INTRODUCTION
Giardia and Cryptosporidium are protozoan parasites that are considered as an important cause of diarrhoea worldwide. In industrialized countries Giardia is detected in up to 14% (Homan and Mank, Reference Homan and Mank2001) and Cryptosporidium in up to 2·2% (Guerrant, Reference Guerrant1997) of immunocompetent patients with diarrhoea. Prevalence peaks in young children, due to lower hygienic habits in this age group, the immature immune system and first contact with the parasite (Griffiths, Reference Griffiths1998; Hoque et al. Reference Hoque, Hope, Scragg and Kjellström2003). Next to age, several other risk factors for Cryptosporidium and Giardia infections have been identified, including contact with farm animals or with infected persons, swimming and travelling abroad (Hoque et al. Reference Hoque, Hope, Kjellstrom, Scragg and Lay-Yee2002; Hunter et al. Reference Hunter, Hughes, Woodhouse, Syed, Verlander, Chalmers, Morgan, Nichols, Beeching and Osborn2004).
Human cryptosporidiosis is mainly due to infections with either the human specific C. hominis or the zoonotic C. parvum. Epidemiologic research following outbreaks has suggested the existence of a human-specific cycle next to a zoonotic cycle of C. parvum. Subgenotype analysis at the 60 kDa glycoprotein (GP60) locus, confirmed that both human-specific and zoonotic subgenotypes occur within C. parvum (Peng et al. Reference Peng, Matos, Gatei, Das, Stantic-Pavlinic, Bern, Sulaiman, Glaberman, Lal and Xiao2001). Human giardiosis is caused by infection with either G. duodenalis assemblage A or B, with the latter being more prevalent (Amar et al. Reference Amar, Dear, Pedraza-Diaz, Looker, Linnane and McLauchlin2002; van der Giessen et al. Reference van der Giessen, de Vries, Roos, Wielinga, Kortbeek and Mank2006). Furthermore, both assemblages have zoonotic potential (Wielinga and Thompson, Reference Wielinga and Thompson2007).
To elucidate the zoonotic potential, the genetic heterogeneity and the transmission pathways, multilocus genotyping is advocated as a standard approach for molecular epidemiology of Cryptosporidium and Giardia isolates (Cacciò et al. Reference Caccio, Thompson, McLauchlin and Smith2005; Wielinga and Thompson, Reference Wielinga and Thompson2007), in combination with epidemiological data. Therefore, multiple genes have been targeted for the molecular characterization of Cryptosporidium and Giardia in the present study. As most data on molecular epidemiology of Cryptosporidium and Giardia are obtained after an outbreak, isolates from non-outbreak related cases of diarrhoea from 4 different medical centres were selected, to avoid a bias towards a limited number of infection sources.
MATERIALS AND METHODS
Origin of the samples
Stool samples from 373 human patients originating from the province of East Flanders (Belgium), showing clinical symptoms (including abdominal pain and/or diarrhoea), and presenting themselves at their general practitioner were collected. Samples were stored at 4°C and examined for the presence of Cryptosporidium oocysts and Giardia cysts within 48 h, using the commercial MERIFLUOR® Cryptosporidium/Giardia immunofluorescence assay (IFA; Meridian Diagnostics Inc., Cincinnati, Ohio), as previously described (Geurden et al. Reference Geurden, Claerebout, Vercruysse and Berkvens2004). A sample was considered to be positive when at least 1 Cryptosporidium oocyst or 1 Giardia cyst were found on the IFA slide.
