Introduction
Neglected tropical diseases caused by parasites are a current health issue that affects millions of people (Hotez et al., Reference Hotez, Fenwick, Ray, Hay and Molyneux2018). Molluscs have been widely identified as intermediate hosts and vectors of different parasites which may have deleterious effects on human public health (Clausen et al., Reference Clausen, Madsen, Murrell, Manh, Viet and Dalsgaard2012; Malek, Reference Malek2018). Applesnails (Caenogastropoda, Ampullariidae) comprise about 150 species, grouped in nine genera (Hayes et al., Reference Hayes, Cowie and Thiengo2009) which are present in tropical and subtropical aquatic ecosystems of the Americas, Africa and Asia (Berthold, Reference Berthold1991). South American Ampullariidae include four genera: Pomacea, Asolene, Felipponea and Marisa, being the former the most diverse genus with 97 species (Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras, Martin, Qiu, Thiengo, Vega, Wada, Yusa, Burela, Cardiemo, Cueto, Dellagnola, Dreon, Frassa, Giraud-Billoud, Godoy, Itualte, Koch, Matsukura, Pasquevich, Rodriguez, Seveanu, Seuffert, Strong, Sun, Tamburi, Tiecher, Turner, Valentine-Darby and Cowie2015). Also, Pomacea canaliculata and Pomacea maculata have been introduced to Southeast Asia, North America and Europe, where they have become a plague for rice and other crops (Cowie, Reference Cowie and Barker2002; Oscoz et al., Reference Oscoz, Tomds and Duron2010).
Apple snails host different symbiotic organisms (Vega et al., Reference Vega, Damborenea, Gamarra-Luques, Koch, Cueto and Castro-Vazquez2006; Damborenea et al., Reference Damborenea, Brusa, Negrete, Joshi, Cowie and Sebastian2017) and they have been identified as intermediate host and vectors of different diseases (Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras, Martin, Qiu, Thiengo, Vega, Wada, Yusa, Burela, Cardiemo, Cueto, Dellagnola, Dreon, Frassa, Giraud-Billoud, Godoy, Itualte, Koch, Matsukura, Pasquevich, Rodriguez, Seveanu, Seuffert, Strong, Sun, Tamburi, Tiecher, Turner, Valentine-Darby and Cowie2015). For example, P. canaliculata has become an alternative host for the nematode Angiostrongylus cantonensis, the aetiological agent of eosinophilic meningitis, an emergent zoonotic disease in South East Asia, Pacific islands, Australia (Tesana et al., Reference Tesana, Srisawangwong, Sithithaworn and Laha2008; Lv et al., Reference Lv, Zhang, Liu, Hu, Yang, Steinmann, Chen, Wang, Utzinger and Zhou2009; Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras, Martin, Qiu, Thiengo, Vega, Wada, Yusa, Burela, Cardiemo, Cueto, Dellagnola, Dreon, Frassa, Giraud-Billoud, Godoy, Itualte, Koch, Matsukura, Pasquevich, Rodriguez, Seveanu, Seuffert, Strong, Sun, Tamburi, Tiecher, Turner, Valentine-Darby and Cowie2015) and more recently in South America and the Caribbean (Valente et al., Reference Valente, Robles, Navone and Diaz2018).
Apple snails are also selected as vectors by several trematode parasites. Human dermatitis-producing schistosomes has been found in the apple snail Pomacea paludosa (Cercaria pomaceae; Leedom and Short, Reference Leedom and Short1981). Pomacea glauca is a natural host for Echinochasmus zubedakhaname (Nasir and Diaz, Reference Nasir and Diaz1968). Cercaria udoi and Cercaria paraudoi have been reported in P. glauca and Marisa cornuarietis (Nasir et al., Reference Nasir, Tulio Díaz and Hamana1969). Also, different larval stages of Echinostoma parcespinosum (in the mantle cavity and digestive gland) and Dietiziella egregia (in the pericardial cavity) have been found in P. canaliculata (Martorelli, Reference Martorelli1987; Damborenea et al., Reference Damborenea, Brusa and Paola2006). Ostrowski de Nuñez (Reference Ostrowski de Nuñez1979) reported unidentified echinocercariae and xiphidiocercariae in the digestive gland of P. canaliculata, whereas Keawjam et al. (Reference Keawjam, Poonswad, Upatham and Banpavichit1993) found metacercariae in the kidney, heart and foot from P. canaliculata collected from Thailand. However, to date, molecular databases have not nucleic acid sequences of apple snails’ cercariae.
In the present work, we studied the morphology of trematode parasites that inhabit the digestive gland of a natural population of the apple snail Asolene platae (Lake Regatas, Buenos Aires, Argentina). Also, we explored the phylogenetic relationships of these trematode parasites by polymerase chain reaction (PCR) and sequencing of the genes that encode for the large subunit ribosomal RNA (28S rRNA), the nuclear ribosomal Internal Transcribed Spacer 1 (ITS1), and the mitochondrial Cytochrome c Oxidase I (mtCOXI).
