INTRODUCTION
Copepods are a group of ca. 12 000 planktonic species of the phylum Crustacea (Brusca and Brusca, Reference Brusca and Brusca2003). Approximately 50% of these species are considered to live in symbiotic associations (including parasitism) with a broad spectrum of aquatic animals, ranging from sponges to marine mammals (Boxshall, Reference Boxshall and Rohde2005). To date, the majority of research has concentrated on ectoparasitic copepods of fish, such as the salmon louse, Lepeophtheirus salmonis. Over the past 30 years, L. salmonis has become a major worldwide problem of salmonid aquaculture, costing ca. US$480 million p.a. in terms of loss of production and parasite control (Costello, Reference Costello2009a). There is also evidence suggesting that salmon farms may be responsible for declines in wild populations of salmonids as a result of parasitic sea lice transfer (e.g. Krkošek et al. Reference Krkošek, Ford, Morton, Lele, Myers and Lewis2007; Costello, Reference Costello2009b), but this idea remains controversial (e.g. Riddell et al. Reference Riddell, Beamish, Richards and Candy2008). Furthermore, sea lice are thought to act as reservoirs of infection and vectors of transmission for various viral (Nylund et al. Reference Nylund, Hovland, Hodneland, Nilsen and Lovik1994), bacterial (Cusack and Cone, Reference Cusack and Cone1986), and other parasitic (Nowak et al. Reference Nowak, Bryan and Jones2010) diseases.
Our knowledge of ectoparasitic copepods in commercially important shellfish is far more limited. For example, lobsters harbour various species of copepod (Shields et al. Reference Shields, Stephens, Jones and Phillips2006), but research into their effect on lobsters, in terms of health and survival, is very limited. One parasitic copepod species, Nicothoë astaci (‘lobster louse’), has been recorded in European lobsters, Homarus gammarus, from a wide variety of locations, including the UK, Ireland, Sweden, Norway, Germany, Netherlands, Portugal, France and Morocco (e.g. Gotto, Reference Gotto1954; Faure, Reference Faure1958; Mason, Reference Mason1959; Sindermann and Rosenfield, Reference Sindermann and Rosenfield1967; Holmes et al., Reference Holmes, Costello, Connor, Howson and Picton1997; ICES 2007; GBIF Data Portal, data.gbif.org, accessed 23-11-10). However, very little is known about its pathology and subsequent effect on lobster populations. Even the full life cycle, including the male copepod, remain elusive. Early studies, however, have revealed that the copepod attaches to lobster gill filaments via a suctorial mouth in order to feed on host haemolymph (blood) (Leigh-Sharpe, Reference Leigh-Sharpe1926; Gurney, Reference Gurney1930; Mason, Reference Mason1959). Since lobster fisheries in the UK are worth an estimated £26 million p.a. (Marine Management Organisation, 2009) and the fact that this parasite could provoke severe detrimental effects on its host, there is a need to improve our understanding of N. astaci infections at both the host and ecosystem level.
The current study is an assessment of N. astaci infection in the European lobster, H. gammarus collected from Lundy Island, Bristol Channel, UK. Particular emphasis was placed on the intensity of the N. astaci infection, together with the nature of the host-parasite interaction, and subsequent tissue damage and host response.
MATERIALS AND METHODS
Lobster collection
European lobsters (H. gammarus) were collected using baited commercial pots from waters surrounding Lundy Island, Bristol Channel, UK, during July and September 2010. In total, 23 lobsters (16 males and 7 females) were processed for histopathology. All lobsters were transported back to Swansea University for analysis. Lobsters collected in July were sampled immediately while those collected in September were maintained under aquarium conditions (<7 days) with fish and shellfish feed, prior to sampling.
Parasite prevalence and intensity
Gross external characteristics of each lobster including size, sex and condition (berried, epibiont shell fouling, limb loss, injury, shell erosion/disease) were recorded. Lobsters were then sacrificed after ca. 10 min at −20°C with either an intra-haemocoelic injection of 30–40 ml of absolute ethanol chilled to −20°C or by injection of the same volume of Davidson's fixative (30% distilled water, 30% ethanol, 20% formaldehyde (37% stock), 10% glycerol, 10% glacial acetic acid). The animals were fully dissected and tissue samples (gills, hepatopancreas, gonad and muscle) routinely taken for histology. During dissection, the outer carapace (branchiostegite) of both gill chambers was removed and the gills excised. The presence or absence of adult Nicothoë astaci parasites was noted, and both parasitized and un-parasitized gills taken for detailed histological analysis. Intensity of infection was assessed in lobsters by recording the number and location of parasites within each gill filament.
