INTRODUCTION
Cryptosporidium parvum, a protozoan parasite of the phylum Apicomplexa that causes the gastrointestinal disease cryptosporidiosis, is transmitted directly as infective oocysts by the fecal-oral route, or by ingestion of contaminated food or water (Juranek, Reference Juranek1997; Fayer et al. Reference Fayer, Speer, Dubey and Fayer1997). The infection causes a self-limiting watery diarrhoea in immunocompetent individuals including animals, but can become persistent or fatal, leading to chronic diarrhoea and wasting in immunocompromised hosts (Fayer et al. Reference Fayer, Speer, Dubey and Fayer1997; Clark, Reference Clark1999). Effective drug therapies to treat C. parvum infection are limited.
A considerable effort has been made to understand the basic biology of C. parvum in order to control the disease. Oocysts of C. parvum are excreted with the feces of the host and contain 4 potentially infective sporozoites. Upon ingestion of oocysts, exposure to stomach acid and then bile salts results in the release of sporozoites, which target enterocytes using an active process termed gliding motility, consisting of circular and helical gliding (O'Donoghue, Reference O'Donoghue1995; Wetzel et al. Reference Wetzel, Schmidt, Kuhlenschmidt, Dubey and Sibley2005). During this active process, numerous proteins from organelles including micronemes, dense granules and the rhoptry are secreted onto the apical surface of sporozoites (Mercier et al. Reference Mercier, Adjogble, Däubener and Delauw2005; Smith et al. Reference Smith, Nichols and Grimason2005). The apical discharge of the secretory organelles has been indicated to be essential for cellular invasion, to be dependent on temperature, cytoskeleton and intracellular calcium, and to be capable of proceeding in the absence of host cells (Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and LaRusso2004). However, information on the biochemical and morphological changes taking place within sporozoites during these processes has been scarce.
Sporozoites do not survive well in culture medium, and there is no quick, easy, reliable or inexpensive method of determining their viability, although numerous systems are available for evaluating oocyst viability including the excystation assay, vital dye staining, assays of infectivity in laboratory animals, and cell culture assays (Campbell et al. Reference Campbell, Robertson and Smith1992; Slifko et al. Reference Slifko, Friedman, Rose and Jakubowski1997; Fricker and Crabb, Reference Fricker and Crabb1998). To date, the viability of Cryptosporidium sporozoites has been assessed based on morphology or motility using phase-contrast microscopy (Woodmansee et al. Reference Woodmansee, Powell, Pohlenz and Moon1987) and, more recently, fluorogenic stains (Brown et al. Reference Brown, McDonald, Denton and Coombs1996; Schmidt and Kuhlenschmidt, Reference Schmidt and Kuhlenschmidt2008). It was suggested that excysted sporozoites became smaller and swollen in cell-free medium (King et al. Reference King, Hoefel, Lim, Robinson and Monis2009). However, information about the morphological changes and viability of post-excystation sporozoites has been limited. In the present study, excysted sporozoites of C. parvum were examined ultrastructurally in vitro and their viability was evaluated using a fluorescent dye.
MATERIALS AND METHODS
Parasites
Oocysts of C. parvum, strain HNJ-1, originally obtained from the feces of a patient in Japan (Abe et al. Reference Abe, Kimata and Iseki2002), were passaged in severe combined immunodeficient (SCID) mice, purified by the sugar flotation method, and stored at 4°C in phosphate-buffered saline (PBS, pH 7·2) until 1 month before use. C. parvum sporozoites were prepared as reported (Matsubayashi et al. Reference Matsubayashi, Kimata, Iseki, Lillehoj, Matsuda, Nakanishi, Tani, Sasai and Baba2005). Briefly, the oocysts were washed twice with RPMI 1640 medium (Invitrogen) by centrifugation at 5300 g, 4°C for 3 min. A million (106) oocysts were pre-treated in 0·01N HCl at 37°C for 30 min. After 2 washes with RPMI 1640 medium, the sporozoites were excysted in 0·1% tarodeoxycholatic acid (Sigma) in RPMI 1640 medium containing 10% fetal calf serum (FCS) for 15–20 min. Sporozoites were then washed twice with the RPMI 1640 medium containing 10% FCS by centrifugation at 8300 g, 4°C for 3 min.