Material and methods
Collection site and snail sampling
Lake Regatas (34°33′19.65″S, 58°26′4.33″W) is a lentic aquatic environment communicating with the Río de la Plata estuary, and is located in Palermo, the largest urban park of Buenos Aires city. The Río de la Plata basin, which encompasses the Paraná River and Uruguay River basins is the second largest basin in South America (Berbery and Barros, Reference Berbery and Barros2002). The malacological fauna of Lake Regatas (Dellagnola, Reference Dellagnola2015) includes three ampullariid species, P. canaliculata (Lamarck, 1822), P. scalaris (d'Orbigny, 1835) and A. platae (Anton, 1838).
Samplings of the three ampullariid species were made in January 2007, February 2011 and February 2015 (Southern summer), going once over the entire perimeter of the lake and collecting all ampullariids seen within 2 m from the border.
In a fourth sampling (February 2017), 50 Asolene platae individuals were collected on several days.
Tissue sampling and histological procedures
Snails collected in the 2011 and 2015 samplings were put in an ice/water bath (~4 °C, for 15 min) for inducing relaxation and minimizing pain before careful shell cracking. Samples of the digestive gland (about 3 mm3) were obtained and fixed in 4% paraformaldehyde in PcABS solution (43 mm NaCl, 1.8 mm KCl, 10 mm HEPES and 30 mm EDTA; pH 7.6 (Cueto et al., Reference Cueto, Rodriguez, Vega and Castro-Vazquez2015)) for 2 days at 4 °C. Samples were dehydrated in an ethanol series, cleared with xylene and embedded in a resin-paraffin mixture (1:1, Histoplast®, Argentina). Sections of 5 µm were stained with a trichrome stain (Nuclear Fast Red, Alcian Blue 8GX, eosin) (Dellagnola et al., Reference Dellagnola, Vega and Castro-Vazquez2017), in which the nuclei were stained bright red, glycosaminoglycans were stained deep blue and cytoplasms appeared light blue to purple. Micrographs were taken with a Nikon Eclipse 80i microscope provided with a Nikon DS-Fi1-U3 digital camera.
Digestive gland crushing
Also, pieces of the digestive gland from infected snails were crushed between a glass slide and a coverslip, and the parasites were photographed with an AmScope MU 1000 MP digital camera (Tokyo, Japan), attached to an Olympus Bx51 microscope and measured with ImageJ software (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). The main morphological features of trematode larval forms were identified, including the Neutral Red staining of cephalic glands. Then, each larval form was drawn with the aid of a camera lucida attached to an Olympus CX31 microscope.
Digestive gland fractionation
Digestive glands from the three studied species were collected in the 2011 sampling, and processed according to Vega et al. (Reference Vega, Gamarra-Luques, Koch, Bussmann and Castro-Vazquez2005). This procedure was first described to isolate the C and K morphotypes of an intracellular prokaryont found in ampullariid snails (Castro-Vazquez et al., Reference Castro-Vazquez, Albrecht, Vega, Koch and Gamarra-Luques2002; Vega et al., Reference Vega, Damborenea, Gamarra-Luques, Koch, Cueto and Castro-Vazquez2006; Dellagnola, Reference Dellagnola2015), but it was found that the trematode's larvae contaminate the K fraction. Briefly, the gland was dissected out and glass-homogenized in 4 mL of TE-Az buffer (10 mm Tris, 1 mm EDTA, 0.1% sodium azide; pH = 7.4) per gram of tissue, and the homogenate was cloth-filtered and centrifuged at 750 g for 10 min (and the supernatant was discarded). The precipitate was resuspended in 5 mL of TE-Az buffer and allowed to sediment in a cold water bath for 30 min. Four sequential cycles of washing the precipitate in TE-Az buffer were done, with decantation times of 60, 30 and 15 (×2) minutes at 1 g (in that order). The last supernatant was discarded while the precipitate thus obtained (‘fraction K’, also containing trematode larvae) was kept frozen for DNA extraction.
Cercarial emergence
Fifty specimens of A. platae from Regatas Lake were collected in February 2017. To promote the emergence of cercariae, snails were placed separately in vials containing 100 mL of tap water, and then directly illuminated with a light bulb for 3 h. The cercariae that emerged were either photographed or used for camera lucida drawings and measurements. The main morphological features of trematode larval forms (either unstained or Neutral Red stained) were identified. Other cercariae released from the snail were stored in 96° ethanol at −20 °C until DNA extraction (see next paragraph).
DNA extraction, PCR amplification and sequencing studies of the cercariae from Asolene platae
Trematode larvae were obtained from the digestive glands of A. platae using a modification of the subcellular fractionation method described by Vega et al. (Reference Vega, Gamarra-Luques, Koch, Bussmann and Castro-Vazquez2005). Genomic DNA from a pool of 15–20 cercariae from each infected snail (xiphidiocercariae, N = 1; echinocercariae, N = 3) was extracted using Wizard® Genomic DNA Purification Kit (Promega; https://worldwide.promega.com/resources/protocols/technical-manuals/0/wizard-genomic-dna-purification-kit-protocol/), according to the manufacturer's protocol. Alternatively, trematode larvae obtained from the digestive glands of two A. platae individuals (according to Vega et al., Reference Vega, Gamarra-Luques, Koch, Bussmann and Castro-Vazquez2005) were pooled, washed and centrifuged at 2300 g, and then genomic DNA was extracted using the CTAB method. In both protocols, the DNA was suspended in DNAse free water overnight and then incubated for 20 min at 65 °C. The DNA quality was evaluated using the 260/280 absorbance ratio.