Additionally, all samples were examined for bacterial and other parasitic pathogens according to standard techniques. In brief, the sample was inoculated onto appropriate culture plates (CIN agar bioMérieux 43203 for Yersinia spp.; Hektoen enteric agar bioMérieux 43111 for Salmonella and Shigella spp.; EMB agar for enterobacteriaceae such as Escherichia spp.; Campylobacter agar bioMérieux 43361; Clostridium difficile agar bioMérieux 43213). For detection of Salmonella and Shigella spp. samples were also plated in a Hektoen enteric agar bioMérieux 43111 after previous enrichment culture in a Rappaport broth bioMérieux 42073. For further typing of isolated bacteria TSI agar (BD 221039) ureum (bioMérieux 55752), Api 20 E and agglutination tests were used. Furthermore, all samples were concentrated using ether/formalin concentration and examined microscopically at 400x magnification for eggs, (oo)cysts and/or trophozoites of gastrointestinal parasites.
All patients received a written questionnaire that included questions about the source of drinking water (tap or bottle), exposure to recreational water (outdoor and indoor), recent foreign travel and contact with animals or a symptomatic person. Animals were described as livestock or pets (cat or dog). For risk factors odds ratios with 95% confidence interval (CI) were calculated.
An additional 59 Giardia positive and 20 Cryptosporidium positive samples, not incorporated in the epidemiological study, were provided by 3 hospitals in Ghent and withheld for genotyping. Again these samples were obtained from human patients showing clinical symptoms (including abdominal pain and/or diarrhoea), and presenting themselves at their general practitioner.
Molecular identification
DNA was extracted using the QIAamp® Stool Mini Kit (Qiagen) according to the manufacturer's instructions, incorporating an initial step of 3 freeze-thaw cycles (freezing in liquid nitrogen for 5 min and heating at 95°C for 5 min) in the protocol to maximize disruption of (oo)cysts. For the identification of Cryptosporidium DNA, previously described primers targeting the 70 kDa heat shock protein (hsp-70) gene were used (Spano et al. Reference Spano, Putignani, McLaughlin, Casemore and Crisanti1997). For the subgenotyping of C. parvum positive samples, the 60 kDa glycoprotein (GP60) was targeted (Peng et al. Reference Peng, Matos, Gatei, Das, Stantic-Pavlinic, Bern, Sulaiman, Glaberman, Lal and Xiao2001). For the identification of Giardia DNA the β-giardin gene (Lalle et al. Reference Lalle, Pozio, Capelli, Bruschi, Crotti and Caccio2005), the triose phosphate isomerase (TPI) gene (Sulaiman et al. Reference Sulaiman, Fayer, Bern, Gilman, Trout, Schantz, Das, Lal and Xiao2003), and the glutamate dehydrogenase (GDH) gene (Homan et al. Reference Homan, Gilsing, Bentala, Limper and van Knapen1998) were used. In all above-mentioned PCR reactions bovine serum albumin (BSA) was added to a final concentration of 0·1 μg BSA/μl reaction mixture. Amplification products were visualized on 1·5% agarose gels with ethidium bromide. A positive (plasmid DNA) and negative (PCR water) control sample were included in each PCR reaction. PCR products were purified using the Qiaquick PCR purification kit (Qiagen) and fully sequenced using the Big Dye Terminator V3.1 Cycle sequencing Kit (Applied Biosystems). Sequencing reactions were analysed on a 3100 Genetic Analyzer (Applied Biosystems) and assembled with the program Seqman II (DNASTAR, Madison WI, USA). To determine the (sub)genotype the fragments were aligned with the homologous sequences available in the GenBank database, using MegAlign (DNASTAR, Madison WI, USA).