Parasite morphology and pathology
Samples of excised gills (parasitized and un-parasitized) were fixed in either Davidson's fixative (exchanged for 70% ethanol after 18 h) or Bouin's seawater fixative (71% seawater saturated picric acid, 24% formaldehyde (37% stock), 5% glacial acetic acid) for histological analysis. Tissues were then dehydrated, embedded in histological wax, and 6–7 μm thick sections cut and stained with Cole's haematoxylin and eosin. In an attempt to improve parasite embedding and wax infiltration, a subset of samples were either; (i) double embedded in 1% necoloidine solution, (ii) tips of parasite egg sacs and lateral wings excised prior to wax embedding, or (iii) wax blocks soaked in Mollifex tissue softener for ca. 30 min at 4°C to soften the tissues for sectioning (Wynnchuk, Reference Wynnchuk1992).
In addition, laser scanning confocal microscopy (LSCM) was employed to reveal further detail on N. astaci morphology. An adapted protocol of Michels (Reference Michels2007) allowed autofluorescence of Davidson's fixed adult and cyclopid larvae (excised from adult egg sacs) to be captured using a Carl Zeiss LSM 710 laser scanning confocal microscope. The 488 nm and 543 nm lasers were used for excitation, and green and red autofluorescence was observed using band-pass filters 493–538 nm and 548–685 nm, respectively.
Data analysis
Statistical analyses were performed using GraphPad Prism 5.00 for Windows (GraphPad software).
RESULTS
Parasite prevalence and intensity
Lobsters collected in July (9 males, 1 female) had a mean size (i.e. carapace length; CL) of 126·8±5·8 mm (mean±s.e.) while those collected in September (7 males, 6 females) had a mean CL of 115·8±3·3 mm (mean±s.e.). Lobsters included visually healthy individuals, as well as those exhibiting epibiont shell fouling, chelae damage or loss, and low severity shell disease (a bacterial condition that damages the cuticle and can result in intra-haemocoelic secondary infections; Vogan et al. Reference Vogan, Powell and Rowley2008). All individuals collected in both July and September (N=23) were found to harbour adult N. astaci in their gills (Fig. 1A–C). The intensity of N. astaci infection in lobsters was highly variable, ranging from 4 to 137 copepods/lobster (47·3±10·1, mean±s.e.). Significantly more parasites were found in the basal region of the gill (Fig. 2A) compared with either the middle (P<0·01) or tip (P<0·001), with no significant difference between the latter two regions (repeated measures one-way ANOVA with Bonferroni's multiple comparison post-test, Fig. 2A). Within each gill, parasites were found attached towards the base of individual gill filaments, with their egg sacs and wings protruding (Figs. 1A–C). The specific parasite attachment point on the gill filament was not recorded. Pleurobranch gills harboured significantly greater numbers of parasites than podobranch or arthrobranch gills (P<0·001 for both, repeated measures one-way ANOVA with Bonferroni's multiple comparison post-test, Fig. 2B). No significant difference was observed between the number of parasites in podobranch and arthrobranch gills. Pleurobranchs, P2, P3 and P4, displayed particularly high total numbers of N. astaci (6·8±1·8, 8·9±2·5 and 7·9±1·7 respectively, mean±s.e., Fig. 2C). No parasites were observed on the diminutive podobranch associated with maxilliped 2 (M2, Figs. 1A and 2C). In addition, there was no significant difference in the number of parasites between the left and right sets of gills (21·9±5·2 vs 25·4±5·3 respectively, mean±s.e., P=0·2123, paired t-test) or between males and females (61·1±43·6 vs 31·2±7·6 respectively, mean±s.e., P=0·1468, unpaired t-test). Similarly, the presence or absence of epibiont shell fouling (e.g. barnacles, serpulids), which was used to indicate the relative time since moult, did not result in a significant difference in the number of copepods/lobster (fouled vs non-fouled, 73·5±23·8 vs 35·7±8·6 respectively, mean±s.e., P=0·0836, unpaired t-test) nor did the presence of shell disease lesions (shell-diseased vs non shell diseased, 38·3±9·8 vs 57·8±18·9 respectively, mean±s.e., P=0·358, unpaired t-test).