Trematode mitochondrial and nuclear gene sequences were amplified and sequenced for phylogenetic studies (Schulenburg et al., Reference Schulenburg, Englisch and Wägele1999; Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003; Van Steenkiste et al., Reference Van Steenkiste, Locke, Castelin, Marcogliese and Abbott2015): the large rRNA subunit (28S rRNA gene), the mitochondrial Cytochrome c Oxidase subunit I (mtCOXI) and the complete non-coding nuclear region of the ITS1, which is placed between the 18S rRNA gene and the beginning of the 5.8S rRNA gene. Oligonucleotide primers and the reaction conditions (cycles of denaturation, annealing and extension) used for each PCR are shown in Supplementary Data 1 (Tables 1 and 2, respectively). All PCRs were performed in a total volume of 20 µL, containing 10 ng of template DNA, 0.2 µ m primers, 0.8 mm nucleotides mix and 1 U of recombinant Taq polymerase (Invitrogen). The reactions were performed in a Mastercycler Gradient thermal cycler (Eppendorf). The PCR products were loaded and run in 0.9% low melting point agarose gel electrophoresis, and then stained with ethidium bromide. All PCR amplicons were purified according to the manufacturer's protocol of the PuriPrep-GP Kit (Inbio Highway, Argentina) and then sequenced by dideoxynucleotide method at Instituto de Biotecnología (INTA-Castelar, Argentina) and Macrogen Inc. (Korea). The electropherograms were analysed, assembled and corrected using Chromas 2.6.2 (Technelysium Pty Ltd.).
Sequence alignment and phylogenetic analyses
The sequences of 28S rRNA, ITS1 and mtCOXI were deposited in GenBank (accession numbers MH532417, MH532418, MH532419, MH532420, MH532421, MH532422, MH532423, MH532424, MH532425, MH532426, MH532427, MH532428, MH532429, MH532430 and MH532431), aligned and then the percentage of identity was calculated (Supplementary Data 2, Tables 3–7) using Clustal Omega (Sievers et al., Reference Sievers, Wilm, Dineen, Gibson, Karplus, Li, Lopez, McWilliam, Remmert and Söding2011). Also, the homologies of the sequences obtained in this work, and those deposited in GenBank® database were analysed using BLASTn search (Zhang et al., Reference Zhang, Schwartz, Wagner and Miller2000).
Homologous sequences of Trematoda and Cestoda were downloaded, aligned (Clustal Omega) and edited with MEGA 7 (Kumar et al., Reference Kumar, Stecher and Tamura2016) in an iterative process. The alignment was then trimmed to the length of the shortest sequence. The final alignments for 28S rRNA, ITS1 and mtCOXI were made with MAFFT version 7 (Katoh et al., Reference Katoh, Rozewicki and Yamada2017). Parameters and nucleotide substitution models were calculated with Smart Model Selection (Lefort et al., Reference Lefort, Longueville and Gascuel2017) using AIC likelihood-based statistical criteria.
Maximum likelihood (ML) trees were inferred using PhyML 3.0 (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). For all trees, the general time reversible model (gamma distributed with invariant sites) was predicted as the best estimator by the Akaike information criterion. A bootstrap analysis (100 replicates) was performed by each phylogenetic hypothesis. Two sequences of Cestoda (for each ML tree) were chosen as outgroup. For 28S rRNA, the proportion of invariable sites was 0.312; the number of discrete gamma categories was 4, and the gamma shape parameter was 0.841. The 28S rRNA ML tree was constructed using 98 taxa with 1454 positions in the last alignment dataset. For ITS1, the proportion of invariable sites was 0.148; the number of discrete gamma categories was 4, and the gamma shape parameter was 1.474. The ITS1 ML tree was constructed using 38 taxa with 1846 positions in the last alignment dataset. For mtCOXI, the proportion of invariable sites was 0.312; the number of discrete gamma categories was 4, and the gamma shape parameter was 0.841. The mtCOXI tree was constructed using 36 taxa with 625 positions in the last alignment dataset.
Results
Trematode larval forms in the digestive gland of A. platae, P. canaliculata and P. scalaris
Asolene platae showed a high overall prevalence of trematode larval forms in the 2007, 2011, 2015 samplings (47%; N = 19 snails). The larvae were found in the haemocoelic spaces and connective tissue of the digestive gland (Fig. 1D). Also, cercariae with an alcianophilic sheath and a well-developed tail (Fig. 1E) were found. On the contrary, P. canaliculata (N = 10 snails) and P. scalaris (N = 13 snails) did not show trematode larval forms in their digestive glands.

