Published online by Cambridge University Press: 09 October 2003
We examined a host-finding factor of the monogenean Heterobothrium okamotoi oncomiracidia to develop an alternative prophylaxis. H. okamotoi oncomiracidia attached preferentially to gill filaments and skin mucus from the tiger puffer Takifugu rubripes compared with corresponding material from other tested fishes (amber jack Seriola dumerili, red sea bream Pagrus major, Japanese flounder Paralichthys olivaceus and spotted halibut Verasper variegatus). The body mucus pH of the tiger puffer was 6·40±0·09 (mean±S.D.), whereas that for the other tested fishes was 7·2–7·4. To find if this difference in pH could account for the specific targeting of tiger puffer by H. okamotoi oncomiracidia, the attachment response of the oncomiracidia to pieces of agar buffered at various pH between 6·0 and 7·4 was examined. The number of attaching oncomiracidia was maximal at pH 6·4. We produced gynogenetic tiger puffers from a single female. These gynogenetic individuals showed a variety of body mucus pH and they were exposed to the oncomiracidia. Thirteen days after exposure, more young H. okamotoi were found on the gills of gynogenetic tiger puffer with mucus at pH 6·3–6·6, than on gills of fish with mucus at pH 6·0–6·3 and 6·6–7·2. H. okamotoi exploits the body mucus pH to identify the host. The simplicity of pH as a lure may lead to development of a simple and economical method to control H. okamotoi outbreaks.
The tiger puffer Takifugu rubripes (Harada & Abe, 1994) is an important cultured fish in Japan, where it is a prized delicacy. Puffer fish have evolved impressive defences against predators, including a tough skin, the ability to expand its size several-fold when threatened, and the production of the deadly nerve poison tetrodotoxin (Koizumi, Levine & Brooks, 1967). However, the tiger puffer has a high mortality rate in aquaculture (Hirazawa, Ohtaka & Hata, 2000) from attack by a specific parasite, Heterobothrium okamotoi, which is a monogenean (Ogawa, 1991). H. okamotoi is problematic because of its obvious pathogenicity and low susceptibility to chemicals (Ogawa & Yokoyama, 1998). Ogawa & Inouye (1997) described the infection cycle for this parasite. The free-swimming oncomiracidia hatch 6–10 days after laying at 20 °C, attach to the gills and grow on the gill filaments, then mature on the branchial cavity wall. Maturation of H. okamotoi takes approximately 49 days from attachment to the gill when the water temperature is between 16·8 and 26·8 °C, with an average of 21·1 °C. This parasite feeds on the host's blood, causing death of the fish through anaemia (Ogawa & Inouye, 1997). Cultured tiger puffer have been treated by bath treatments with formalin to prevent infestation, but this is not a satisfactory procedure because of the resultant pollution to the seawater. In recent years, the use of formalin for off-shore culture has been prohibited by law. Effective methods should be developed urgently to control this parasite.
In-feed caprylic acid, a medium-chain fatty acid, has an anti-parasitic efficacy against H. okamotoi (see Hirazawa et al. 2000), but it cannot control this parasite completely in seasons when the water is at a high temperature (Hirazawa et al. 2001). Ideally, in addition to chemical treatments, biological control measures with no unfavourable effects on the environment should be incorporated into the control strategy. However, much remains to be studied of the parasite's biology and, with the aim of developing an alternative prophylaxis, we explored the possibility that the unusually low pH of the body mucus of the tiger puffer is exploited by the oncomiracidium of H. okamotoi for host identification.
The source of H. okamotoi and its propagation on the tiger puffer has been described previously (Hirazawa et al. 2001). Parasite eggs found attached to the drainpipe and the aeration tube of a tank in which tiger puffer were maintained were removed from the tank and were incubated in a 300 ml plastic beaker containing seawater filtered with sand at 20 °C for 8 days in the dark. The seawater used for incubation was changed every day. Oncomiracidia that hatched within 12 h were used for the experiments.
Laboratory-reared tiger puffer, amber jack Seriola dumerili, red sea bream Pagrus major, spotted halibut Verasper variegatus and commercially supplied Japanese flounder Paralichthys olivaceus were used for the experiment and the body weight of each species was 0·2 kg, 5 kg, 0·2 kg, 0·2 kg and 1 kg on average, respectively.
Fish mucus from the skin surface of tested fishes was obtained by gently rubbing the surface of a living fish. Gill filaments from each tested fish were excised from a fish freshly killed by pitting. The filaments were washed twice in filtered seawater (450 nm membrane filter) and were then cut into 7 mm lengths in a tissue culture dish. The mucus and gill filaments were used for the experiment.