Fig. 1. Copepod parasites on European lobster, Homarus gammarus gills. (A) Lobster branchial chamber showing female adult Nicothoë astaci copepods attached to outer podobranchs (M2-3 and P1-4) and inner pleurobranch (P5) gills (anterior of lobster on the left of photo). Gills are separated by membranous epipods (Ep). Po, podobranch; Pl, pleurobranch. The dwarf podobranch (P3) is an abnormality. (B) Excised lobster gills showing dark material (arrow) at the site of parasite attachment. Regions of gill used to document parasite location are labelled. B, base; M, middle; T, tip. (C) Damaged, melanized gill filaments (arrow) due to presence of an adjacent attached parasite. Scale bars=1 cm (A, B) and 1 mm (C).

Fig. 2. Intensity and location of Nicothoë astaci in Homarus gammarus gills. (A) Number (mean±s.e.) of N. astaci adult copepods attached to the base, middle and tip region of lobster gills. N=13, *** P<0·001, ** P<0·01 (repeated measures one-way ANOVA with Bonferroni's multiple comparison post-test). (B) Number (mean±s.e.) of N. astaci attached to the 3 different gill types. N=13, *** P<0·001 (repeated measures one-way ANOVA with Bonferroni's multiple comparison post-test). (C) Number (mean) and location of N. astaci on each of the 20 gills. H. gammarus possess 20 gills in each branchial chamber. There are 6 external podobranchs, 10 arthrobranchs (i.e. middle gills, divided into 1 anterior row and 1 posterior row) and 4 inner pleurobranchs. The final pleurobranch (P5) is the only gill in association with pereopod 5, therefore appears as an external gill rather than an inner gill.
Morphology and pathology of N. astaci
Morphology
All N. astaci observed on the gills of lobsters were adult females that possessed 3 main body regions; the cephalothorax, paired lateral expansions (‘wings’) and 2 egg sacs (Fig. 3A). There was no indication of male N. astaci. The ventral surface of the cephalothorax possessed a cuticle-lined oral cone and suctorial disc (Fig. 3B), in which there were denticle-like structures in association with the small central opening (Fig. 3B). The margin of the oral cone/suctorial disc was surrounded by 2 radial fringes of setule-like extensions (Fig. 3B). The cuticle-lined oesophagus was shown to contain host haemolymph and to be in continuity with the lobate ‘stomach’ within each wing (Fig. 3C). It also contained a tooth-like structure (Fig. 3D). All N. astaci had distended ‘stomachs’ replete with host haemolymph in which the host haemocytes had aggregated (Fig. 3C). The wings also contained oocytes of various sizes (ca. 10–60 μm in diameter) and stages of development. Immediately behind the wings was a small abdomen containing paired spermathecae and many densely-stained spermatozoa (Fig. 3E), which was connected to 2 large egg sacs (Fig. 3A). The stage of oocyte development within the egg sacs varied between individual parasites, and ranged from early 4–8 cell stage oocytes, to embryos with developing limb buds, and finally to fully formed cyclopid larvae ready for release. LSCM of cyclopid larvae removed from the egg sacs revealed a protruding oral cone composing a spiral structure with small denticle-like and setule-like structures around the margin (Fig. 4A).