Fig. 1. Digestive epithelial cells associated with symbiotic corpuscles and trematodes (trichrome stain) in three sympatric ampullariid species from Regatas Lake (Buenos Aires, Argentina): (A) Asolene platae (Maton, 1809), (B) Pomacea canaliculata (Lamarck, 1822) and, (C) Pomacea scalaris (d'Orbigny, 1835). (D) Haemocoelic spaces and connective tissues of the digestive gland of an individual of A. platae containing numerous trematode larval stages. (E) Individual cercaria of A. platae with an alcianophilic body wall and a well-developed tail. cer, cercariae; gb, germinal balls; hae, haemocoelic spaces; tal; tubuloacinar lumen.
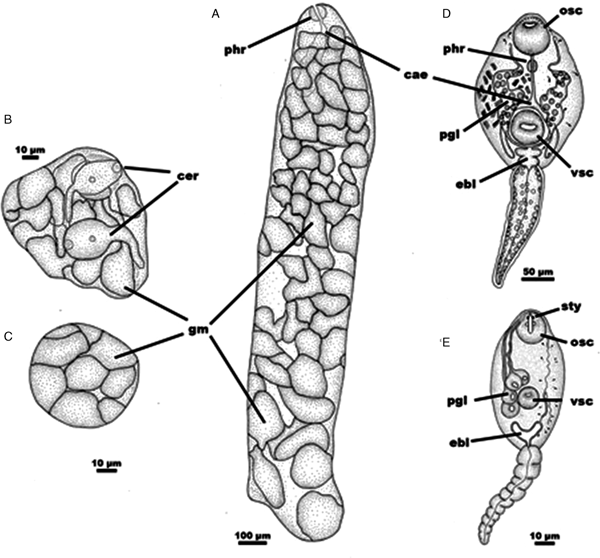
The crushing of digestive gland pieces of infected snails showed many rediae or sporocysts (Fig. 2). Redia had a sac-like body with two posterior appendages, a small pharynx connected with caecum in the first third of the body, and was filled with germinal balls and developing echinocercariae (Fig. 2A). On the other hand, sporocysts containing 2–3 developing xiphidiocercariae and germinal balls were observed (Figs. 2B and C).

Fig. 2. Drawings of the trematode larval stages from Asolene platae (Regatas Lake, Buenos Aires, Argentina). (A) Redia containing numerous germinal balls and immature echinocercariae. (B) Sporocyst containing well-developed xiphidiocercariae. (C) Sporocyst with immature xiphidiocercariae (germinal balls). (D) Mature echinocercaria. (E) Mature xiphidiocercaria. cae, caecum; cer, cercariae; ebl, excretory bladder; gm, germinal balls; osc, oral sucker; pgl, penetration glands; phr, pharynx; sty, stylet; vsc, ventral sucker.
In an independent sampling of A. platae (2017), we found that only 10% of the snails (N = 50) were able to shed cercariae (both xiphidiocercariae and echinocercariae) when placed under constant illumination.
It should be added that numerous C and K symbiotic elements (Castro-Vazquez et al., Reference Castro-Vazquez, Albrecht, Vega, Koch and Gamarra-Luques2002; Vega et al., Reference Vega, Damborenea, Gamarra-Luques, Koch, Cueto and Castro-Vazquez2006; Dellagnola, Reference Dellagnola2015) were observed in all sampled glands of the three species.
Morphological description of echinocercariae from A. platae
Body slender, 221 µm (range = 194–235) long and 158 µm (range = 145–190) wide, without spines. Abundant cystogenous glands containing stick-shaped inclusions were located between the pharynx and posterior end of the ventral sucker and arranged in four bands, two laterals and two central, near the oesophagus. Head collar prominent without spines. Tail as long as body, 199 µm (range = 157–223) long and 53 µm (range = 48–60) wide. Oral sucker 52 µm (range = 46–58) in diameter with 10 small concretions on the anterior margin. Ventral sucker postequatorial similar in size to oral sucker 49 µm (range = 44–56) in diameter. Prepharynx short; pharynx muscular; oesophagus relatively long; intestinal caeca bifurcated anteriorly to the ventral sucker; and wide intestinal caeca reaching the posterior end of the body. Excretory system stenostomate; flame cell formula 2[(2 + 2) + (2 + 2)] = 16, primary excretory ducts twisted between excretory bladder and lateral border of oral sucker and filled with 18–20 large spherical refractive granules; excretory bladder formed by two chambers, the anterior being smaller; excretory bladder continues anteriorly into a short duct that immediately bifurcates into primary excretory ducts; posterior chamber opens into an excretory duct, that opening at the extreme of the posterior tip of tail.