Approximately 500 oncomiracidia in 30 ml of seawater were added to a culture dish (diameter 9 cm) containing 5 cut gill filaments, one from each of the 5 fish species. The dish was incubated at 20 °C for 1 h. Then, each gill filament was removed from the dish and was placed on a glass slide. Oncomiracidia on the gill filament were counted under a light microscope. Formalin (700 μl) was added to the dish containing unattached oncomiracidia and then the fixed oncomiracidia were counted by using a stereo-microscope to estimate the total attachment rate for each tested fish.
Skin mucus (20 mg) from each of the 5 fish species was added to each of 5 out of 8 recessed areas of a glass slide (DC8SC glass slide: Neuro Probe, Inc., USA). Each recessed area was circular, with a diameter of 6·4 mm and an area of 32 mm2. The glass slide was put into a tissue culture dish (diameter 9 cm) containing approximately 500 oncomiracidia in 30 ml of seawater and was incubated at 20 °C for 1 h. Then the glass slide was removed from the dish and the attached oncomiracidia in each recessed area of the glass slide were counted under a light microscope. The unattached oncomiracidia were counted as for the gill filament.
Each experiment was done 3 times.
The fish body mucus pH of each tested fish was measured by using a surface pH meter (LAB F-22 pH-meter, 6262-10 C pH-electrode, Horiba, Ltd, Japan). The pH electrode touched the cephalic, abdominal and caudal parts of a live fish body surface and the values were averaged. Four fish were measured for each species.
Eight agar blocks (7×7×21 mm), each buffered to a different pH i.e. 6·0, 6·2, 6·4, 6·6, 6·8, 7·0, 7·2, 7·4, with 100 mM sodium-phosphate, were all placed in a tissue culture dish (diameter 9 cm). Seawater (30 ml) containing approximately 1000 oncomiracidia was added to the dish. The dish was incubated at 20 °C and the number of oncomiracidia adhering to each block was counted under an inverted microscope after 1, 2 and 5 h. The experiment was done 3 times.
Gynogenetic diploids (reviewed by Purdom, 1993) of the tiger puffer were made to expand the phenotypic variation of the individual body mucus pH. Eggs from a single tiger puffer female were fertilized with genetically inactivated tiger puffer sperm by exposure to ultraviolet (UV) irradiation at 0·45 J·cm−2 for 90 sec. The fertilized eggs were exposed to hydrostatic pressure shock at 69 MPa for 5 min by using a French press (type 5615, Ohtake Ltd, Tokyo, Japan) at 5 min after the fertilization to retain the second polar body chromosome set. The chromosome set of the second polar body will contribute to the development of a fish as an alternative of the chromosome set of the sperm. Homozygous loci increase by this process, leading to the increase of phenotypic variances with the expression of recessive genes. Hatched tiger puffer larvae (7 days after fertilization at 18 °C) were transferred to a tank with 2-metric tons capacity (2 t tank) and were reared. The aerated 2 t tank containing the tiger puffers was supplied with seawater from the open sea and since this water was a potential source of H. okamotoi infection (Hirazawa, Goto & Shirasu, 2003), the seawater was sand-filtered and UV-irradiated. Gynogens of the tiger puffer (23 individuals weighing approximately 100 g) were tagged to recognize them individually. A column-shaped transponder (TX1400L: Electronic ID, Inc., USA), which was pre-programmed with a individual ID code, with a length of 11 mm and thickness of 2·1 mm was injected into the back side muscle of each fish. When the transponder is activated by a low-frequency radio signal, it transmits the ID code to the reader (Mini portable reader MPR: Electronic ID, Inc., USA). Body mucus pH of the tiger puffer was measured 3 times at intervals of 2 weeks. Their mucus pH was stable and they were divided into 3 groups according to their body mucus pH (low pH group: 3 fish, 6·0–6·3; normal pH group: 11 fish, 6·3–6·6; high pH group: 9 fish, 6·6–7·2) and were used for the challenge trial. The body mucus pH of normal diploid fish, weighing 100–300 g and produced at our laboratory, was also measured for comparison with that of gynogenetic fish.
All fish were transferred to a tank with 200 l capacity supplied with sand-filtered and UV-irradiated seawater (approximately 2·4 l/min) and were acclimated for 7 days. After the acclimation, 7000 oncomiracidia were put into the tank, while the seawater supply was discontinued for 1 h, to complete the infection. The challenge trial was done for 13 days. At 13 days after the exposure, fish were sampled and the number of the parasites on the gills was counted. The water temperature was 18·8–19·8 °C during the experiment.
The infection value of each fish was converted into the number of parasites per 1 g gill to compare the intensity of infection and was tested by using the T-test between the normal pH group and the other groups. A probability level of P<0·05 was considered significant. All calculations were done by using the StatView statistical software version 4.5 (Abacus Concepts, Inc. USA).
The attachment response of H. okamotoi oncomiracidia to gill filaments of the tested fishes were compared (Table 1). Markedly more H. okamotoi oncomiracidia attached to gill filaments and mucus from the tiger puffer compared with the other tested fishes.