For the species-specific amplification of the Giardia TPI gene the primary PCR reaction was performed as described by Sulaiman et al. (Reference Sulaiman, Fayer, Bern, Gilman, Trout, Schantz, Das, Lal and Xiao2003). In the nested PCR reaction, new species-specific primers were used: the G. duodenalis assemblage A specific primers to amplify a 332 bp PCR product were previously described (Geurden et al. Reference Geurden, Geldhof, Levecke, Martens, Berkvens, Casaert, Vercruysse and Claerebout2007). The G. duodenalis assemblage B specific primers (Bf: 5′-GTT GTT GTT GCT CCC TCC TTT-3′ and Br: 5′-CCG GCT CAT AGG CAA TTA CA-3′) were designed based on Genbank Accession numbers DQ789114, AY228628 to AY228638, and AY368162 to AY368171, for the amplification of a 400 bp PCR product. The reaction mixture for the secondary species-specific PCR consisted of a master mix containing 0·25 μl of Taq DNA polymerase, 0·5 μl of dNTP mixture, BSA to a final concentration of 0·1 μg BSA/μl reaction mixture, 2·5 μl of standard Taq buffer, 10 pm of each primer and 2·5 μl of template DNA in a total volume of 25 μl. Subsequent steps were initial denaturation for 10 min at 94°C, followed by 35 cycles of denaturation for 45 sec at 94°C, annealing for 45 sec at 62°C and an extension for 45 sec at 72°C. Amplification products were subsequently visualized on 1·5% agarose gels with ethidium bromide. A positive (plasmid DNA) and negative (PCR water) control sample was included in each PCR reaction. To verify the specificity of the species-specific primers, a subset of the samples was selected for sequencing, both from the G. duodenalis assemblage A and assemblage B positive samples. The PCR products were purified and sequenced as previously described, and compared with homologous sequences available in the GenBank database, or aligned with Clustal W.
RESULTS
Occurence of Cryptosporidium and Giardia in patients with diarrhoea
Campylobacter was found to be the most prevalent pathogen (32·25%) in the 373 patients with diarrhoea. Furthermore, 15 patients (4·01%) were positive for Giardia and 5 patients (1·34%) were positive for Cryptosporidium. None of the patients had a mixed Cryptosporidium and Giardia infection, and none of the patients positive for Cryptosporidium or Giardia were positive for any other pathogen. Cryptosporidium was diagnosed both in young children (n=3) and in adults (n=2), whereas all but 1 of the Giardia-positive patients were adults. No significant correlation between age and infection was found in the present study. Other infective agents that were found in the present study include Salmonella (3·74%), Endolimax nana (1·34%), Escherichia coli (1·34%) and Entamoeba spp. (1·07%). In less than 1% of the patients, Blastocystis, Clostridium difficile or Taenia spp. were identified.
In total, 49% of the patients (n=182) responded to the questionnaire, including all 5 Cryptosporidium-positive patients and 11 out of 15 Giardia-positive patients. None of the patients reported direct contact with livestock or with symptomatic patients. Contact with pet animals did not differ between infected and non-infected patients. All patients reported the consumption of bottled water rather than tap water. Exposure to recreational water was more frequently reported in Giardia-positive patients (45%: both outdoor and indoor) than in the negative patients (25%). For Cryptosporidium, there was a significant (P<0·05; OR 10·809: 1·178–99·191) correlation between exposure to recreational water in a public indoor swimming pool and infection. Travel to foreign countries was more frequent in Cryptosporidium and Giardia-positive patients than in negative patients, although not significantly.
Molecular identification
DNA extraction and subsequent genotyping was successful for 72 Giardia and 24 Cryptosporidium positive samples. However, not all samples could be characterized at each of the targeted loci (Tables 1, 2 and 3).
Table 1. Molecular characterization of Cryptosporidium isolates at the heat shock protein (HSP-70) and the 60 kDa glycoprotein gene (GP60)
(Species, subgenotypes and GenBank Accession numbers are indicated. NA, no amplification.)

Table 2. Multilocus genotyping of Giardia duodenalis assemblage B isolates at the β-giardin gene (β-giardin), triose phosphate isomerase gene (TPI) and glutamate dehydrogenase gene (GDH)
(All samples were amplified using a novel TPI species-specific PCR to detect mixed A and B infections (TPI-mixed). na, no amplification; * 1 single nucleotide polymorphism.)