Morphological description of xiphidiocercariae from A. platae
Body ovoid, 79 µm (range = 66–89) long and 38 µm (range = 30–50) wide, entirely covered with small spines, and with abundant cystogens glands. Tail shorter than the body, 61 µm (range = 45–74) long and 14 (range = 12–18) μm long respectively, without fin-folds. Stylet sclerotized, with thickened base and pointed anterior end that reaches anterior body margin, 14 µm (range = 13–15) long and 3 µm (range = 2–3) wide at the base. Oral sucker sub-terminal, 20 µm (range = 18–22) in diameter. Ventral sucker equatorial, small tan oral sucker, 11 µm (range = 10–12) in diameter. The digestive system was not observed due to a large number of cystogenic cells that make the body very opaque. Four pairs of penetration glands at mid-body arranged lateral to ventral sucker, and with finely granular cytoplasmic content; ducts open at anterior extremity symmetrically on both sides of the stylet. Genital primordium not present. Excretory system mesostomate, with excretory ducts bifurcating at both sides of the ventral sucker; flame cell formula 2[(3 + 3 + 3) + (3 + 3 + 3)] = 36; excretory bladder epitheliocystid V-shaped and continuing into the caudal duct that ends at the tip of the tail.
PCR mediated amplification of DNA encoding for the trematode 28S rRNA, ITS1 and mtCOXI genes
Genomic DNA extracted from trematodes of infected A. platae individuals yielded bands of expected sizes, regardless of the extraction and purification protocols used (Supplementary Figs. 1–2). The PCR assays, using the primers sets LSU5f/LSU1500r and DICE1f/DICE14r, showed single bands of approximately 1300 bp and 900 bp for 28S rRNA and mtCOXI genes, respectively. The amplification of ITS1 rRNA, using the primers S20T2f/5.8S1r, showed two bands of ~900 and ~700 bp for echinocercariae and xiphidiocercariae, respectively. No mtCOXI gene sequence was amplified in the presence of template DNA from xiphidiocercariae.
Similarity between DNA isolates of echinocercariae released from A. platae
The percentage of identity amongst echinocercariae sequences showed the existence of two molecular types. The isolates 1 (MH532427, MH532421 and MH532417) and 3 (MH532429, MH532423 and MH532419) were identical, but they differed from that of isolate 2 (MH532428, MH532422 and MH532418) for each molecular marker (28S rRNA, ITS1 and mtCOXI) (Supplementary Data 2, Tables 3–7).
Phylogenetic relationships of trematodes found in Asolene platae (28S rRNA tree)
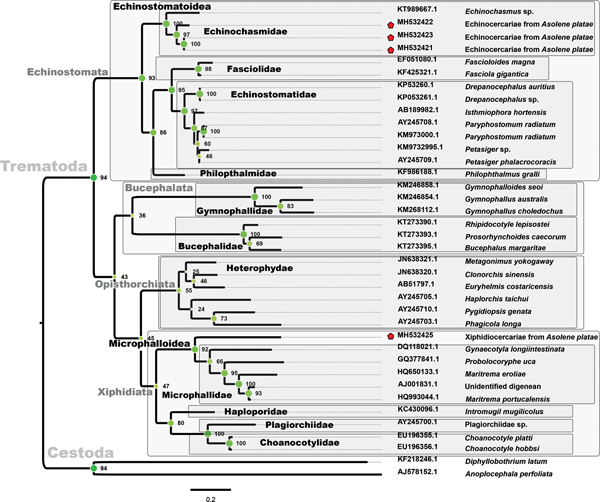
Three sequences of echinocercariae (MH532427, MH532428 and MH532429) and one of a xiphidiocercaria (MH532430) from A. platae, 92 sequences of Trematoda and two of Cestoda were used to infer the phylogenetic position of the parasites (Fig. 3). The ML phylogenetic tree, constructed using 1454 positions in the final alignment dataset, showed a strong support for the order Plagiorchiida (bootstrap value of 100); this order included three suborders, Echinostomata, Opisthorchiata and Xiphidiata. Echinostomata grouped representatives of five families (Echinochasmidae, Psilostomidae, Fasciolidae, Echinostomatidae and Hismanthlidae). The arrangement of Echinochasmidae showed two highly-supported sister clades (bootstrap value of 98): (1) members of the genera Echinochasmus and Stephanoprora, and (2) echinocercariae of A. platae, together with Echinochasmus japonicus, E. costatus, Uroproctepisthmium bursicola and Microparyphium facetum. Three sequences of echinocercariae (MH532427, MH532428 and MH532429) were grouped together (bootstrap value of 67), but only the sequences MH532427 and MH532429 sequences had a high bootstrap value (bootstrap value of 100).

Fig. 3. Phylogenetic relationships of cercariae (red stars) from Asolene platae based on 28S rRNA. Bootstrap values are indicated in each node. The colour value (or saturation*) of each circle filling is proportional to bootstrap support values. Grey-filled squares enclose trematode monophyletic taxa.