The body mucus pH of the tiger puffer was low compared with the other tested fishes: 6·40±0·09 (mean±S.D.) for the tiger puffer compared with 7·2–7·4 for the other tested fishes (Table 2).

The attachment response of H. okamotoi oncomiracidia to pieces of agar buffered at various pH values between 6·0 and 7·4 was examined (Fig. 1). The number of attaching oncomiracidia was maximal at pH 6·4, which is the same pH as the average for tiger puffer mucus. However, the number of attaching oncomiracidia was extremely low at pH 7·2 and pH 7·4, which correspond with the average pH of mucus from the other tested fishes.

Fig. 1. Attachment response of Heterobothrium okamotoi oncomiracidia to agar blocks at different pH values at 20 °C. Values are the mean and standard deviation of triplicate experiments.
The body mucus pH of normal diploid fish was 6·42±0·16 (mean±S.D., n=20) and the minimum and maximum values were 6·10 and 6·82, respectively. However, the body mucus pH of gynogenetic fish was 6·58±0·26 (n=23) and the minimum and maximum values were 6·02 and 7·16, respectively. The range of pH variation in gynogenetic fish was wider than that of normal diploid fish.
Fig. 2 shows the relationship between body mucus pH of the gynogenetic tiger puffer and the number of immature H. okamotoi on the gills. A relatively heavy infection of H. okamotoi was found on fish with pH 6·3–6·6 at 13 days after the exposure to oncomiracidia. The number of immature H. okamotoi on the gills of the high pH group was significantly lower than that for the normal pH group. The number of larvae on the gills of the low pH group also tended to be low compared with the normal pH group, but the difference between the two groups was not statistically significant (P=0·178).