Morphological observations
The C. parvum sporozoites prepared as above were incubated in RPMI 1640 medium containing 10% FCS or HEPES Balanced Salt Solution (HBSS) at 37°C for 0, 1, 2 and 3 h, and examined by light microscopy. Two hundred sporozoites were counted and, based on morphology, divided into 4 groups ((1) banana-shaped and more than 5 μm in length, (2) cashew nut-shaped and less than 5 μm in length, (3) rod-shaped and less than 5 μm in length and (4) round) (Fig. 1). These experiments were repeated 5 times.
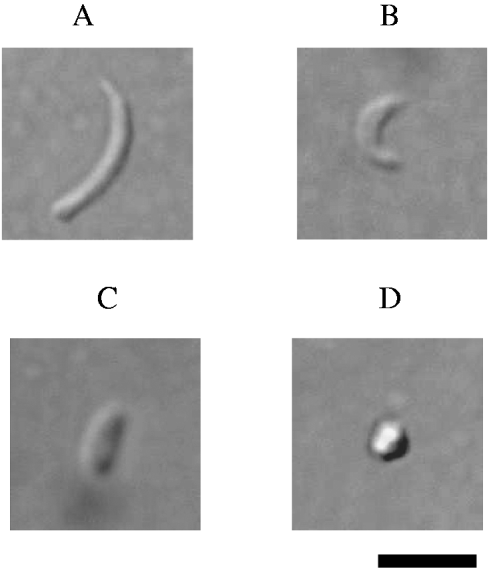
Fig. 1. Photographs of Cryptosporidium parvum sporozoites after incubation for 0–3 h. (A) Banana-shaped and more than 5 μm in length, (B) cashew nut-shaped and less than 5 μm in length, (C) rod-shaped and less than 5 μm in length and (D) round. Scale bar =5 μm.
Transmission electron microscopy (TEM)
The sporozoites were incubated in RPMI 1640 medium containing 10% FCS at 37°C for 0 or 2 h, and prepared for transmission electron microscopy as reported (Matsubayashi et al. Reference Matsubayashi, Takase, Kimata, Nakagawa, Tani, Sasai and Baba2008) except for a few modifications. Briefly, they were fixed with 2% glutaraldehyde (GA, Electron Microscopy Sciences) and 2% paraformaldehyde (PFA, TAAB laboratories Equipment Ltd.) in 0·1 m phosphate buffer (PB, pH 7·4) at room temperature for 2 h, and additionally at 4°C overnight. They were washed with 0·1 m PB containing 0·1 m sucrose (Wako), post-fixed with 1% OsO4 in 0·1 m PB containing 0·1 m sucrose (Wako) at 4°C for 2 h, dehydrated in an ethanol series and embedded in Epon resin. Ultrathin sections were stained with saturated uranyl acetate (TAAB Laboratories Equipment Ltd.) in distilled water for 20 min and Reynold's lead citrate for 3 min, and examined with an electron microscope (H-7500, Hitachi).
Viability assay of C. parvum sporozoites
Sporozoites were incubated in HBSS at 37°C for 0, 1, 2 and 3 h. For each period, sporozoite viability was estimated using the LIVE/DEAD® Reduced Biohazard Viability/Cytotoxicity Kit (L-7013, Invitrogen) according to the manufacturer's instructions except for a few modifications. The basis for the assay is the difference in permeability between live and dead cells. The kit contains a green fluorescent nucleic acid stain (SYTO® 10) that is a highly membrane-permeant dye and labels all cells including those with intact plasma membranes, and a red fluorescent nucleic acid stain (DEAD Red) that is a cell-impermeant dye and labels only cells with compromised membranes. Briefly, after incubation in each tube, an aliquot was centrifuged at 8300 g, 4°C for 3 min, the supernatant was removed, and 200 μl of working dye solution prepared according to the manufacturer's instructions was added. Sporozoites were stained in the dark at room temperature for 15 min and centrifuged at 8300 g, 4°C for 3 min. The pellets were resuspended in 100 μl of HBSS, and 100 μl of 4% GA was added for fixation. Tubes containing samples were stored at room temperature in the dark for 60 min, and aliquots were observed by differential interference contrast and fluorescence microscopy (Nikon). One hundred sporozoites were examined on each microscope slide. The green fluorescence dye stained all sporozoites (FITC filter cube; Ex=480/40, Em= 535/50) while the red fluorescence dye stained only dead sporozoites (TRITC filter cube; Ex=545/30, Em= 620/60). At the same time, the morphological determinations described above were made. These experiments were repeated 3 times.