The suborder Xiphidiata included ten well-supported families (bootstrap value of 88): Plagiorchiidae, Choanocotylidae Omphalometridae, Telorchiidae, Haematoloechidae, Lecithodendriidae, Phaneropsolidae, Microphallidae, Prosthogonimidae and Pleurogenidae. Two sister clades conformed a monophyletic arrangement (bootstrap value of 93) with the superfamily Microphalloidea Ward, 1901 sensu Tkach et al. (Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003): (1) the families Microphallidae, Prosthogonimidae and Pleurogenidae, and (2) the families Lecithodendriidae and Phaneropsolidae. The xiphidiocercariae from A. platae (MH532430) grouped with the genus Phaneropsolus (bootstrap value of 99) belonging to the family Phaneropsolidae Mehra, 1935.
ITS1 tree
Three sequences of echinocercariae (MH532421, MH532422 and MH532423) and one of a xiphidiocercaria (MH532424) from A. platae, 32 GenBank sequences of Trematoda and two of Cestoda (which were used as outgroup) were used to infer their phylogenetic position (Fig. 4). As expected, the ML phylogenetic tree showed a strong support for the order Plagiorchiida (bootstrap value of 94) with four suborders, Echinostomata, Bucephalata, Opisthorchiata and Xiphidiata. Echinostomata formed a monophyletic group (bootstrap value of 93) with four families (Echinochasmidae, Fasciolidae, Echinostomatidae and Philopthalmidae). Echinochasmidae included the echinocercariae from A. platae and one sequence of Echinochasmus sp. (bootstrap value of 100). Three sequences of echinocercariae (MH532421, MH532422 and MH532423) were grouped together (bootstrap value of 97), although MH532421 and MH532423 sequences had a bootstrap value of 100. The suborder Xiphidiata clustered around four families (Microphallidae, Haploporidae, Plagiorchiidae and Choanocotylidae). The xiphidiocercaria isolated from A. platae (MH532424) formed a clade (bootstrap value of 92) outside of the Microphallidae.

Fig. 4. Phylogenetic relationships of cercariae (red stars) from Asolene platae based on ITS1. Bootstrap values are shown in each node. The colour value (or saturation) of each circle filling is proportional to bootstrap support values. Grey-filled squares enclose trematode monophyletic taxa.
MtCOXI tree
Three sequences of echinocercariae isolated from A. platae, 31 sequences of Trematoda, and two of the outgroup (Cestoda) were used for phylogenetic inference (Fig. 5). The order Diplostomida (sensu Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003) was sister to the order Plagiorchiida (including suborders Pronocephalata, Echinostomata and Xiphidiata), and they were strongly supported (bootstrap value of 100). The Echinostomata was formed by three families (Echinostomatidae, Fasciolidae and Echinochasmidae). Indeed, Echinostomatidae and Fasciolidae conformed a monophyletic group (bootstrap value of 88). The Echinochasmidae included the echinocercariae from A. platae and two sequences of Echinochasmus japonicus (bootstrap value of 98). Three sequences of echinocercariae (MH532417, MH532418 and MH532419) were grouped together, but only the MH532417 and MH532419 sequences had a high bootstrap value (100)

Fig. 5. Phylogenetic relationships of cercariae (red stars) from Asolene platae based on mitochondrial cytochrome oxidase subunit I gene sequences. Bootstrap values are shown in each node. The colour value (or saturation) of each circle filling is proportional to bootstrap support values. Grey-filled squares enclose trematode monophyletic taxa.
Discussion
The study of symbiotic associations of apple snails is important because these gastropods are globally extended in tropical and subtropical aquatic ecosystems and they may be vectors of different human diseases. The search for trematode parasites in three Neotropical ampullariids that coexist in the same environment provided some insights into the interspecific parasite relationships. Echinocercariae and xiphidiocercariae were found (prevalence = 47%) in the digestive gland of A. platae and these parasites were phylogenetically close to the genera Echinochasmus and Phaneropsolus, respectively. This is the first study of trematode parasites in the genus Asolene.
Trematode larval forms in apple snails of Lake Regatas
Infected individuals of A. platae showed a high abundance of trematode larval forms, mainly its rediae and sporocysts, occupying the connective tissue and haemocoelic spaces of the digestive gland (Fig. 1). These parasites seemed to compress the acinar lumina, which may affect their secretory/absorptive function. Epithelial cell starvation provoked by the parasitic blockade of the acinar lumina can start a process of cell autolysis and acinar disorganization, as observed by James (Reference James1965) in Littorina saxatilis (Littorinimorpha, Littorinidae). In an ampullariid as A. platae, it is also possible that protease activity of the intracellular C and K symbiotic elements (Godoy et al., Reference Godoy, Castro-Vazquez and Vega2013) contribute to cell disruption and acinar disorganization. It is notable that no hemocyte aggregations were formed around the trematode larvae in the digestive gland of A. platae, which contrasts with the nodule formation elicited by other immune challenges in the ampullariid P. canaliculata (Rodriguez et al., Reference Rodriguez, Prieto, Vega and Castro-Vazquez2018).