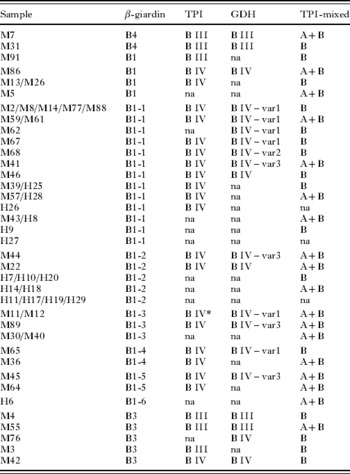
Table 3. Subgenotypes at the β-giardin locus identified in the present study
(Polymorphic sites are numbered with reference to the full-length gene. Bold characters refer to polymorphism that define new subgenotypes. The positions marked in grey are described for the first time in the present study.)

The results of the molecular characterization for Cryptosporidium are presented in Table 1. A slight majority of the Cryptosporidium positive samples was identified as C. hominis (54·2%) but C. parvum was also commonly identified (45·8%). Different subgenotypes within C. hominis and C. parvum were identified by sequence analysis of the GP60 gene, including a recently described C. parvum subgenotype (IIdA16G1; GenBank Acc. no. EU847735).
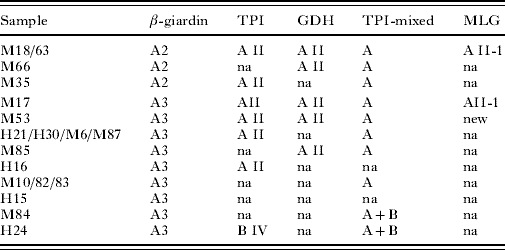
In the case of G. duodenalis, genotyping based on sequencing showed that 74·4% of infections were due to assemblage B, and 25·6% to assemblage A. At the β-giardin gene, sequencing showed that assemblage B isolates corresponded to both previously described as well as to 6 new subgenotypes (Table 2). The single nucleotide substitutions defining the β-giardin gene subgenotypes are shown in Table 3. Because the 6 new subgenotypes were most similar to the previously described subgenotype B1, they were designated as B1-1 to B1-6 (Genbank Acc. nos EU881697 to EU881701). The subgenotype B1-1 was found to be identical to isolates ISSGdA722 from a macaque (Acc. no. EU637581) and ISSGd167 of human origin (Acc. no. EU637579), that were recently submitted to GenBank. For assemblage A isolates, genotyping at the β-giardin gene identified the previously described A2 and A3 subtypes (Table 5).
At the TPI gene, assemblage B isolates were typed as subgroup BIV (81·2%) and BIII (18·8%), whereas assemblage A isolates were all subtype AII. Little heterogeneity was found in the TPI gene, and in 2 isolates only (M11 and M12) a single nucleotide polymorphism was identified (on position 302: G substituted by A). The new TPI subtype was submitted to GenBank (Acc. no. EU834845).
At the GDH gene, assemblage B isolates were typed as subgroup BIII and BIV (Table 2), and 3 new variants of BIV were identified (GenBank, Acc. nos EU834843, EU834844, and EU847734). The new variants showed several single nucleotide substitutions compared to previously described sequences (Table 4). For assemblage A isolates (Table 5), only subtype AII was identified. In the present study, all mutations at each of the 3 investigated genes were synonymous. Using the TPI species-specific assay, 23 of the 54 (41·8%) isolates typed as assemblage B by sequencing, but only 2 of the isolates typed as assemblage A, were identified as mixed A+B infections (Table 2).
Table 4. Intra-genotypic substitutions at the GDH gene for assemblages BVI-like and BIV, based on the polymorphisms outlined in Table 2b in Wielinga and Thompson (Reference Wielinga and Thompson2007)
(The 3 new variants have been added to the list of polymorphisms. Genbank Accession numbers are provided.)