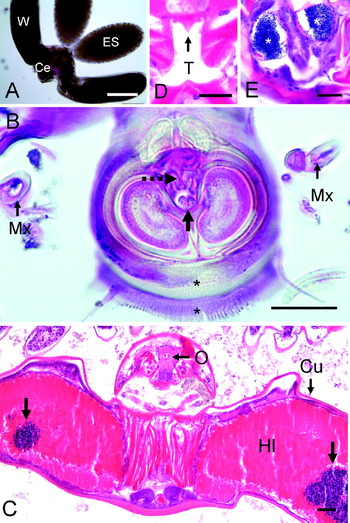
Fig. 3. Morphology of adult female Nicothoë astaci. (A) N. astaci adult removed from a gill, showing cephalothorax (Ce), paired ‘wings’ (W) and egg sacs (ES). Scale bar=500 μm. (B) Micrograph showing the ventral oral cone/suctorial disc and 1st maxilla (Mx). The small oral opening (solid arrow), associated denticle-like structures (dashed arrow) and radial fringes of setule-like structures (*) are clearly visible. Scale bar=25 μm. (C) Low-power micrograph of N. astaci, showing oral region (O) of cephalothorax and lobate wings replete with host haemolymph (Hl) and clumped host haemocytes (unlabelled arrows). Parasite cuticle (Cu). Scale bar=100 μm. (D). Histological section through oral region showing tooth-like (T) structure in oesophagus. Scale bar=25 μm. (E). Densely-stained spermatozoa (*) in paired spermathecae in abdomen. Scale bar=25 μm.

Fig. 4. LSCM micrographs of Nicothoë astaci. (A) Autofluorescence of cyclopid larvae removed from egg sac showing protruding oral cones (OC) composing a spiral structure with small denticle-like and setule-like structures around the margin. (B) Autofluorescence of adult N. astaci attached to a gill filament (G) with its maxillipeds (Mp) and other thoracic appendages (Th). An antennule (A) is also clearly visible. Scale bar=100 μm.
Pathology
Observations of N. astaci parasites in situ on the gills revealed dark material within the vicinity of the parasite (Fig. 1B, C). Histology showed that this material was a flocculent matrix containing a rich epibiotic community. The community included protozoan cysts, filamentous bacteria, ciliates, turbellarians, crustacean larvae, and isopods (not shown). While such communities were prominent within the site of N. astaci attachment, they were very occasionally seen in other areas of the gills.
Nicothoë astaci were commonly found attached to individual H. gammarus gill filaments via their ventral suctorial disc (Fig. 5). LSCM studies, however, revealed that they also use their maxillae and maxillipeds to secure attachment to the parasitized gill filament (Fig. 4B). Whilst some N. astaci were found to be physically attached to the filament (Fig. 5A), others were observed in intimate association with the gill filament, but with the suctorial disc not actually attached. In such cases, however, there was evidence of previous attachment in the very close vicinity to the parasite. During the attachment process, the parasite appears to penetrate the gill cuticle, forming an invasive (feeding) channel through which it can extract host haemolymph (Fig. 5A–C). Figure 5A shows a feeding channel in active use by N. astaci. Both the gill cuticle and epithelium have been breached, with the gill cuticle responding with thickening on either side of the feeding channel, and a gap in the sheet-like gill epithelium (that secretes the cuticle) underneath the channel. There was also a very intimate association between the parasite and host cuticle (Fig. 5A). Other feeding channels were characterized by excessive thickening and melanization (i.e. yellow-brown pigmentation) of the gill cuticle (Fig. 5B and C). On the majority of occasions, the channel was funnel shaped through the thickened cuticle (Fig. 5B), with the narrow end of the funnel (5–10 μm diameter) towards the parasite, and the wide end (ca. 30 μm diameter) opening into the host gill filament (Fig. 5B). There was also evidence of host haemolymph within the funnel/feeding channel (Fig. 5B). Figure 5C shows an imprint of an oral cone/suctorial disc on a gill filament. The melanized central channel through the gill cuticle is clearly visible, as is the imprint of the setule fringes.

Fig. 5. Attachment and invasion of gill filaments by Nicothoë astaci. (A). Attachment of N. astaci to a gill filament (G) showing the invasive feeding channel (*) through the gill cuticle. Note thickened gill cuticle either side of feeding channel (solid arrow), and lack of intact gill epithelium (Ep) immediately below channel. The parasite's cuticle (dashed arrow) is in very close association with host gill cuticle. (B) Funnel-shaped feeding channel through thickened gill filament cuticle (GC) with dashed arrow indicating direction of blood flow from gill filament into the parasite. Note excessive thickening and melanization (yellow-brown pigmentation) of the gill cuticle (GC) either side of the feeding channel and closely associated gill epithelium (Ep). (C) Imprint of N. astaci suctorial disc on the surface of a gill filament. Note the central channel (Ch) through host gill cuticle and surrounding dark melanized cuticle. Imprint of setule-like fringe (*) is also visible. Scale bars=50 μm (A, B) and 10 μm (C).