It has been stated that most digenean parasites select hosts within a single family or even a single host species within the family (Adema and Loker, Reference Adema and Loker2015). In this study, trematodes parasites appear to be specific to the genus Asolene. Jokela and Lively (Reference Jokela and Lively1995) have proposed that snail infection by trematodes is associated with shallow-water habits where the hosts spend most of their time. It is probably that A. platae spends more time into the water than species of the genus Pomacea because the former has a lesser degree of specialization for air-breathing (Tiecher et al., Reference Tiecher, Burela and Martín2014) and also has an underwater egg-laying behaviour, contrasting with the aerial egg-laying behaviour of Pomacea species (Hayes et al., Reference Hayes, Burks, Castro-Vazquez, Darby, Heras, Martin, Qiu, Thiengo, Vega, Wada, Yusa, Burela, Cardiemo, Cueto, Dellagnola, Dreon, Frassa, Giraud-Billoud, Godoy, Itualte, Koch, Matsukura, Pasquevich, Rodriguez, Seveanu, Seuffert, Strong, Sun, Tamburi, Tiecher, Turner, Valentine-Darby and Cowie2015). Assuming that miracidia could enter the three ampullariid hosts that coexist in the same environment, the parasites reported here could not avoid the internal defences of both P. scalaris and P. canaliculata. Amongst them, the immune system of P. canaliculata is the only one that has been studied at both the cellular and organ levels (Cueto et al., Reference Cueto, Vega and Castro-Vazquez2013, Reference Cueto, Rodriguez, Vega and Castro-Vazquez2015; Rodriguez et al., Reference Rodriguez, Prieto, Vega and Castro-Vazquez2018), but a variety of symbionts (sensu lato) are still able to dwell in this species (Vega et al., Reference Vega, Damborenea, Gamarra-Luques, Koch, Cueto and Castro-Vazquez2006).
Echinochasmus sensu lato from Asolene platae
Echinocercariae of A. platae (Fig. 2D) resemble other echinostomatoidean cercariae found in different caenogastropod snails. In North America, Echinochasmus donaldsoni from Amnicola limosa (Beaver, Reference Beaver1941) and Cercaria ornatostoma from Goniobasis semicarinata (Cable, Reference Cable1938) were reported. In Venezuela, Echinochasmus zubedakhaname from Pomacea glauca (Nasir and Diaz, Reference Nasir and Diaz1968), and Cercaria udoi and Cercaria paraudoi from P. glauca and Marisa cornuarietis, were reported (Nasir et al., Reference Nasir, Tulio Díaz and Hamana1969). In Argentina, the echinocercariae of A. platae were similar to Cercaria gymnocephala from Heleobia sp. (Ostrowsky de Núñez, Reference Ostrowsky de Núñez1975; Martorelli, Reference Martorelli1985) and Cercaria Echinostomatoidea sp. from Heleobia conexa (Etchegoin and Martorelli, Reference Etchegoin and Martorelli1998). Also, four adults of Echinochasmus have been reported in birds in Argentina; E. wernickii (Marcó del Pont, Reference Marcó del Pont1926), E. suspensun, (Ostrowski de Núñez, Reference Ostrowski de Núñez1974) mentioned as Episthmium suspensum, E. talaensis (Martorelli, Reference Martorelli1985) and Echinochasmus sp. (Labriola and Suriano, Reference Labriola and Suriano1998). The echinocercariae of A. platae showed a morphological organization similar to other Echinochasmus cercariae, but without sensory hairs on their body and tail.
The three ML trees (28S rRNA, ITS1 and mtCOXI) placed the echinocercariae from A. platae inside the superfamily Echinostomatoidea Looss, 1899. Recently, the systematic framework of Echinostomatoidea based on comparative morphology (Kostadinova et al., Reference Kostadinova, Jones, Jones, Bray, Gibson, Jones, Bray and Gibson2005) has been changed by a phylogenetic framework using 28S rRNA data (Tkach et al., Reference Tkach, Kudlai and Kostadinova2016). Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) reported that family Echinostomatidae is a polyphyletic taxon with three strongly supported, family-level clades. Also, they proposed to elevate the subfamily Echinochasminae to full family status, which included sequences of Echinochasmus, Stephanoprora, Uroproctepisthmium and Microparyphium genera. In this work (Fig. 3), two well-supported clades within the family Echinochasmidae were apparent: (a) echinocercariae of A. platae together with Microparyphium facetum, Echinochasmus japonicus, E. coaxatus and Uroproctepisthmium bursicola, and (b) genera Stephanoprora and other sequences from polyphyletic genus Echinochasmus (Tkach et al., Reference Tkach, Kudlai and Kostadinova2016; Besprozvannykh et al., Reference Besprozvannykh, Rozhkovan and Ermolenko2017). Although the mtCOXI and ITS1 databases of Trematode are more limited than the 28 rRNA database, the study of both phylogenetic markers showed that the echinocercariae of A. platae belongs to the family Echinochasmidae closely to the genus Echinochasmus (Figs 4 and 5). The sequenced mitochondrial genome of E. japonicus supports this point of view (Le et al., Reference Le, Nguyen, Nguyen, Doan and Blair2016).
