also been reported in infections with other parasites (Kershaw, Leytham and Dickerson, 1959; Kavaliers and Colwell, 1995; Kavaliers, Colwell and Galea, 1995), with infected subjects displaying a retardation of ability to learn in comparison with controls. Witting (1979) assessed learning and memory in T. gondii-infected mice using a series of maze experiments, and reported severe memory impairment in infected animals compared to controls. The level of impairment was also significantly correlated with the number of tissue cysts present in the brain.
Of those studies cited above using mice, behavioural testing was carried out using outbred strains, which could potentially explain the absence of statistical significance in the results. In the present study, we used inbred mice, genetically identical within strains, which could explain why T. canis-induced behavioural alterations were more pronounced. The evidence of memory impairment in T. canis-infected mice in this study is of particular interest since it was evident in BALB/c mice – the chosen susceptible strain – suggesting that heavier cerebral larval burdens lead to significantly more pronounced behavioural alterations.
Exp. 1 identified BALB/c and NIH mice as susceptible and resistant strains (respectively) to cerebral T. canis infection, with BALB/c mice carrying a significantly higher larval burden in the brain, compared with NIH mice, on days 7, 35 and 42 p.i. The choice of strains was supported by previous studies where BALB/c mice have been reported to be more susceptible to T. canis infection (Bardon, Cuellar and Guillen, 1994; Epe et al. 1994), and NIH mice have been reported to show a relatively higher level of resistance to T. canis infection than outbred CD1 mice (Abo-Shehada and Herbert, 1989), and have been reported to clear infections with Trichuris muris and Trichinella spiralis more rapidly than other inbred strains (Lee and Wakelin, 1982; Alizadeh and Wakelin, 1983; Dehlawi and Goyal, 2003). In Exp. 2, larval burdens in the brains of the two chosen strains were similar and they no longer appeared divergent. However, when larval recoveries were expressed as percentages of total burdens, it was evident that a significantly higher percentage of total larvae were recovered from the brains of BALB/c mice compared with NIH mice, on days 14, 35 and 97 post-infection, indicating that these mice are still more susceptible to cerebral infection. These results suggest that actual larval burdens may not be a reliable indicator of susceptibility and resistance to cerebral toxocariasis, and that perhaps a more suitable end-point characteristic would be percentage of total larvae, since it takes into account any variation in the numbers of eggs that hatched successfully after inoculation.
The behavioural changes observed in this study may be due to a number of factors related to larval burden. It is possible that the position of larvae in the brain may influence the types of behaviours altered. Previous studies have reported the presence of larvae in the telencephalon (Good, Holland and Stafford, 2001) and cerebellum (Burren, 1971) of infected mice, which is noteworthy considering these areas of the brain are associated with learning and memory, and co-ordination and control of voluntary movement (respectively). The presence of larvae in the cerebellum is also of relevance to the mice in this study, as by day 97 p.i., infected BALB/c mice were exhibiting central nervous symptoms – i.e. lack of co-ordination and balance.
The quality and quantity of immune response elicited by the infected host may be another factor influencing the magnitude of behavioural changes. Although the systemic immune response to T. canis infection has been widely studied (Del Prete et al. 1991; Wang et al. 1995; Kuroda et al. 2001), the immune response in the brain has received no attention. Studies on other parasitic infections of the CNS have demonstrated that, often, cytokines produced in response to infection are responsible for the induction of pathology and neurodegeneration (Restrepo et al. 1998; Brown et al. 1999; Gazzinelli et al. 1993). It could be, therefore, that infected BALB/c mice in this study are displaying the consequences of an immune response produced against the parasite. Immunological data obtained from the present study highlight differences in the humoral and cellular responses between the strains, and suggest a link with behavioural changes, but this will be reported elsewhere.
In conclusion, this study has demonstrated that cerebral infection with T. canis leads to some level of memory impairment in infected murine hosts. These results are interesting, not only because of the implications they may carry for the animal's survival in the wild, but also because of the wider implications with respect to human infection. Evidence for cerebral toxocariasis in humans is patchy but there are some indications that larval involvement in the human brain may have some subtle public health implications (Hill, Denham and Scholtz, 1985; Marmor et al. 1987; Magnaval et al. 1997). The clinical significance of cerebral infection remains unclear, however, since there are usually other factors involved when the patient presents – such as epilepsy, dementia, and mental retardation (Kaplan et al. 2004; Magnaval et al. 1997; Glickman et al. 1979). Nevertheless, given the results of this study, and the others cited, it is evident that cerebral T. canis infection can lead to behavioural alterations, and although these results are in rodents, they still carry implications for human health, especially given the high seroprevalence of T. canis in some countries (Worley et al. 1984; Thomson et al. 1986; Holland et al. 1995), and the close partnership between dog and man. Furthermore, this model system may also provide insights into the impact of chronic geohelminth infection on cognitive development, something that has proved to be very difficult to investigate in human subjects (Holland and Hamilton, 2005).
The authors would like to thank Dr M. Brown and Mr S. Wright for comments on earlier drafts of this manuscript. We also thank M. O'Regan for expert statistical advice, and B. Teahan for help with the larval counts. This project was funded by a Basic Research Award from the Irish Research Council for Science, Engineering and Technology (IRCSET).