The presence of N. astaci caused physical damage to multiple gill filaments, distant from its attachment site (Figs. 1C and 6). These gill filaments appeared swollen in response to damage, with the gill cuticle forming a melanized layer below the damaged area (Fig. 6A). This layer instigated occlusion of the damaged distal gill section from the underlying ‘healthy’ region (Fig. 6A, B). Melanization was followed by haemocyte ensheathment (sometimes accompanied by large numbers of free haemocytes; Fig. 6A, B) and a reforming epidermis from within the ‘healthy’ region (Fig. 6A). These additional cell layers appear to complete the occlusion of the damaged distal section. The isolated damaged area was characterized by cuticular remnants and loss of cellular content (Fig. 6A, B).

Fig. 6. Damaged gill filaments in close vicinity of Nicothoë. astaci. (A) Destruction of gill filaments within close vicinity (but not the site of attachment) of N. astaci (P), with melanization (M) and haemocyte infiltration (solid arrows) as host response mechanisms. Occlusion (*) of the distal region of another gill filament is also visible. Scale bar=200 μm. (B) Details of occlusion process, showing filament swelling, melanization of cuticle (MC) and underlying haemocyte ensheathment (arrow), as well as reforming cuticular epidermis (E). All these layers facilitate isolation of the damaged gill section from the ‘healthy’ gill filament below. The isolated damaged gill section (*) contains cuticle remnants and acellular material. Scale bar=50 μm.
Further pathological effects of N. astaci were observed in the central axes of the gills (Fig. 7). They ranged from very limited haemocyte infiltration to major tissue damage. In the absence of parasite attachment, the ‘normal’ vascular system in the central axis region of the gill is a complex network of afferent and efferent blood vessels with surrounding connective tissue containing limited haemocytes (Inoue and Ueno, Reference Inoue and Ueno1995; Inoue et al. Reference Inoue, Ueno and Baba1997; Fig. 7A). In contrast, parasite attachment caused extensive disorganization and disruption of the vascular system within the central axis, resulting from infiltration of haemocytes into this area close to the parasite (Fig. 7B). This disruption, however, was never observed within the immediate vicinity of the attachment site in the gill filament but in the underlying central axis. Within the dense infiltration of haemocytes associated with the disruption were nodules (granuloma) consisting of melanized inner cores and surrounding sheaths of haemocytes (Fig. 7C, D).

Fig. 7. Tissue damage within central axis of lobster gill as a result of Nicothoë astaci attachment. (A) Central axis region of an un-parasitized region of gill showing central axial blood vessel (CV) and accompanying smaller blood vessels (V). Note lack of dark flocculent material (*) in between gill filaments (F). (B) Central axis region of a parasitized gill (directly underneath the vicinity of parasite attachment site), showing complete disruption of blood vessels and tissues, and extensive haemocyte infiltration. Note the presence of dark flocculent material (*) in between filaments (F). (C, D) Higher power micrographs showing extensive haemocyte infiltration and haemocytic nodules in central axis region underlying N. astaci. Nodules consist of a central melanotic core surrounded by a sheath of haemocytes (arrow). Scale bars=200 μm (A, B) and 50 μm (C, D).
DISCUSSION
In the current study, all lobsters collected from Lundy Island waters during July and September 2010 harboured copepod parasites in their gills. Morphological details, of both adult and juvenile parasites, very closely resembled those documented in the early studies of Leigh-Sharpe (Reference Leigh-Sharpe1926), Gurney (Reference Gurney1930) and Mason (Reference Mason1959), thus confirming the ectoparasite to be Nicothoë astaci.