Interestingly, the three molecular markers showed the existence of two echinocercarial entities, which were morphologically undistinguishable. Also, other nuclear and mitochondrial DNA analyses have shown the existence of cryptic trematode species, only distinguished by molecular characters (Miura et al., Reference Miura, Kuris, Torchin, Hechinger, Dunham and Chiba2005; Vilas et al., Reference Vilas, Criscione and Blouin2005; Leung et al., Reference Leung, Keeney and Poulin2009).
Phaneropsolus sp. from Asolene platae
The morphologic features of the xiphidiocercariae (Fig. 2E) are consistent with the characteristics of the xiphidiocercariae armatae belonging to the Opisthoglyphe type (Schell, Reference Schell1970; Grabda-Kazubska, Reference Grabda-Kazubska1971). Although the xiphidiocercaria of A. platae is similar to those described in Heleobia conexa of the Mar Chiquita lagoon (Etchegoin and Martorelli, Reference Etchegoin and Martorelli1998), it does not has a well-developed digestive system. In Argentina, two adults of phaneropsolid species have been reported in chiropterans; Limatulum oklahomense (Milano, Reference Milano2016) and Postorchigenes cf. joannae (Falconaro et al., Reference Falconaro, Vega and Viozzi2017).
The three ML trees placed the xiphidiocercariae of A. platae inside the superfamily Microphalloidea Ward 1901, one of the most diverse clades of digenetic trematodes whose cercariae have a conspicuous, penetrating stylet (Olson et al., Reference Olson, Cribb, Tkach, Bray and Littlewood2003). Although microphalloideans cercariae are common in infected caenogastropod snails (Galaktionov and Skirnisson, Reference Galaktionov and Skirnisson2007; Martorelli et al., Reference Martorelli, Fredensborg, Leung and Poulin2008; Kudlai et al., Reference Kudlai, Stunžėnas and Tkach2015), this is the first report of microphalloidean species in an ampullariid snail. The inclusion of five families into Microphalloidea (Fig. 3) is consistent with the phylogenetic hypothesis proposed in previous studies (Tkach et al., Reference Tkach, Littlewood, Olson, Kinsella and Swiderski2003; Kanarek et al., Reference Kanarek, Zaleśny, Sitko and Tkach2017). The xiphidiocercariae of A. platae was close to the genus Phaneropsolus forming a clade within the family Phaneropsolidae. The ITS1 tree (Fig. 4) placed the xiphidiocercariae of A. platae outside of the family Microphallidae; however, the ITS1 and mtCOXI-based taxonomic structure remain unresolved since these sequence resources are yet scarce.
Human health concerns
Both genera Echinochasmus and Phaneropsolus cause food-borne illness in humans. Echinochasmus japonicus and relatives cause gastrointestinal diseases (Seo et al., Reference Seo, Lee, Chai and Hong1985; Fried et al., Reference Fried, Graczyk and Tamang2004; Toledo and Esteban, Reference Toledo and Esteban2016), and it has been associated with the presence of caenogastropod snails and consumption of raw infected freshwater fishes (second intermediate host). Phaneropsolus species are also found in the human gastrointestinal tract (Manning and Lertprasert, Reference Manning and Lertprasert1973; Tesana et al., Reference Tesana, Srisawangwonk, Kaewkes, Sithithaworn, Kanla and Arunyanart1991; Chai et al., Reference Chai, Han, Shin, Sohn, Yong, Eom, Min, Um, Park and Hoang2009). Although the life cycle is poorly understood, the disease has been associated with the presence of several primate reservoirs, caenogastropod snails and with the consumption of freshwater fishes that predate over insect naiads infected with metacercariae (Manning and Lertprasert, Reference Manning and Lertprasert1973).
Apple snails have been proposed as an alternative protein source for farmed species and they are often consumed by people in several places of Asia and America (Diupotex-Chong et al., Reference Diupotex-Chong, Cazzaniga, Hernández-Santoyo and Betancourt-Rule2004; Hayes et al., Reference Hayes, Joshi, Thiengo and Cowie2008; López-Soriano et al., Reference López-Soriano, Salgado and Tarruella2009; Heuzé and Tran, Reference Heuzé, Tran, Joshi, Cowie and Sebastian2017). From a public health view, the use of apple snails as a protein supplement should consider the detection of parasites in natural aquatic ecosystems and the identification of their complete life cycle, with apple snails acting as intermediate or definitive hosts. In all cases, these molluscs must be fully cooked before consumption, to avoid infections with possible metacercariae or other larval helminth stages they could harbour.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182019000027.
Author ORCIDs
Federico A. Dellagnola, 0000-0002-2192-0477
Acknowledgements
We thank Dr Cristian Rodriguez for careful reading of the manuscript and advice. Also, we thank Marcia Montes for the drawings included in Fig. 2.
Financial support
This work was supported by grants from Universidad Nacional de Cuyo and Agencia Nacional de Promoción Científica y Tecnológica (PICT-2013-1190). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None.
Ethical standards
Sacrifices procedures and tissue sampling were carried out in accordance with the policies of the Committee for Animal Care and Use of the Facultad de Ciencias Médicas de la Universidad Nacional de Cuyo (Approval Protocol N° 55/2015).