Statistics
The statistical analysis was conducted using Student's t-test with Statcel2 software. The level of significance was set at a P value of <0·05. The mean and standard error were determined.
RESULTS
The C. parvum sporozoites excysted from oocysts changed morphologically in RPMI 1640 medium or HBSS over 3 h at 37°C (Fig. 2). At 0 h, most sporozoites were banana-shaped, but by 3 h, this type had decreased to 1·4% in RPMI 1640 medium and 4·2% in HBSS as a proportion of the total population of sporozoites. Meanwhile, the numbers of rod-shaped and round sporozoites were increasing. Therefore, the sporozoites appeared to change morphologically, becoming rod-shaped and rounded. There was no significant difference in the proportion of each type of sporozoite between the RPMI 1640 medium and HBSS (P>0·05).

Fig. 2. Morphological type of each sporozoite (%) (banana-shaped, cashew nut-shaped, rod-shaped, and round) after incubation for 0–3 h. Sporozoites were incubated in RPMI 1640 medium (A) and HBSS (B). The experiments were performed 5 times.
We analysed ultrastructurally C. parvum sporozoites at 0 and 2 h in RPMI 1640 medium. At 0 h, sporozoites were banana-shaped, and rhoptry, dense granules, and amylopectin granules at the apical end, and nuclei in the posterior region were seen (Fig. 3A). After 2 h, 4 types of sporozoites (banana-shaped, cashew nut-shaped, rod-shaped and round) were present. In the cashew nut-shaped sporozoites, the distance between the apical end and nucleus was markedly shortened, dense granules were present close to the rhoptry in the apical region, amylopectin granules had disappeared, and micronemes were observed in some zoites (Fig. 3B–D). The rod-shaped sporozoites were shortened more and changed to a club-like appearance (Fig. 3D). In Fig. 3E, some dense granules existed, membranes of a round sporozoite were found to be less clear than those of other sporozoites, and the inner membrane complex of the sporozoite in some part had disappeared.

Fig. 3. Transmission electron micrographs of Cryptosporidium parvum sporozoites after 0 h (A) and 2 h (B–E) shown as traverse sections. (A) Banana-shaped, (B) cashew nut-shaped, (C and D) rod-shape and (E) round. R, rhoptry; M, micronemes; DG, dense granules; AG, amylopectin granules; N, nuclei. Scale bars=1 μm.
We examined the morphological influence of the LIVE/DEAD® Reduced Biohazard Viability/Cytotoxicity Kit. Figure 4 shows the time-course of changes to sporozoites in the dye solution or in HBSS without the working solution as a control. Each group of sporozoites was similar in both conditions (Fig. 4). Banana-shaped sporozoites were decreased while rod-shaped and round sporozoites increased like that shown in Fig. 1. The proportion of each type of sporozoite showed no significant difference between in the fluorescent dye solution and HBSS (P>0·05). Therefore, the working dye solution for evaluating viability appeared not to influence the morphological changes to sporozoites.

Fig. 4. Morphological rates of 4 groups (banana-shaped, cashew nut-shaped, rod-shaped, and round) after incubation for 0–3 h of post-excysted sporozoites. Sporozoites were incubated in HBSS (A) and fluorescent dye solutions (B). The experiments were performed 5 times.
Using the viability assay kit, the survival of excysted sporozoites was evaluated. At 0–3 h, the rate of viability decreased from 89% to 56% (Fig. 5). The viability of banana-shaped sporozoites was high (84·3%), while that of the cashew nut-shaped (73·3%), rod-shapes (67·3%) and round forms (49·2%) was lower (Table 1).

Fig. 5. Viability of excysted sporozoites of Cryptosporidium parvum using fluorescent staining.