Examined lobsters included males and females, with visually healthy individuals, as well as those exhibiting varying levels of epibiont shell fouling, chelae damage and low severity shell disease. Although the focus of the present study is histopathological in nature, rather than epidemiological, our results suggest that N. astaci prevalence and intensity are independent of host sex and condition. This, however, may be an artefact of our relatively small sample size. Our study recorded an intensity of parasitization of between 4 and 137 N. astaci per lobster. Previous studies on N. astaci are very limited, but other intensity recordings in the European lobster, H. gammarus, range from 0 to 756 N. astaci/lobster (Gotto, Reference Gotto1954; Faure, Reference Faure1958; Mason, Reference Mason1959). Our mean intensity of ca. 47 N. astaci/lobster is at the lower end of reported N. astaci densities, suggesting that this parasite is probably not currently damaging the Lundy lobster population to any great significance. This idea is further supported by negligible levels of culturable haemolymph bacteria and the histologically normal appearance of other tissues (unpublished data). Heavy infestations of gill parasites may potentially harm the host by affecting respiratory function and/or by causing secondary infections. Previous studies have shown that adverse effects may be enhanced under stressful conditions, as illustrated by N. astaci-associated lobster mortalities at 2 growing-on facilities in Ireland (ICES, 2007), and co-infection of N. astaci with gaffkaemia (a fatal bacterial disease) in stored lobsters (Gibson, Reference Gibson1961). In both studies, high lobster densities may have exacerbated the situations. However, whether the mortalities were a direct result of N. astaci infection or, in fact, resultant of secondary infections due to gill damage is unknown.
Lobsters possess trichobranchiate gills (McLaughlin, 1983), forming 3 layers within each branchial chamber. The external layer is composed of podobranch gills, while the inner layer is made up of pleurobranchs and smaller arthrobranch gills separate the two. All gills are associated with a particular appendage (maxilliped 2 or 3, or pereopod 1-5) and are classified according to their attachment position (either on the appendage or thorax; McLaughlin, 1983). Our study revealed that inner pleurobranchs, in particular gills P2-4, harboured significantly more N. astaci than either podobranchs or arthrobranchs. In addition, parasites were significantly more abundant on the base of each gill, than either the middle or the tip region. Investigations into the distribution of parasitic copepods on fish gills have also shown clear attachment site differences (e.g. Davey, Reference Davey1980; Roubal, Reference Roubal1999; Bennett and Bennett, Reference Bennett and Bennett2001; Scott-Holland et al. Reference Scott-Holland, Bennett and Bennett2006). These differences were related to the strength of the ventilation current and the probability of coming into contact with a suitable attachment site. Parasites will try and avoid strong and turbulent water flow, as well as orientating themselves between gill filaments in a way to reduce friction drag on their body, thus stabilizing attachment (Bennett and Bennett, Reference Bennett and Bennett2001). Similar factors may be influencing N. astaci distribution within lobster gills. Water enters the lobster branchial chamber equally at the base of the gill-possessing appendages (maxillipeds and pereopods), and travels up each gill from base to tip (McLaughlin, 1983). The first point of contact for planktonic copepodids, therefore, is the gill base, and dominance of parasites within this region (often in large clusters) suggests that their attachment is opportunistic. Higher numbers of N. astaci on inner pleurobranchs, may result from the surrounding arthrobranchs and podobranchs providing protection from the water current. The attachment of N. astaci on the basal region of individual gill filaments may reduce friction and drag, but also allow efficient dispersal of offspring from the protruding eggs sacs.
All N. astaci observed during this study were adult females with egg sacs containing cyclopid larvae at various stages of development. In accordance with Mason (Reference Mason1959), male N. astaci were never observed. Although the study by Mason (Reference Mason1959) never found evidence of testes, all last-stage copepodids investigated possessed spermathecae and spermatozoa prior to settlement on the lobster host. In agreement with Mason (Reference Mason1959) all adults examined in the current study possessed spermatozoa (but no evidence of sperm production). Mason (Reference Mason1959) suggested that males develop during the transition from cyclopid larvae (released from egg sacs) to last-stage copepodids (ready for settlement on lobsters). Thus, it appears that males impregnate females at a very early stage of development and do not attach to lobster gills. The deposited spermatozoa in juvenile females must therefore be used to fertilize successive generations of oocytes.
The complete life cycle of N. astaci remains elusive. Whether cyclopid larvae released from eggs sacs parasitize an intermediate host prior to infecting lobsters is unknown. Mason (Reference Mason1959) was unable to achieve direct infection of lobsters with cyclopid larvae, and his examination of invertebrates and fish within lobster habitats failed to locate developing cyclopids. However, the presence of a well-developed sucker, and the ability to attach and move using this sucker during in vitro experiments (Mason, Reference Mason1959), highlight the feasibility of an ectoparasitic life style for such larvae.