Fig. 2. Relationship between body surface mucus pH of gynogenetic tiger puffer Takifugu rubripes and the number of immature Heterobothrium okamotoi on their gills. Values are the mean and standard deviation. Significant difference from the value of the normal pH group is indicated by * P<0·01).
Oncomiracidia of monogeneans swim around to attach to the host surface. Successful host-finding and attachment are crucial for their survival. Some reports have shown that monogenean oncomiracidia respond to environmental stimuli i.e. light, gravity, mechanical disturbance, water currents and chemical substances, indicating that such responses increase the probability of successful host-finding (Ktari, 1969; Paling, 1969; Kearn, 1974, 1980; Whittington & Kearn, 1986, 1989). Chemical substances that exist in the fish epithelium have been identified as attractants for monogenean oncomiracidia (Kearn, 1967; Yoshinaga et al. 2000). In this study, the results show that the body mucus pH of tiger puffer is low compared with the other tested fishes and this unique pH of the tiger puffer has been recognized as an important factor for host identification by H. okamotoi oncomiracidia. We assume that the trait, such as pH, of mucus between the gills and body surface in tiger puffer may be similar and thus the number of immature H. okamotoi on the gills of gynogenetic fish with low and high body mucus pH was significantly lower than that for gynogenetic fish with normal pH. It was difficult to measure the pH of gill mucus. As the tiger puffer has a unique branchial mantle and the spout hole from the branchial cavity is small, it was necessary to excise the branchial mantle anatomically to expose the gills. In addition, we were not able to use the pH meter for the gills because the gills bled when the pH meter electrode touched the gill surface. However, the pH of the gill mucus of normal diploid fishes showed around 6·4 in a measurement by using pH test-paper, although this value is not accurate (personal observation). Furthermore, Chigasaki et al. (2000) showed that oncomiracidia of H. okamotoi settled both on the gills and on the skin, and recognized the settlement-inducing substance contained both in the gill mucus and in the skin mucus. These facts suggest that the mucus trait of the gills and body surface in tiger puffer may be similar. We also assume that the oncomiracidia may possibly migrate to the gills from the body surface. Chigasaki et al. (2000) also assumed this phenomenon. The number of the infected oncomiracidia on the skin of the normal pH group may be higher than that for the low and high pH groups and the parasites may migrate to the gills and thus the number of immature H. okamotoi on the gills of the normal pH group was significantly higher than that for the low and high pH groups. On the other hand, our results also show that H. okamotoi possibly have other host finding factors besides body mucus pH, such as the above environmental stimuli, because H. okamotoi infected gynogenetic fish with low and high body mucus pH.
The cercaria of the digenean Opisthorchis viverrini infects fish. Haas, Granzer & Brockelman (1990) showed that skin surface mucus of the goldfish Carassius auratus stimulated their attachment and that the effectiveness of the mucus in stimulating attachment decreased with increasing pH when the pH of the skin surface of the fish increased from 6·5–7·5 to 7·5–8·0 after death. This shows that the pH of body mucus is important for some parasites as a factor in the identification of the host.
The pH of the body mucus of the tiger puffer is low compared with that of the other tested fishes. This feature may be advantageous to protect the fish from bacterial infections. This suggestion is supported by the following facts. Aquaculture of the tiger puffer has a long history and started in the 1950s (Okamoto, 1963). Annual production has exceeded 5000 metric tons in recent years. As with other farmed fishes, many parasitic diseases occur and 20 species of parasites have been recognized (Ogawa & Yokoyama, 1998), although, among them, H. okamotoi is possibly the most problematic because of its pathogenicity. Despite the increasing reports of parasites, only one pathogenic bacterium, Listonella anguillarum, has been identified (Kusuda & Kawai, 1998). This suggests that the tiger puffer is relatively resistant to bacterial infections. However, many species of pathogenic bacteria have been recognized for the four other tested fishes in this study: L. anguillarum, Photobacterium damsela, Lactococcus garvieae, Nocardia seriolae, Streptococcus iniae and Flexibacter maritimus in amber jack; Edwardsiella tarda, L. anguillarum, Vibrio alginolyticus, L. garvieae and F. maritimus in red sea bream; E. tarda, L. anguillarum, Vibrio ichthyoenteri, L. garvieae, S. iniae and F. maritimus in Japanese flounder (Kusuda & Kawai, 1998; Miyashita & Kumai, 2000); E. tarda, Vibrio spp. and F. maritimus in spotted halibut (personal observation). The optimal pH range for the growth of these pathogenic bacteria is 7·2–8·0 (Hoshina, 1962; Simizu & Egusa, 1972; Kusuda et al. 1976; Ishihara & Kusuda, 1982). According to Levit & Stock (1999), bacteria can follow a pH gradient to migrate to more favourable environments. We speculate that tiger puffer has evolved a low mucus pH to protect itself from bacteria, but H. okamotoi has exploited this feature to identify the host.
We used the gynogenetic fish in our experiment to expand the phenotype of the body mucus pH of the tiger puffer. The range of quantitative variations in gynogenetic fish is wider than that of normal diploid fish, and useful quantitative characters, such as a resistance to infectious diseases, growth, etc. are obtainable (Tave, 1993; Inada et al. 1997). In this study, the range of pH variation of body mucus in the gynogenetic tiger puffer was wider than that of the normal diploid tiger puffer and we produced gynogenetic tiger puffers that had a low body mucus pH. The number of H. okamotoi larvae in the low pH group tended to be low compared with the normal pH group in the challenge trial. These results suggest that a brood line with lower body mucus pH might be produced by using inbreeding. Such a fish would be less vulnerable to H. okamotoi, and would also be expected to have a lower susceptibility to bacterial infection.
H. okamotoi oncomiracidia were adhered to an agar block buffered at pH 6·4 which was the same pH as the average for the tiger puffer. This feature could be used as a trap to collect and eliminate the oncomiracidia from fish culture systems. Such a trap, combined with selective breeding to obtain fish for culture with body mucus of low pH, may provide an economical, more environmentally friendly, control against outbreaks of H. okamotoi in fish farms.

Table 1. Attachment response of Heterobothrium okamotoi oncomiracidia to gill filaments and mucus of tested fishes

Table 2. pH of body surface mucus of tested fishes
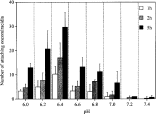
Fig. 1. Attachment response of Heterobothrium okamotoi oncomiracidia to agar blocks at different pH values at 20 °C. Values are the mean and standard deviation of triplicate experiments.

Fig. 2. Relationship between body surface mucus pH of gynogenetic tiger puffer Takifugu rubripes and the number of immature Heterobothrium okamotoi on their gills. Values are the mean and standard deviation. Significant difference from the value of the normal pH group is indicated by * P<0·01).