Table 5. The results of the multilocus genotyping of Giardia duodenalis assemblage A-positive isolates, using primers targetting the β-giardin gene (β-giardin), triose phosphate isomerase gene (TPI) and glutamate dehydrogenase gene (GDH)
(All samples were also amplified using a novel species-specific PCR, based on the triose phosphate isomerase gene, to detect mixed assemblage A and B infections (TPI-mixed). For those isolates with a good amplification and sequencing results on all genes the multilocus genotyping (MLG) was determined. na, no amplification.)

DISCUSSION
The results of the present study confirm that both Cryptosporidium and Giardia occur frequently in patients with gastro-intestinal symptoms and should be considered in the aetiology of non-outbreak related diarrhoea. As all cases of diarrhoea were indeed not related to an outbreak, the source of infection was difficult to identify. The frequent identification of C. hominis suggests anthroponotic transmission, although 46% were identified as the potentially zoonotic C. parvum, which is considerably higher than in other studies (Cacció et al. Reference Caccio, Thompson, McLauchlin and Smith2005). The GP60 gene revealed the presence of multiple subgenotypes, although 1 subgenotype predominates both within C. hominis (IbA10G2) and C. parvum (IIaA15G2R1). All subgenotypes were previously described in human stool samples in other countries (Sulaiman et al. Reference Sulaiman, Hira, Zhou, Al-Ali, Al-Shelahi, Shweiki, Iqbal, Khalid and Xiao2005; Gatei et al. Reference Gatei, Das, Dutta, Sen, Cama, Lal and Xiao2007), including the IIdA16G1 subgenotype that was recently described for the first time in the Netherlands (Wielinga et al. Reference Wielinga, de Vries, van der Goot, Mank, Mars, Kortbeek and van der Giessen2008). The zoonotic subgenotype IIaA15G2R1 of C. parvum was previously found to be the most prevalent one in calves (Geurden et al. Reference Geurden, Geldhof, Levecke, Martens, Berkvens, Casaert, Vercruysse and Claerebout2007) in Belgium. Nevertheless, the identification of the IIaA15G2R1 subtype in the human patients does not conclusively implicate animals as a source of infection. The high prevalence and widespread occurence of the IIaA15G2R1 subgenotype in humans (Sulaiman et al. Reference Sulaiman, Hira, Zhou, Al-Ali, Al-Shelahi, Shweiki, Iqbal, Khalid and Xiao2005; Alves et al. Reference Alves, Xiao, Antunes and Matos2006; Feltus et al. Reference Feltus, Giddings, Schneck, Monson, Warshauer and McEvoy2006; Xiao et al. Reference Xiao, Zhou, Santin, Yang and Fayer2007; Zintl et al. Reference Zintl, Proctor, Read, Dewaal, Shanaghy, Fanning and Mulcahy2008) might even suggest that this subgenotype also circulates in human populations without frequent zoonotic transmission. The results from the questionnaire seem to provide additional indications that the C. parvum infections in the present study are not animal-derived, as none of the Cryptosporidium-positive patients reported direct contact with livestock in the questionnaire. Swimming in a public pool was the only significant (P<0·05) risk factor associated with Cryptosporidium infection, despite the limited number of positive samples (n=5). As humans are a more likely a source of infection of swimming pools compared to animals, this further suggests the anthroponotic hypothesis. Similarly in the US and in Australia, swimming pools have frequently been associated with human cryptosporidiosis (Griffiths, Reference Griffiths1998; Hoque et al. Reference Hoque, Hope, Kjellstrom, Scragg and Lay-Yee2002). The use of GP60 to trace the source of infection should therefore always be combined with additional epidemiological data, to elucidate the zoonotic origin of infection.