The current investigation has shown, for the first time, the nature of the attachment of N. astaci to the lobster host and the subsequent pathology and host response. Some parasites were physically attached to gills, whilst others were not. This may be a processing artefact, or an indication of N. astaci relocating. There was no evidence of multiple attachment sites, or sealed-off feeding channels (by melanized host cuticle), so it appears that parasite attachment is permanent and that there is no forced detachment by the host and subsequent parasite relocation. Detailed examination of the host-parasite interface found that parasites attach to the host gill via their ventral suctorial disc, together with their maxillae and maxillipeds. The majority of ectoparasitic copepods use these mouthparts for attachment (e.g. Kabata, Reference Kabata1981; Boxshall, Reference Boxshall and Rohde2005). The suctorial disc on the oral cone is a very complex structure, and in Nicothoidae is formed from the fusion of the labrum and labium (Boxshall and Lincoln, Reference Boxshall and Lincoln1983). Detailed morphology of the disc was difficult to discern during our study; however, we did observe denticle-like structures in the disc centre, and 2 fringes of setule-like structures. The documented styliform mandibles of N. astaci (and all other Nicothoidae species; Boxshall, Reference Boxshall and Rohde2005) were absent in all specimens examined, although larger protrusions were occasionally observed. The styliform mandibles of N. astaci are thought to protrude through the oral cone, penetrating the host gill cuticle and epidermis, thus creating a feeding channel into the host haemocoel (Leigh-Sharpe, Reference Leigh-Sharpe1926; Gurney, Reference Gurney1930). Even though N. astaci are thought to infect recently moulted lobsters, when the gill cuticle is soft and easily penetrable (Mason, Reference Mason1959), a physical piercing action is probably still required. The current finding that the ‘stomach’ of N. astaci was full of host haemolymph confirmed that this parasite is capable of piercing through the cuticular lining of the gill to gain entry to the haemocoel and thus feeds on host blood. We also observed complex musculature in association with the oral cone and gullet which is probably essential for its suction feeding mechanism. An additional feature, not previously documented, was the presence of a tooth-like structure within the oesophagus. Its function, at present, is unknown; however, it could be involved in breaching host cuticle and epidermis. Our results clearly reveal that further studies are required to fully elucidate the structures and mechanisms involved in host invasion by N. astaci.
In the current study, the pathology and host response to N. astaci in lobster gills was very variable and included haemocyte infiltration, occlusion of gill filaments adjacent to the parasite, and major disruption to the central vascular system of the gill. These responses by the host may interfere with its blood supply to the parasite, thus potentially leading to parasite starvation. In contrast, a host response was not observed within the immediate vicinity of the parasite and instead was displaced to a neighbouring region of the gill. This suggests that the parasite may be modulating the host's haemostatic responses. Further indication of modulation by the parasite was the lack of host blood coagulation within the gill filament, and resultant blockage of the parasite's feeding channel. Hence, there appears to be complex interplay between the host and parasite, and this may prove an interesting avenue of research.
In conclusion, the current study has provided an insight into the relationship between the lobster louse, N. astaci and its host, H. gammarus. Although we now have a clearer understanding of the localized pathology, there is still much to learn. The physiological effects of N. astaci infection require investigation, paying special attention to respiratory function, stress and overall health of the host. Full epidemiological studies are also vital in discerning implications at the population level, particularly with respect to climate change, fisheries and aquaculture development and initiation of marine reserves. Finally, the complete life cycle of N. astaci must be resolved in order to fully understand the future impact of this parasite on European lobsters.
ACKNOWLEDGEMENTS
We thank the skipper and crew of the FV Walrus (Geoff and Chopper), Ian Tew, Keith Naylor and Dr Kristina Hamilton of Swansea University for their sterling support at sea, and Sarah Clark from Devon and Severn IFCA for her invaluable advice and assistance. The Lundy warden, Nicola Saunders, and her staff on Lundy are also gratefully acknowledged for their support, as is the permission of Devon and Severn IFCA and Natural England to allow lobster sampling in the No-Take Zone of Lundy Island. Finally, we thank Sally James (Institute of Life Science, Swansea University) for assistance with the LSCM studies.
FINANCIAL SUPPORT
This work was supported by Interreg 4A Ireland Wales Programme 2007–2013 (Susfish) grant #42.