Table 1 Viability of sporozoites of each morphology group at 0–3 h in HBSS with dye solution

DISCUSSION
Post-excysted sporozoites of C. parvum are thought to use active motility to invade host cells. Thus, many researchers have focused on biochemical and physiological analyses of sporozoites to study cryptosporidiosis. However, not much has been done to clarify the biology of sporozoites after excystation. In the present study, we examined the morphological changes to C. parvum sporozoites after excystation in cell-free medium. The sporozoites became shortened and rounder in only 2–3 h, and transformed from a banana-shaped into a rounded one. A few reports suggested that C. parvum sporozoites after excystation swelled and discharged apical region-associated molecules within 2 h (Chen et al. Reference Chen, O'Hara, Huang, Nelson, Lin, Zhu, Ward and LaRusso2004; King et al. Reference King, Hoefel, Lim, Robinson and Monis2009). Our results support these findings in cell-free medium.
The cultivation of C. parvum in host cell-free cultures has been attempted (Hijjawi et al. Reference Hijjawi, Meloni, Ng'anzo, Ryan, Olson, Cox, Monis and Thompson2004; Girouard et al. Reference Girouard, Gallant, Akiyoshi, Nunnari and Tzipori2006; Karanis et al. Reference Karanis, Kimura, Nagasawa, Igarashi and Suzuki2008). C. parvum developed in RPMI 1640 medium containing supplements (Hijjawi et al. Reference Hijjawi, Meloni, Ng'anzo, Ryan, Olson, Cox, Monis and Thompson2004; Karanis et al. Reference Karanis, Kimura, Nagasawa, Igarashi and Suzuki2008), but another author failed to propagate Cryptosporidium spp. in cell-free cultures (Girouard et al. Reference Girouard, Gallant, Akiyoshi, Nunnari and Tzipori2006). Thus, a system for the complete development of C. parvum without host cells was not available. We incubated the sporozoites in RPMI 1640 medium containing 10% FCS for 2 h at 37°C and analysed them ultrastructurally. We could see rounded/swollen or distorted zoites with unclear outer membranes, but not zoites of any developmental stage. By light microscopy, the round sporozoites in the present study (49·2% viability) resembled trophozoites or merozoites cultivated in cell-free media (Hijjawi et al. Reference Hijjawi, Meloni, Ng'anzo, Ryan, Olson, Cox, Monis and Thompson2004; Karanis et al. Reference Karanis, Kimura, Nagasawa, Igarashi and Suzuki2008), but were similar to sporozoites in medium examined up to 24 h later by TEM (Harris et al. Reference Harris, Adrian and Petry2003; Petry et al. Reference Petry, Kneib and Harris2009). Thus, it is necessary to analyse and characterize ultrastructurally cultivated zoites in cell-free media based on our findings to confirm the successful development of C. parvum in vitro.
In the present study, we assessed the viability of sporozoites using a fluorescent assay kit, and analysed morphological changes. The permeability to SYTO® 10 of membranes was promoted in sporozoites 2–3 h after excystation, and the viability of post-excysted sporozoites was found to decrease to 56%, leading to morphological changes. These results suggest depolarization of the sporozoite plasma-membrane following apical organelle discharge. Furthermore, our findings support a recent report that sporozoite infectivity in vitro rapidly declined within 2 h of incubation at 37°C which was accompanied by rapid depolarization of the sporozoite membrane (King et al. Reference King, Hoefel, Lim, Robinson and Monis2009). C. parvum sporozoites appear to lack a mitochondrion (Henriquez et al. Reference Henriquez, Richards, Roberts, McLeod and Roberts2005) and might have limited internal energy resources, and instead, the presence of amylopectin has been suggested as a source of energy within sporozoites (Reduker et al. Reference Reduker, Speer and Blixt1985; McDonald et al. Reference McDonald, McCrossan and Petry1995; Fayer et al. Reference Fayer, Speer, Dubey and Fayer1997). By TEM, amylopectin granules were not observed in sporozoites within 2 h of incubation. Thus, the sporozoites after excystation from oocysts could immediately enter host cells before they can no longer infect. To date, viability assays for C. parvum sporozoites have been limited. This fluorescent staining method appeared to be useful, inexpensive and provides an alternative for analysis of abiotic stress effects on the sporozoites, such as pharmaceutical screening, to more costly and intensive flow cytometric assays or infectivity assays with host cells in vitro.
ACKNOWLEDGEMENTS
This work was partly supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 20580357 to K.S., H.T., M.M. and No. 19700609 to M. M.).