As for Cryptosporidium, it was difficult to conclusively identify the source of the Giardia infections. Most of the G. duodenalis isolates were characterized as assemblage B, confirming its relevance in patients with sporadic diarrhoea (Cacció and Ryan Reference Cacciò and Ryan2008a). As this assemblage is not prevalent in livestock and as none of the Giardia-positive patients reported direct contact with livestock, zoonotic transmission from livestock seems unlikely. Pet animals infected with assemblage A and B, are considered as a potential reservoir for human giardiasis in domestic environments (Thompson and Monis, Reference Thompson and Monis2004). Moreover, assemblage A was found to be most prevalent in household dogs in Belgium (Claerebout et al. Reference Claerebout, Casaert, Dalemans, De Wilde, Levecke, Vercruysse and Geurden2008). However, contact with pets did not differ between infected and non-infected patients in the present study. Since both recent travel and exposure to recreational water were more frequently reported in Giardia-infected than in non-infected patients, and have previously been identified as important risk factors for infection, human-to-human transfer was considered to be the most likely route of transmission in this study.
As extensively discussed by Wielinga and Thompson (Reference Wielinga and Thompson2007), the 3 markers used in the present study have a high though variable degree of genetic polymorphism. At each locus, considerably less heterogeneity was observed among assemblage A isolates compared to assemblage B isolates, confirming previous multilocus results (Cacciò et al. Reference Cacciò and Ryan2008a). At the β-giardin locus, the large heterogeneity among assemblage B isolates permitted identification of both previously described as well as 6 new subgenotypes. One of these new subgenotypes (B1-1) was recently also identified in isolates from a macaque and human patients in Italy (Cacció et al. Reference Cacciò, Beck, Lalle, Marinculic and Pozio2008b). The 5 remaining new β-giardin subgenotypes were described for the first time in the present study. Recently, several novel assemblage B subgenotypes on the β-giardin locus were also found in human patients and in non-human primates in Italy (Cacció et al. Reference Cacciò, Beck, Lalle, Marinculic and Pozio2008b), and in captive non-human primates in Belgium and the Netherlands (Levecke B., unpublished results). However, the significance of these β-giardin subgenotypes is still difficult to determine, as there is no obvious correlation between the β-giardin subgenotypes and the subgroups identified at the TPI and GDH locus. Although less compared to the β-giardin locus, variability within assemblage B was also observed on the GDH gene. Most BIV sequences were identical to previously described sequences, but 2 new variants of BIV-like sequences were identified (Wielinga and Thompson, Reference Wielinga and Thompson2007), along with a third new variant which was similar to the BIV subgroup. Surprisingly, little heterogeneity was observed at the TPI locus in the present study.
Using the newly developed TPI assemblage-specific PCR, mixed infections were identified in samples typed as either assemblage A or B by the other markers. Although mixed infections were more frequently found in assemblage B-positive samples, the current results seem to suggest that there is no preferential amplification of one assemblage over the other during a standard PCR protocol, and amplification probably reflects the most abundant DNA target. In total, 32·4% of the Giardia-positive samples were identified as mixed infections. Using a similar approach, mixed assemblage A and E infections have previously been identified in over 30% of calves (Geurden et al. Reference Claerebout, Casaert, Dalemans, De Wilde, Levecke, Vercruysse and Geurden2008). Hence, mixed infections should be considered in (i) evaluating the clinical outcome of giardiasis and the pathogenicity of different assemblages, (ii) evaluating the possibility of zoonotic transmission, (iii) elucidating population genetics or (iv) as a possible cause for assemblage swapping (Cacció et al. Reference Cacciò and Ryan2008a). As an example, one sample studied here was typed as assemblage A3 at the β-giardin gene, as assemblage BIV at the GDH gene, and as a mixed infection using the TPI species-specific PCR.
The results of the present study confirm that Cryptosporidium and Giardia should be considered as important causes of non-outbreak-related diarrhoea. C. hominis was more prevalent than C. parvum in this survey. For Giardia, assemblage B was most prevalent and isolates showed a large heterogeneity, especially at the β-giardin locus.
The authors would like to thank Dr Vincent Van Maele (Sint Lucas Hospital, Ghent), Dr Guy De Cock (Maria Middelares Hospital, Ghent) and Dr Luc Van Nimmen (Jan Palfijn Hospital, Ghent) for providing positive samples.